QuEChERS and HPLC-MS/MS Combination for the Determination of Chloramphenicol in Twenty Two Different Matrices
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC-MS/MS Conditions
2.2. Optimization of Sample Preparation
2.3. Validation Result
3. Experimental Section
3.1. Materials and Methods
3.2. HPLC-MS/MS
3.3. Sample Preparation
3.4. Validation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rejtharová, M.; Rejthar, L. Determination of chloramphenicol in urine, feed water, milk and honey samples using molecular imprinted polymer clean-up. J. Chromatogr. A 2009, 1216, 8246–8253. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No 1430/94 of 22 June 1994 Amending Annexes I, II, III and IV of Council Regulation (EEC) No 2377/90 Laying down a Community Procedure for the Establishment of Maximum Residue Limits of Veterinary Medicinal Products in Foodstuffs of Animal Origin. 1994. Available online: https://ec.europa.eu/health/sites/health/files/files/mrl/regpdf/1994_06_22-1430_en.pdf (accessed on 30 November 2018).
- Commission Decision (2003/181/EC) of 13 March 2003 Amending Decision 2002/657/EC as Regards the Setting of Minimum Required Performance Limits (MRPLs) for Certain Residues in Food of Animal Origin. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32003D0181&from=EN (accessed on 30 November 2018).
- Gaugain, M.; Chotard, M.; Hurtaud-Pessel, D.; Verdon, E. Comprehensive validation of a liquid chromatography—Tandem mass spectrometry method for the confirmation of chloramphenicol in urine including stability of the glucuronide conjugate and efficiency of deconjugation. J. Chromatogr. B 2016, 1011, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Shakila, R.J.; Vyla, S.A.P.; Saravana Kumar, R.; Jeyasekaran, G.; Indra Jasmine, G. Stability of chloramphenicol residues in shrimp subjected to heat processing treatments. Food Microbiol. 2006, 23, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Sniegocki, T.; Gbylik-Sikorska, M.; Posyniak, A. Transfer of chloramphenicol from milk to commercial dairy products—Experimental proof. Food Control. 2015, 57, 411–418. [Google Scholar] [CrossRef]
- RASFF—The Rapid Alert System for Food and Feed. Available online: https://webgate.ec.europa.eu/rasff-window/portal/?event=search ResultList (accessed on 30 November 2018).
- Sniegocki, T.; Gbylik-Sikorska, M.; Posyniak, A. Analytical strategy for determination of chloramphenicolin different biological matrices by liquid chromatography—Mass spectrometry. J. Vet. Res. 2017, 61, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Barreto, F.; Ribeiro, C.; Barcellos, R.; Dalla, T. Determination of chloramphenicol, thiamphenicol, florfenicol and florfenicol amine in poultry, swine, bovine and fish by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2016, 1449, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Guidi, L.R.; Tette, P.A.S.; Fernandes, C.; Silva, L.H.M.; Gloria, M.B.A. Advances on the chromatographic determination of amphenicols in food. Talanta 2017, 162, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Rønning, H.T.; Einarsen, K.; Asp, T.N. Determination of chloramphenicol residues in meat, seafood, egg, honey, milk, plasma and urine with liquid chromatography-tandem mass spectrometry, and the validation of the method based on 2002/657/EC. J. Chromatogr. A 2006, 1118, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Rezende, D.; Filho, N.; Rocha, G. Simultaneous determination of chloramphenicol and florfenicol in liquid milk, milk powder and bovine muscle by LC–MS/MS. Food Addit. Contam. Part A 2012, 37–41. Available online: http://www.tandfonline.com/doi/abs/10.1080/19440049.2011.641161 (accessed on 30 November 2018). [CrossRef] [PubMed]
- Tajik, H.; Malekinejad, H.; Razavi-Rouhani, S.M.; Pajouhi, M.R.; Mahmoudi, R.; Haghnazari, A. Chloramphenicol residues in chicken liver, kidney and muscle: A comparison among the antibacterial residues monitoring methods of Four Plate Test, ELISA and HPLC. Food Chem. Toxicol. 2010, 48, 2464–2468. [Google Scholar] [CrossRef] [PubMed]
- Sniegocki, T.; Posyniak, A.; Zmudzki, J. Determination of chloramphenicol residues in milk by gas and liquid chromatography mass spectrometry methods. Bull. Vet. Inst. Pulawy 2007, 51, 59–64. [Google Scholar]
- Kittler, K.; Radeck, W.; Polzer, J. Investigations on the influence of hydrolysis on the total amount of marker residue and consequences. Conf. Mater. EuroResidue VIII 23–26 May 2016, 2, 129–131. [Google Scholar]
- Berendsen, B.J.; Zuidema, T.; de Jong, J.; Stolker, L.A.; Nielen, M.W. Discrimination of eight chloramphenicol isomers by liquid chromatography tandem mass spectrometry in order to investigate the natural occurrence of chloramphenicol. Anal. Chim. Acta 2011, 700, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Tatarczak-Michalewska, M.; Kowalska, A.; Madejska, A.; Śniegocki, T.; Sroka-Bartnicka, A.; Szymańska-Chargot, M. Effective phospholipid removal from plasma samples by solid phase extraction with the use of copper (II) modified silica gel cartridges. J. Chromatrog. B 2017, 1070, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ellison, S.L.R.; Williams, A. Eurachem/CITAC Guide: Quantifying Uncertainty in Analytical Measurement, Third edition, 2012 ISBN 978-0-948926-30-3. Available online: www.eurachem.org (accessed on 30 November 2018).
- Capability of Detection—Part 2: Methodology in the Linear Calibration Case, This Br. Stand. Is UK Implement. ISO 11843-22000\rincorporating Corrigendum Oct. 2007, Which Should Be Read Conjunction\rwith BS ISO 11843-12000. Available online: https://www.iso.org/obp/ui/#iso:std:iso:11843:-2:ed-1:v1:en (accessed on 30 November 2018).
- SANCO/2004/2726-rev 4 December 2008, Guidelines for the Implementaion of Decision 2002/657/EC. 2008. Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/cs_vet-med-residues_cons_2004-2726rev4_en.pdf (accessed on 30 November 2018).
- Matuszewski, B.K.; Constanzer, M.L. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal. Chem. 2003, 1, 3019–3030. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds chloramphenicol are not available from the authors. |
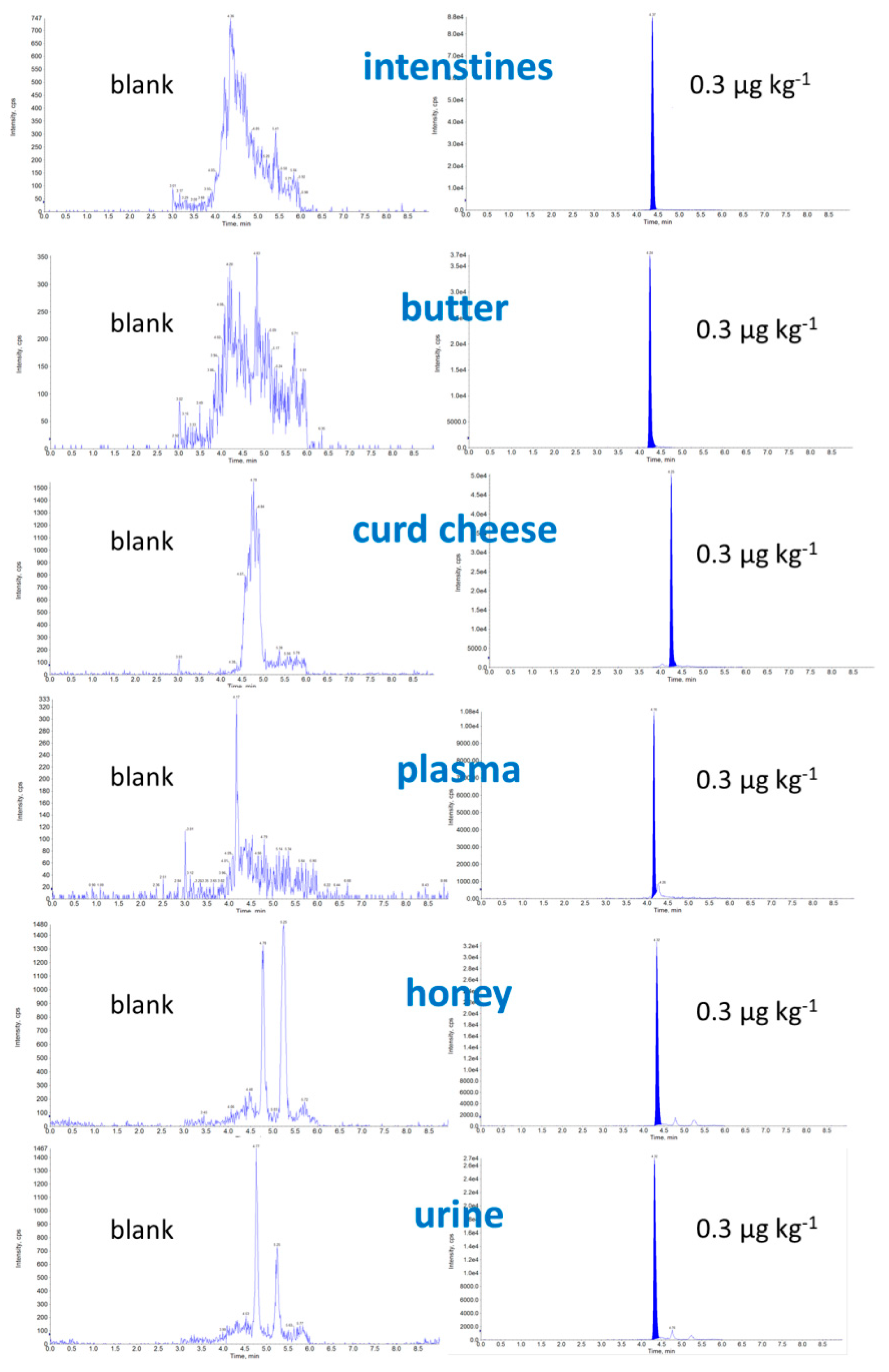
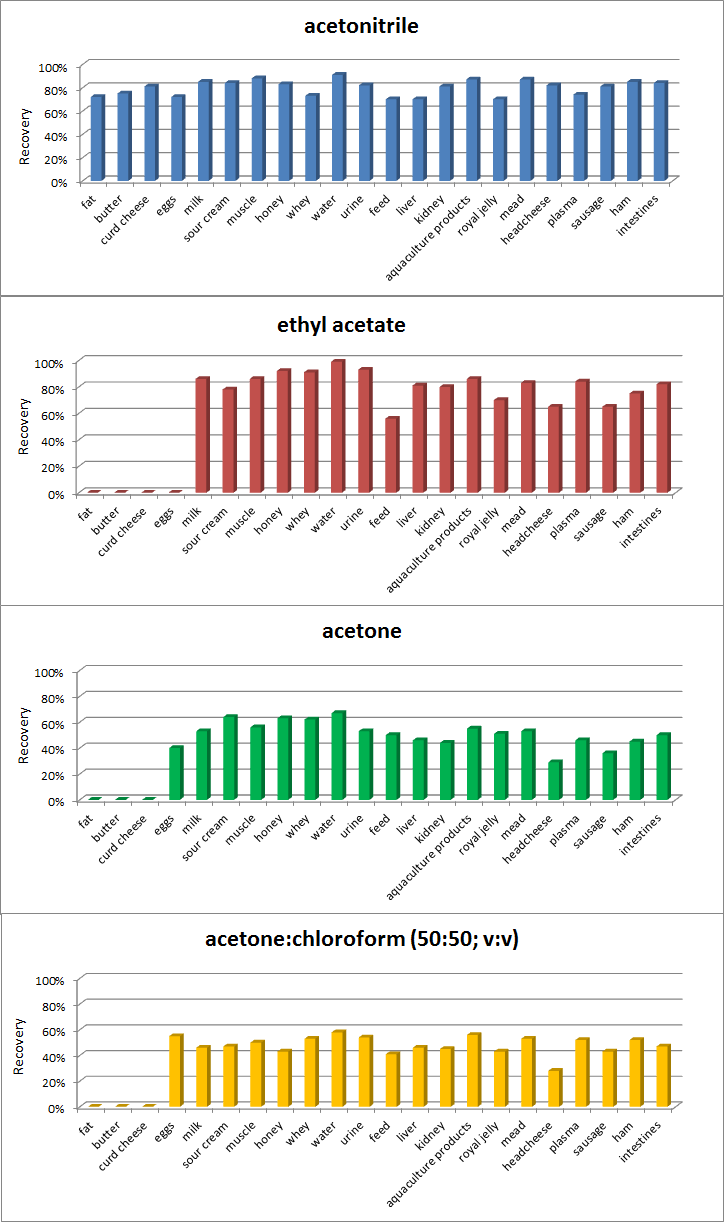
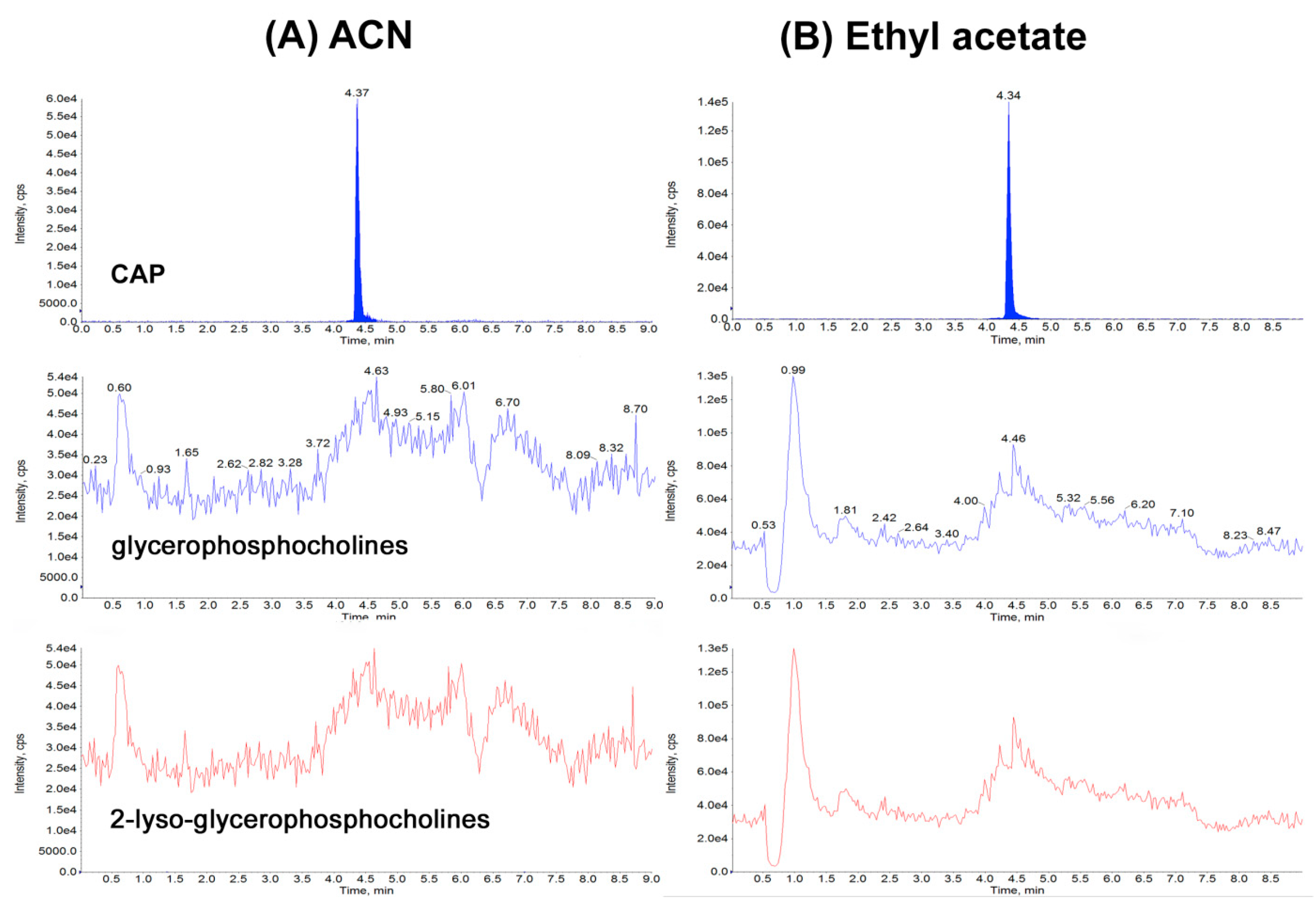
| Matrix | CCα [µg kg−1] | CCβ [µg kg−1] | Matrix Effect (%) | Expanded Uncertainty [µg kg−1] | Concentration Range (ng/mL) | Determination Coefficient | Calibration Curve |
|---|---|---|---|---|---|---|---|
| butter | 0.10 | 0.12 | 8.2 ± 3.1% | 0.30 ± 0.04 | 0.10–2.4 | 0.987 | y = 0.8134 (±0.0412)x + 0.0123 (±0.0006) |
| sour cream | 0.10 | 0.13 | 8.1 ± 2.7% | 0.30 ± 0.03 | 0.10–2.4 | 0.992 | y = 0.7425 (±0.0443)x + 0.0052 (±0.0003) |
| curd cheese | 0.10 | 0.13 | 7.3 ± 3.0% | 0.30 ± 0.04 | 0.10–2.4 | 0.982 | y = 0.7525 (±0.0451)x + 0.0062 (±0.0004) |
| whey | 0.10 | 0.12 | 3.2 ± 1.2% | 0.30 ± 0.04 | 0.10–2.4 | 0.993 | y = 0.7143 (±0.0283)x + 0.0062 (±0.0002) |
| milk | 0.10 | 0.12 | 7.1 ± 2.2% | 0.30 ± 0.04 | 0.10–2.4 | 0.995 | y = 0.7498 (±0.0384)x + 0.0032 (±0.0001) |
| water | 0.10 | 0.12 | 3.1 ± 1.2% | 0.30 ± 0.03 | 0.10–2.4 | 0.998 | y = 0.7604 (±0.0461)x + 0.0067 (±0.0004) |
| feed | 0.15 | 0.18 | 11.1 ± 5.3% | 0.30 ± 0.09 | 0.15–2.4 | 0.983 | y = 0.7724 (±0.0312)x + 0.0228 (±0.0009) |
| urine | 0.15 | 0.18 | 6.9 ± 2.1% | 0.30 ± 0.06 | 0.15–2.4 | 0.985 | y = 0.7424 (±0.0445)x + 0.0148 (±0.0009) |
| plasma | 0.15 | 0.17 | 6.4 ± 2.5% | 0.30 ± 0.04 | 0.15–2.4 | 0.982 | y = 0.8750 (±0.0376)x + 0.0365 (±0.0016) |
| muscle | 0.10 | 0.12 | 5.8 ± 2.9% | 0.30 ± 0.05 | 0.10–2.4 | 0.997 | y = 0.8654 (±0.0519)x + 0.0324 (±0.0019) |
| liver | 0.15 | 0.18 | 9.6 ± 3.2% | 0.30 ± 0.08 | 0.15–2.4 | 0.980 | y = 0.7524 (±0.0381)x + 0.0238 (±0.0014) |
| kidney | 0.15 | 0.17 | 7.5 ± 2.3% | 0.30 ± 0.06 | 0.15–2.4 | 0.984 | y = 0.7124 (±0.0499)x + 0.0103 (±0.0007) |
| fat | 0.10 | 0.12 | 9.1 ± 3.6% | 0.30 ± 0.06 | 0.10–2.4 | 0.993 | y = 0.8023 (±0.0321)x + 0.0224 (±0.0009) |
| eggs | 0.10 | 0.14 | 11.2 ± 3.2% | 0.30 ± 0.06 | 0.10–2.4 | 0.987 | y = 0.7977 (±0.0399)x + 0.0011 (±0.0001) |
| honey | 0.10 | 0.13 | 10.3 ± 3.2% | 0.30 ± 0.08 | 0.10–2.4 | 0.981 | y = 0.8124 (±0.0366)x + 0.0130 (±0.0007) |
| sausage | 0.10 | 0.12 | 8.3 ± 4.4% | 0.30 ± 0.07 | 0.10–2.4 | 0.997 | y = 0.8634 (±0.0518)x + 0.0330 (±0.0019) |
| ham | 0.10 | 0.12 | 6.4 ± 2.6% | 0.30 ± 0.05 | 0.10–2.4 | 0.992 | y = 0.8536 (±0.0427)x + 0.0322 (±0.0019) |
| headcheese | 0.10 | 0.12 | 9.2 ± 4.3% | 0.30 ± 0.06 | 0.10–2.4 | 0.988 | y = 0.8750 (±0.0525)x + 0.0354 (±0.0021) |
| intestines | 0.10 | 0.12 | 9.3 ± 5.0% | 0.30 ± 0.06 | 0.10–2.4 | 0.997 | y = 0.8623 (±0.0321)x + 0.0302 (±0.0012) |
| aquaculture products | 0.10 | 0.13 | 6.3 ± 3.6% | 0.30 ± 0.06 | 0.10–2.4 | 0.996 | y = 0.8670 (±0.0324)x + 0.0352 (±0.0011) |
| royal jelly | 0.10 | 0.13 | 9.9 ± 4.3% | 0.30 ± 0.10 | 0.10–2.4 | 0.984 | y = 0.8029 (±0.0962)x + 0.0123 (±0.0012) |
| mead | 0.10 | 0.12 | 7.3 ± 3.4% | 0.30 ± 0.06 | 0.10–2.4 | 0.987 | y = 0.8122 (±0.0461)x + 0.0098 (±0.0009) |
| Matrix | Repeatability (RSDr, %) | Within-Lab Reproducibility (RSDwR, %) | Apparent Recovery (%) | Repeatability (RSDr, %) | Within-Lab Reproducibility (RSDwR, %) | Apparent Recovery (%) | Repeatability (RSDr, %) | Within-Lab Reproducibility (RSDwR, %) | Apparent Recovery (%) |
|---|---|---|---|---|---|---|---|---|---|
| 0.30 µg kg−1 | 0.45 µg kg−1 | 0.60 µg kg−1 | |||||||
| butter | 6.5 ± 4.0 | 6.6 ± 4.2 | 103.2 ± 2.8 | 6.2 ± 3.8 | 6.6 ± 4.0 | 102.3 ± 3.1 | 6.5 ± 3.2 | 6.3 ± 3.9 | 103.4 ± 2.3 |
| sour cream | 4.7 ± 3.6 | 5.1 ± 4.1 | 102.2 ± 1.5 | 4.6 ± 3.9 | 5.0 ± 4.0 | 101.9 ± 3.5 | 4.3 ± 3.4 | 4.8 ± 3.8 | 101.6 ± 2.9 |
| curd cheese | 6.2 ± 2.3 | 6.5 ± 2.1 | 98.2 ± 3.4 | 6.0 ± 2.1 | 6.3 ± 2.3 | 98.7 ± 3.1 | 6.0 ± 2.0 | 6.0 ± 1.9 | 98.7 ± 2.6 |
| whey | 3.8 ± 5.1 | 5.6 ± 4.3 | 101.3 ± 2.4 | 3.7 ± 4.5 | 5.2 ± 4.1 | 102.3 ± 3.1 | 3.6 ± 4.9 | 4.3 ± 3.3 | 102.9 ± 3.1 |
| milk | 8.0 ± 2.1 | 8.3 ± 2.6 | 98.8 ± 2.5 | 7.5 ± 2.4 | 7.8 ± 2.9 | 98.2 ± 2.9 | 7.7 ± 2.3 | 7.3 ± 3.2 | 99.3 ± 3.2 |
| water | 4.7 ± 3.4 | 5.4 ± 4.1 | 103.5 ± 1.8 | 3.5 ± 3.3 | 4.5 ± 3.9 | 102.7 ± 2.8 | 4.4 ± 3.4 | 4.4 ± 4.0 | 102.9 ± 2.6 |
| feed | 9.1 ± 4.4 | 13.1 ± 5.3 | 91.1 ± 2.8 | 8.9 ± 4.2 | 9.1 ± 5.6 | 94.1 ± 3.2 | 8.6 ± 4.0 | 10.1 ± 4.8 | 94.2 ± 4.6 |
| urine | 8.2 ± 3.9 | 8.9 ± 4.3 | 108.0 ± 4.3 | 7.3 ± 3.9 | 8.3 ± 4.4 | 103.5 ± 3.3 | 8.0 ± 3.2 | 8.7 ± 3.9 | 106.1 ± 3.2 |
| plasma | 8.3 ± 3.5 | 8.6 ± 4.1 | 95.0 ± 6.8 | 7.4 ± 3.6 | 8.4 ± 3.2 | 97.2 ± 5.3 | 7.6 ± 3.4 | 8.0 ± 3.3 | 96.3 ± 4.3 |
| muscle | 6.4 ± 3.2 | 8.4 ± 3.2 | 105.1 ± 4.1 | 6.2 ± 3.1 | 8.1 ± 3.4 | 104.3 ± 3.8 | 6.0 ± 3.5 | 7.9 ± 3.4 | 103.2 ± 3.8 |
| liver | 9.5 ± 4.6 | 10.1 ± 6.3 | 93.1 ± 5.4 | 8.3 ± 4.1 | 9.3 ± 4.4 | 94.5 ± 5.0 | 8.7 ± 4.0 | 9.5 ± 5.4 | 94.2 ± 3.4 |
| kidney | 7.3 ± 3.5 | 8.5 ± 3.1 | 95.2 ± 4.2 | 7.1 ± 3.2 | 8.0 ± 3.2 | 96.1 ± 3.2 | 6.3 ± 3.5 | 8.0 ± 3.3 | 96.3 ± 3.6 |
| fat | 5.5 ± 4.9 | 6.7 ± 4.3 | 104.3 ± 2.4 | 5.0 ± 4.3 | 6.2 ± 4.1 | 104.8 ± 2.7 | 5.2 ± 4.6 | 6.3 ± 3.3 | 102.9 ± 3.1 |
| eggs | 7.0 ± 5.3 | 9.2 ± 5.6 | 94.0 ± 2.3 | 6.3 ± 4.2 | 8.8 ± 5.2 | 96.2 ± 2.3 | 6.2 ± 4.3 | 8.3 ± 5.4 | 96.2 ± 3.3 |
| honey | 9.2 ± 4.3 | 10.7 ± 3.7 | 96.7 ± 3.3 | 7.8 ± 4.1 | 9.9 ± 3.3 | 95.7 ± 3.3 | 8.7 ± 4.5 | 10.1 ± 3.8 | 97.6 ± 2.8 |
| sausage | 9.3 ± 4.3 | 10.6 ± 3.3 | 93.1 ± 3.7 | 8.3 ± 3.6 | 10.0 ± 3.2 | 95.3 ± 2.7 | 9.0 ± 4.7 | 10.0 ± 3.6 | 95.3 ± 3.2 |
| ham | 5.3 ± 3.4 | 6.5 ± 3.4 | 94.1 ± 2.8 | 5.3 ± 3.0 | 6.1 ± 3.0 | 95.2 ± 3.3 | 5.5 ± 3.1 | 6.0 ± 3.1 | 95.2 ± 3.3 |
| headcheese | 5.6 ± 3.2 | 6.9 ± 3.6 | 95.3 ± 4.3 | 5.2 ± 3.2 | 6.4 ± 3.1 | 96.4 ± 3.3 | 5.1 ± 3.5 | 6.7 ± 3.6 | 96.4 ± 4.0 |
| intestines | 6.5 ± 4.0 | 6.5 ± 4.0 | 93.3 ± 5.0 | 5.7 ± 3.7 | 6.1 ± 4.0 | 94.4 ± 4.6 | 5.8 ± 3.3 | 6.2 ± 3.2 | 96.0 ± 4.3 |
| aquaculture products | 5.2 ± 3.4 | 6.3 ± 4.4 | 105.0 ± 2.9 | 4.1 ± 3.3 | 5.9 ± 4.1 | 104.3 ± 3.9 | 4.0 ± 3.6 | 6.3 ± 4.0 | 103.8 ± 3.9 |
| royal jelly | 7.9 ± 4.2 | 11.1 ± 5.1 | 93.1 ± 5.2 | 7.9 ± 4.3 | 10.3 ± 3.9 | 95.5 ± 4.2 | 7.0 ± 4.2 | 9.2 ± 4.1 | 96.4 ± 4.0 |
| mead | 4.2 ± 2.3 | 5.7 ± 4.3 | 97.4 ± 3.8 | 4.0 ± 2.5 | 5.2 ± 3.9 | 96.4 ± 2.8 | 4.6 ± 2.7 | 5.4 ± 3.3 | 98.0 ± 2.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śniegocki, T.; Sell, B.; Giergiel, M.; Posyniak, A. QuEChERS and HPLC-MS/MS Combination for the Determination of Chloramphenicol in Twenty Two Different Matrices. Molecules 2019, 24, 384. https://doi.org/10.3390/molecules24030384
Śniegocki T, Sell B, Giergiel M, Posyniak A. QuEChERS and HPLC-MS/MS Combination for the Determination of Chloramphenicol in Twenty Two Different Matrices. Molecules. 2019; 24(3):384. https://doi.org/10.3390/molecules24030384
Chicago/Turabian StyleŚniegocki, Tomasz, Bartosz Sell, Marta Giergiel, and Andrzej Posyniak. 2019. "QuEChERS and HPLC-MS/MS Combination for the Determination of Chloramphenicol in Twenty Two Different Matrices" Molecules 24, no. 3: 384. https://doi.org/10.3390/molecules24030384
APA StyleŚniegocki, T., Sell, B., Giergiel, M., & Posyniak, A. (2019). QuEChERS and HPLC-MS/MS Combination for the Determination of Chloramphenicol in Twenty Two Different Matrices. Molecules, 24(3), 384. https://doi.org/10.3390/molecules24030384






