Conversion of D-fructose to 5-acetoxymethyl-2-furfural Using Immobilized Lipase and Cation Exchange Resin
Abstract
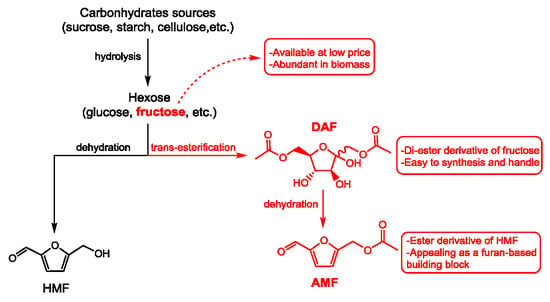
1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Synthetic Procedures and Analysis
3. Results and Discussion
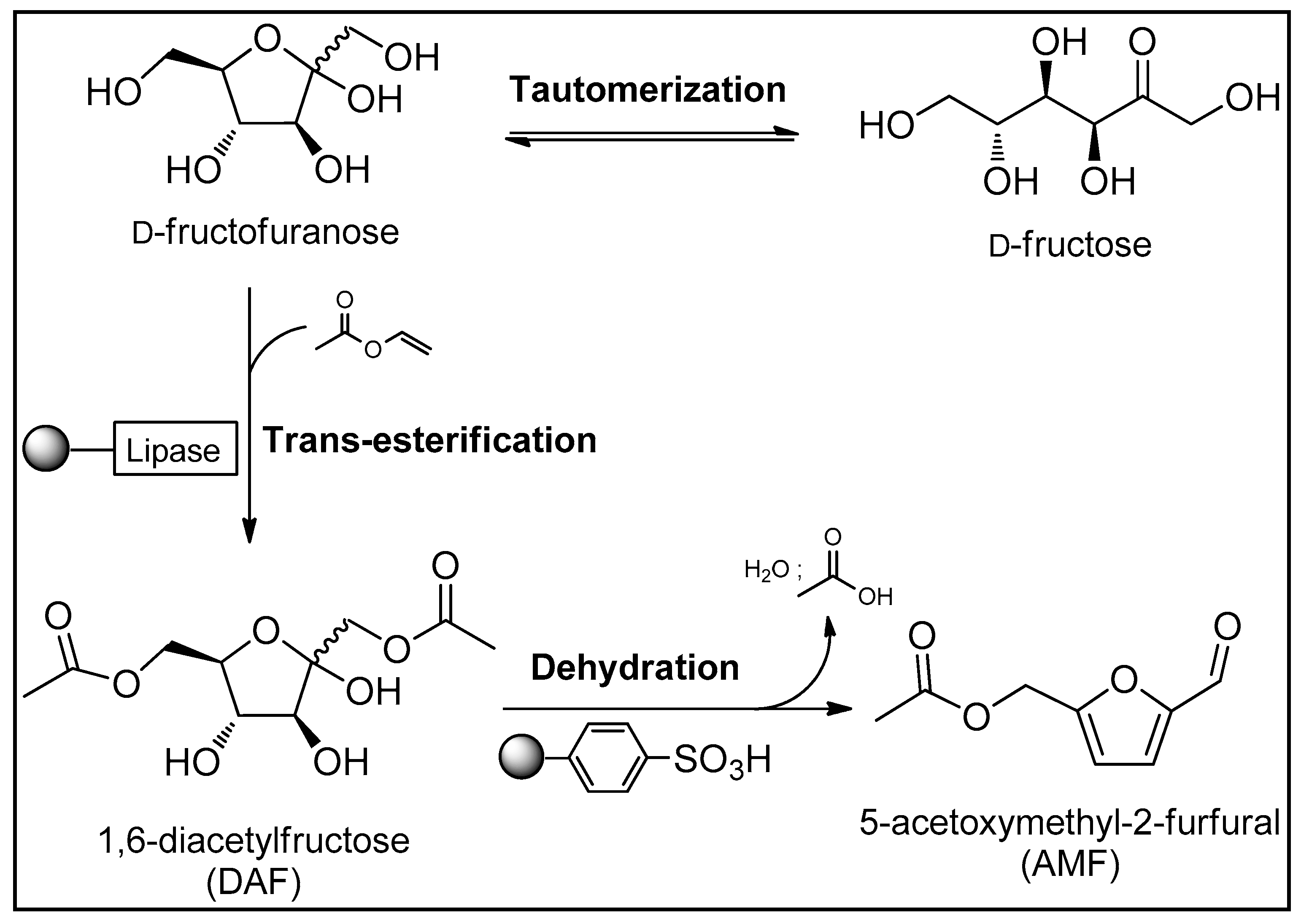
3.1. Trans-Esterification of D-fructose
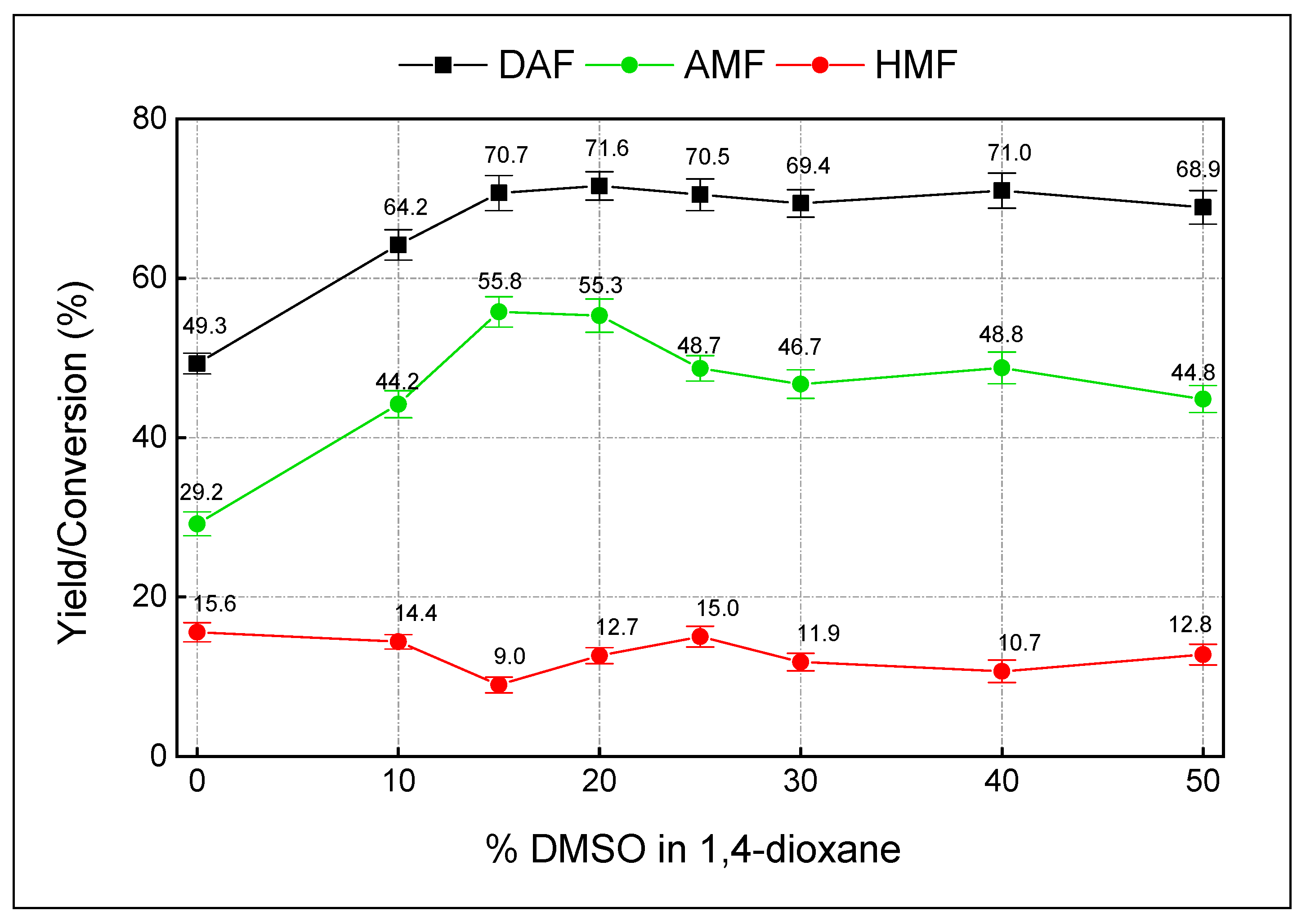
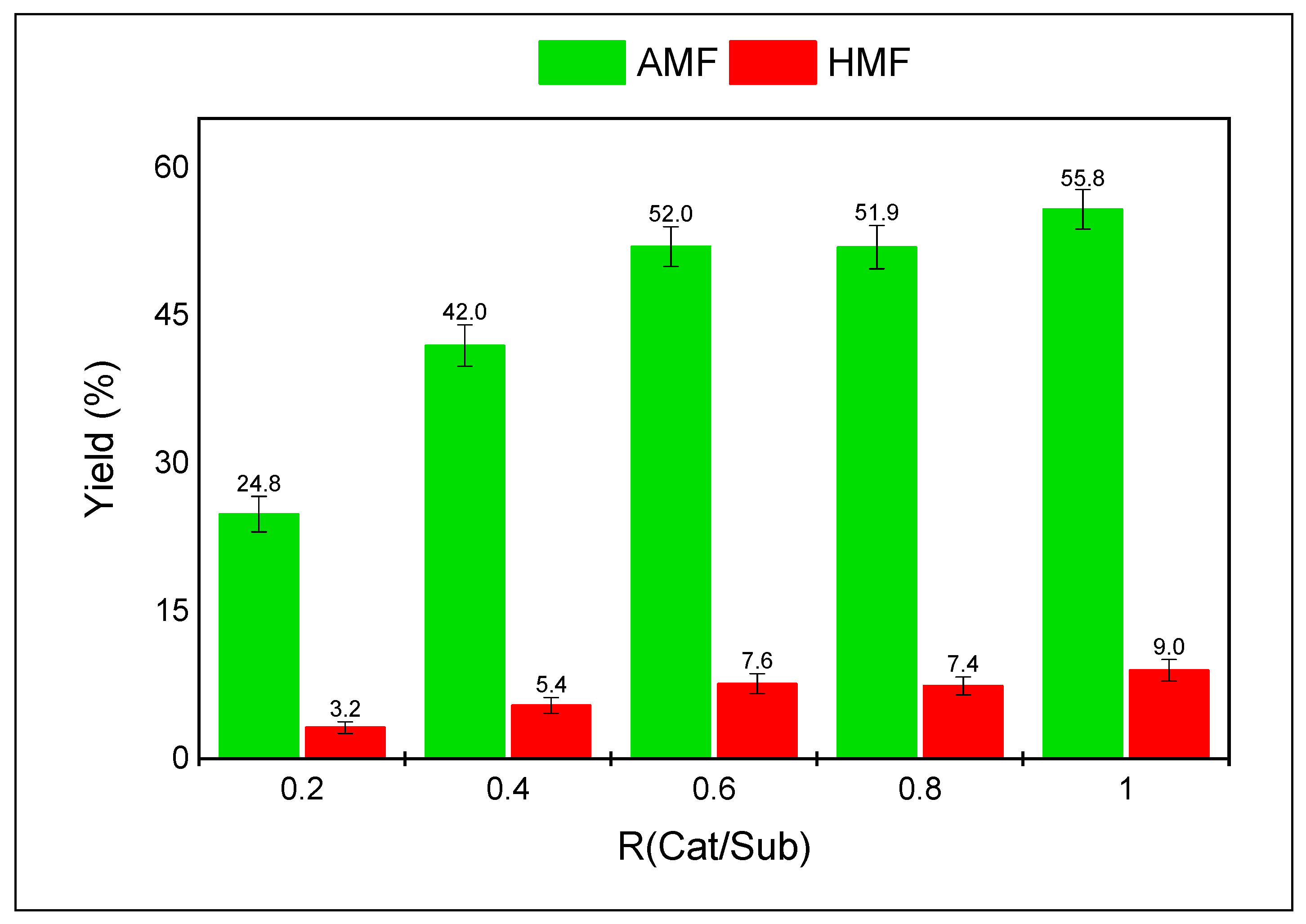
3.2. Dehydration of DAF
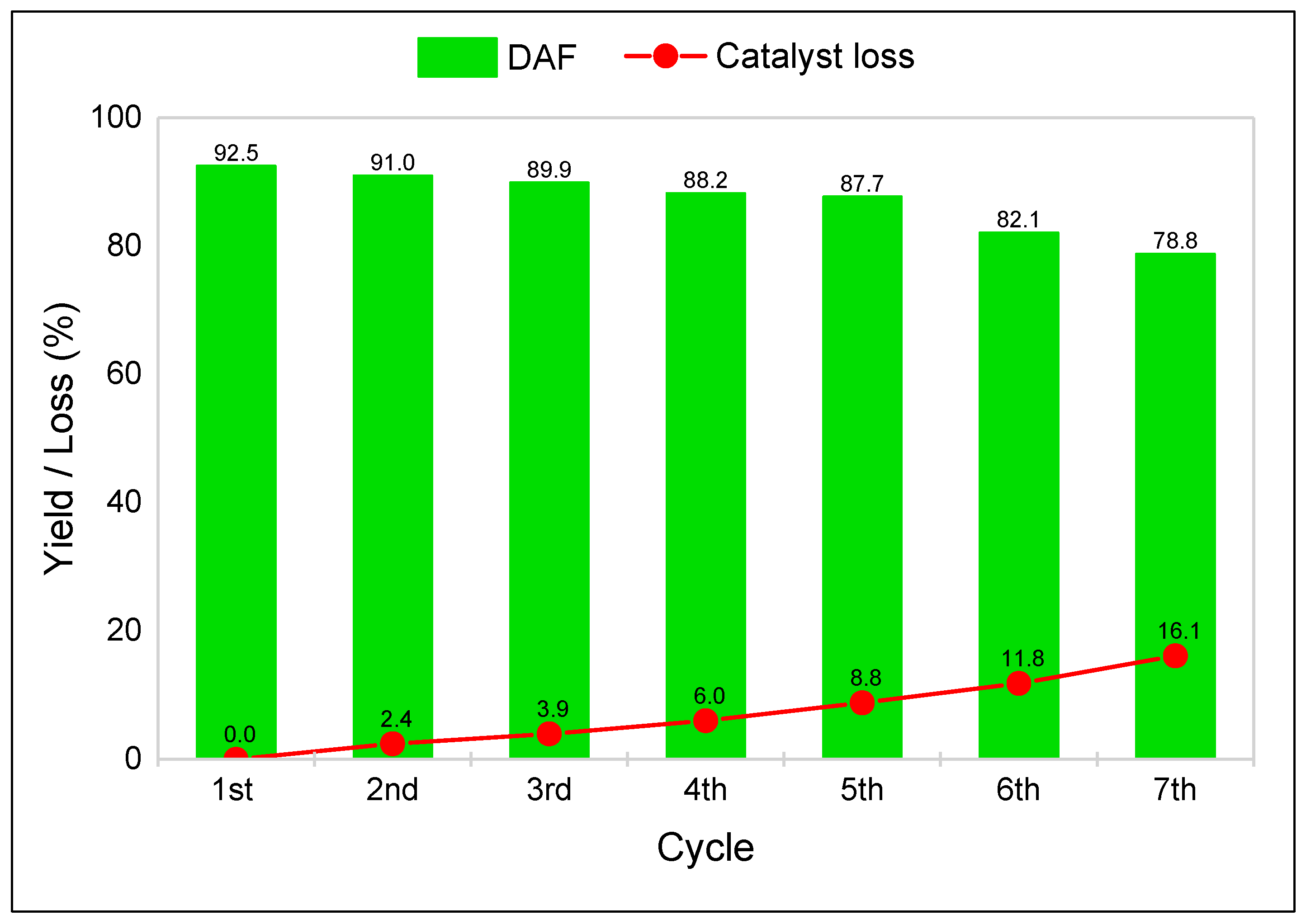
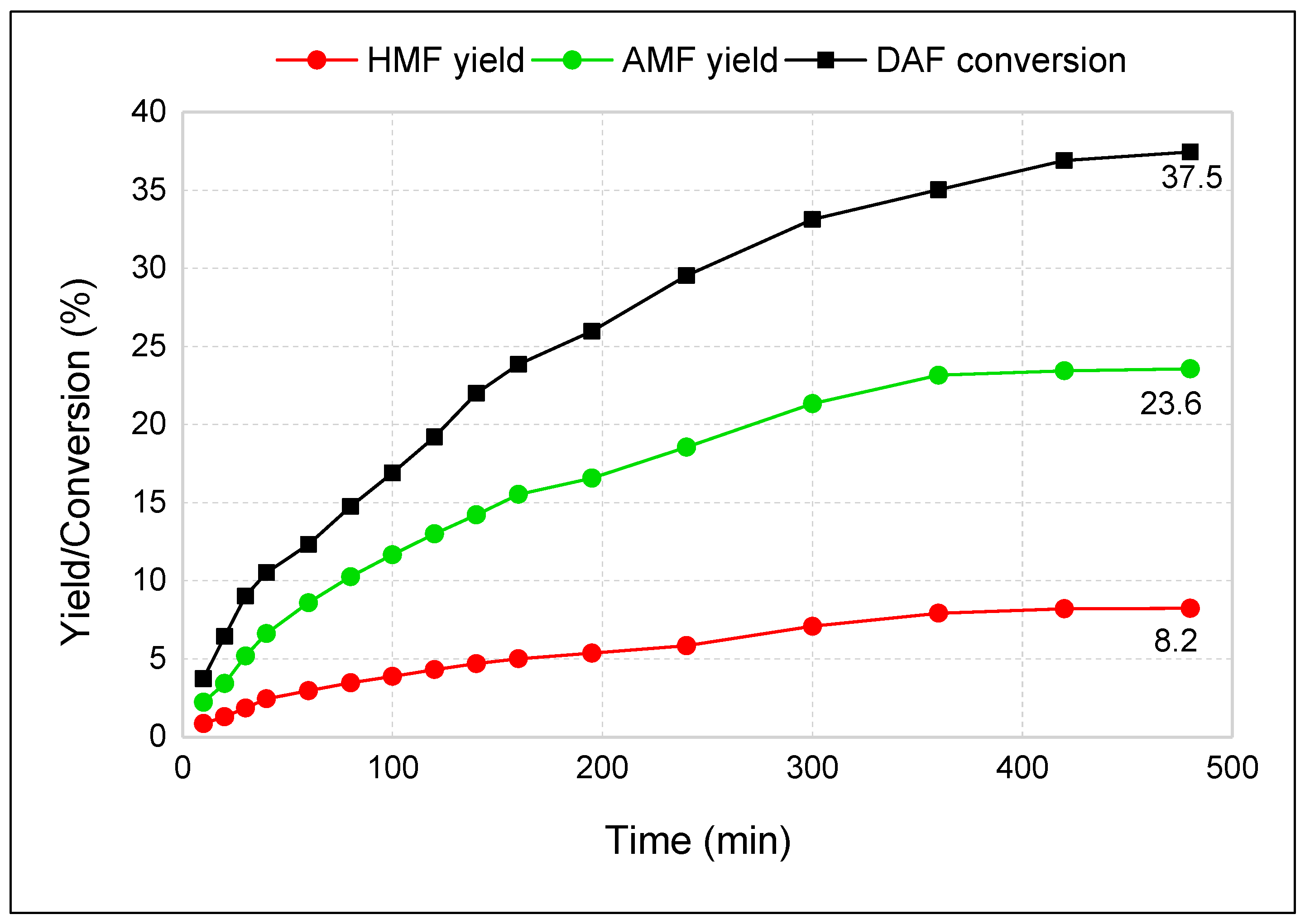
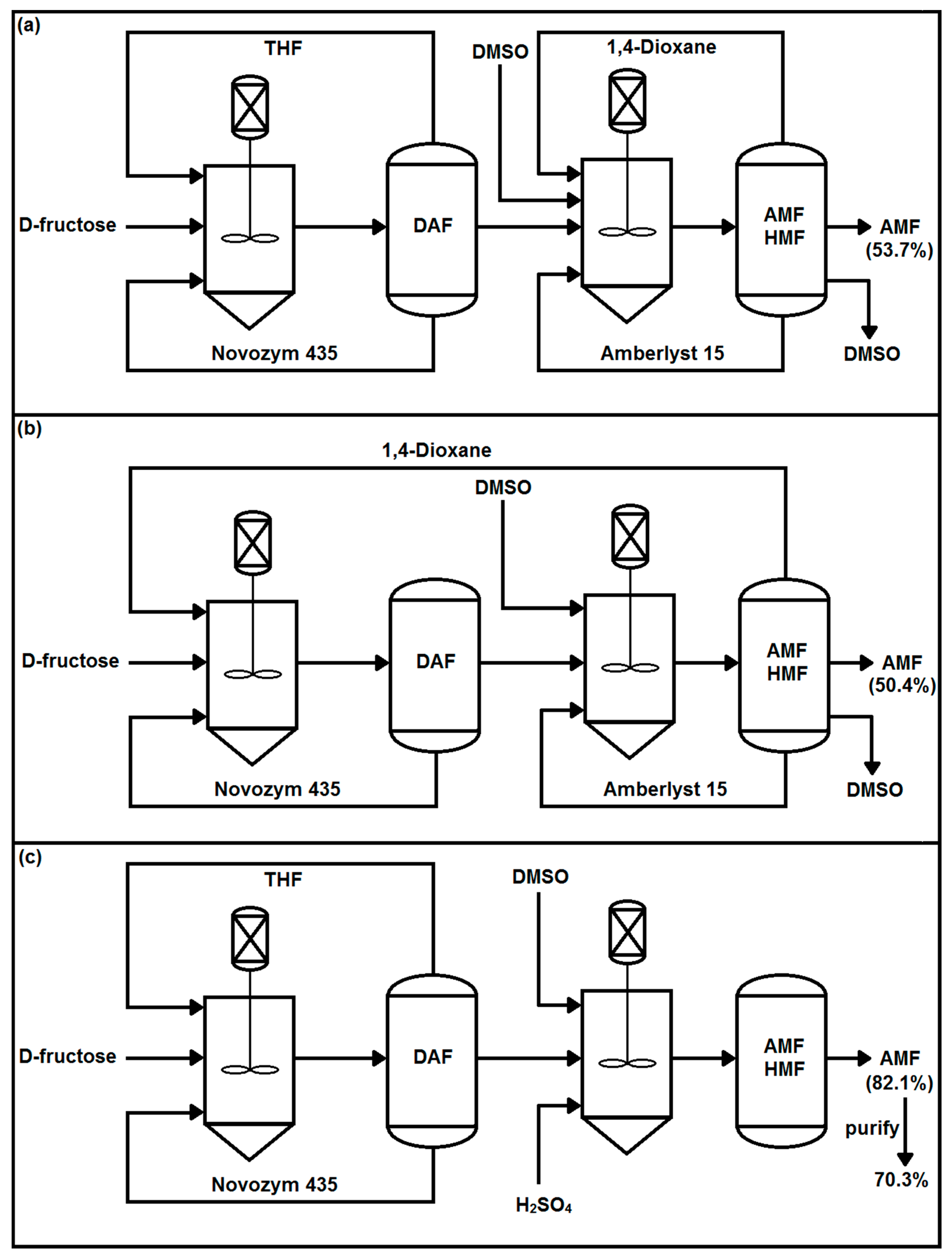
3.3. Two-Step Synthesis Procedure and Separation of AMF
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Narayan, R. Biomass (Renewable) Resources for Production of Materials, Chemicals and Fuels. Symp. A Q. J. Mod. Foreign Lit. 1992. [Google Scholar] [CrossRef]
- Van Putten, R.; van Der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I: Results of Screening for Potential Candidates. In Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab.: Golden, CO, USA, 2004; 76p. [Google Scholar]
- Papers, G. Synthesis, chemistry and applications of 5-hydroxymethyl-furfural and its derivatives. Arkivoc 2005, 2001, 17. [Google Scholar]
- Yu, I.K.M.; Tsang, D.C.W. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Toftgaard Pedersen, A.; Ringborg, R.; Grotkjær, T.; Pedersen, S.; Woodley, J.M. Synthesis of 5-hydroxymethylfurfural (HMF) by acid catalyzed dehydration of glucose-fructose mixtures. Chem. Eng. J. 2015, 273, 455–464. [Google Scholar] [CrossRef]
- Hansen, T.S.; Woodley, J.M.; Riisager, A. Efficient microwave-assisted synthesis of 5-hydroxymethylfurfural from concentrated aqueous fructose. Carbohydr. Res. 2009, 344, 2568–2572. [Google Scholar] [CrossRef] [PubMed]
- Seri, K.; Inoue, Y.; Ishida, H. Highly Efficient Catalytic Activity of Lanthanide (III) Ions for Conversion of Saccharides to 5-Hydroxymethyl-2-furfural in Organic Solvents. Chem. Lett. 2000, 29, 22–23. [Google Scholar] [CrossRef]
- Takagaki, A.; Ohara, M.; Nishimura, S.; Ebitani, K. A one-pot reaction for biorefinery: Combination of solid acid and base catalysts for direct production of 5-hydroxymethylfurfural from saccharides. Chem. Commun. 2009, 41, 6276–6278. [Google Scholar] [CrossRef]
- Bao, Q.; Qiao, K.; Tomida, D.; Yokoyama, C. Preparation of 5-hydroymethylfurfural by dehydration of fructose in the presence of acidic ionic liquid. Catal. Commun. 2008, 9, 1383–1388. [Google Scholar] [CrossRef]
- Shimizu, K.I.; Uozumi, R.; Satsuma, A. Enhanced production of hydroxymethylfurfural from fructose with solid acid catalysts by simple water removal methods. Catal. Commun. 2009, 10, 1849–1853. [Google Scholar] [CrossRef]
- Caes, B.R.; Raines, R.T. Conversion of Fructose into 5-(Hydroxymethyl) furfural in Sulfolane. ChemSusChem 2011, 4, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Catalytic dehydration of fructose into 5-hydroxymethylfurfural by ion-exchange resin in mixed-aqueous system by microwave heating. Green Chem. 2008, 10, 799–805. [Google Scholar] [CrossRef]
- Lansalot-Matras, C.; Moreau, C. Dehydration of fructose into 5-hydroxymethylfurfural in the presence of ionic liquids. Catal. Commun. 2003, 4, 517–520. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Zhou, Y.; Han, B.; Fan, H.; Li, W.; Song, J.; Xie, Y. Conversion of fructose to 5-hydroxymethylfurfural using ionic liquids prepared from renewable materials. Green Chem. 2008, 10, 1280–1283. [Google Scholar] [CrossRef]
- Zakrzewska, M.E.; Bogel-łukasik, E.; Bogel-łukasik, R. Ionic Liquid-Mediated Formation of 5-Hydroxymethylfurfural s A Promising Biomass-Derived Building Block. Chem. Rev. 2011, 111, 397–417. [Google Scholar] [CrossRef] [PubMed]
- Asghari, F.S.; Yoshida, H. Dehydration of fructose to 5-hydroxymethylfurfural in sub-critical water over heterogeneous zirconium phosphate catalysts. Carbohydr. Res. 2006, 341, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Bicker, M.; Hirth, J.; Vogel, H. Dehydration of fructose to 5-hydroxymethylfurfural in sub-and supercritical acetone. Green Chem. 2003, 5, 280–284. [Google Scholar] [CrossRef]
- Carlini, C.; Patrono, P.; Galletti, A.M.R.; Sbrana, G.; Zima, V. Selective oxidation of 5-hydroxymethyl-2-furaldehyde to furan-2,5-dicarboxaldehyde by catalytic systems based on vanadyl phosphate. Appl. Catal. A Gen. 2005, 289, 197–204. [Google Scholar] [CrossRef]
- Kang, E.S.; Hong, Y.W.; Chae, D.W.; Kim, B.; Kim, B.; Kim, Y.J.; Cho, J.K.; Kim, Y.G. From Lignocellulosic Biomass to Furans via 5-Acetoxymethylfurfural as an Alternative to 5-Hydroxymethylfurfural. ChemSusChem 2015, 8, 1179–1188. [Google Scholar] [CrossRef]
- Sanborn, A.J. Archer Daniels Midland Company. Oxidation of Furfural Compounds. U.S. Patent 2,010,034,856, 18 November 2010. [Google Scholar]
- Shaikh, A.; Janka, M.E.; Lange, D.M.; Morrow, M.C.; Bowers, B.R.; Parker, K.R.; Partin, L.R.; Jenkins, J.C.; Moody, P.; Shanks, T.E.; et al. An Oxidation Process to Produce a Crude and/or Purified Carboxylic Acid Product. U.S. Patent 13/228,797, 29 November 2012. [Google Scholar]
- Frank, R.; Jostock, R.; Schick, H.; Theil, F.; Groeger, O.; Kudick, R.; Sonnenschein, H.; Henkel, B. Salts of Substituted Allophanates and Their Use in Drugs. U.S. Patent 8,173,700, 8 May 2012. [Google Scholar]
- D’antona, N.; El-Idrissi, M.; Ittobane, N.; Nicolosi, G. Enzymatic procedures in the preparation of regioprotected D-fructose derivatives. Carbohydr. Res. 2005, 340, 319–323. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Oh, K.; Ko, S.Y.; Kim, J.Y.; Lee, B.; Kim, B.T.; Lee, Y.S.; Min, Y.K.; Park, K. Furan Derivatives For Preventing and Curing Osteoporosis and Pharmaceutical Compositions Containing the Same Technical Field. U.S. Patent 12,125,841, 11 September 2018. [Google Scholar]
- Stergiou, P.Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T. Lipase-catalyzed syntheses of monoacylglycerols. Enzym. Microb. Technol. 1995, 17, 578–586. [Google Scholar] [CrossRef]
- Neena, N.G.; Patil, N.S.; Sawant, S.B.; Joshi, J.B. Lipase Catalyzed Esterification. Catal. Rev. Sci. Eng. 2000, 42, 439–480. [Google Scholar]
- Reetz, M.T. Lipases as practical biocatalysts. Curr. Opin. Chem. Biol. 2002, 6, 145–150. [Google Scholar] [CrossRef]
- Cao, L.; Bornscheuer, U.T.; Schmid, R.D. Lipase-Catalyzed Solid Phase Synthesis of Sugar Esters. Lipid/Fett 1996, 98, 332–335. [Google Scholar] [CrossRef]
- Yahya, A.R.M.; Anderson, W.A.; Moo-Young, M. Ester synthesis in lipase-catalyzed reactions. Enzym. Microb. Technol. 1998, 23, 438–450. [Google Scholar] [CrossRef]
- Jeong, J.; Antonyraj, C.A.; Shin, S.; Kim, S.; Kim, B.; Lee, K.-Y.; Cho, J.K. Commercially attractive process for production of 5-hydroxymethyl-2-furfural from high fructose corn syrup. J. Ind. Eng. Chem. 2013, 19, 1106–1111. [Google Scholar] [CrossRef]
- Ren, L.K.; Zhu, L.F.; Qi, T.; Tang, J.Q.; Yang, H.Q.; Hu, C.W. Performance of Dimethyl Sulfoxide and Brønsted Acid Catalysts in Fructose Conversion to 5-Hydroxymethylfurfural. ACS Catal. 2017, 7, 2199–2212. [Google Scholar] [CrossRef]
- Rong, C.; Ding, X.; Zhu, Y.; Li, Y.; Wang, L.; Qu, Y.; Ma, X.; Wang, Z. Production of furfural from xylose at atmospheric pressure by dilute sulfuric acid and inorganic salts. Carbohydr. Res. 2012, 350, 77–80. [Google Scholar] [CrossRef]
- Kuster, B.F.M. 5-Hydroxymethylfurfural (HMF). A Review Focussing on its Manufacture. Starch—Stärke 1990, 42, 314–321. [Google Scholar] [CrossRef]
- Román-Leshkov, Y.; Chheda, J.N.; Dumesic, J.A. Phase modifiers promote efficient production of hydroxymethylfurfural from fructose. Science 2006, 312, 1933–1937. [Google Scholar] [CrossRef] [PubMed]
- Despax, S.; Maurer, C.; Estrine, B.; Le Bras, J.; Hoffmann, N.; Marinkovic, S.; Muzart, J. Fast and efficient DMSO-mediated dehydration of carbohydrates into 5-hydroxymethylfurfural. Catal. Commun. 2014, 51, 5–9. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Williams, L.T.D.; Ebede, C.C. Mechanism of the dehydration of d-fructose to 5-hydroxymethylfurfural in dimethyl sulfoxide at 150 °C: An NMR study. Carbohydr. Res. 2008, 343, 3021–3024. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, H.E.; Kieboom, A.P.G.; van Bekkum, H. The Conversion of Fructose and Glucose in Acidic Media: Formation of Hydroxymethylfurfural. Starch—Stärke 1986, 38, 95–101. [Google Scholar] [CrossRef]
- Kunin, R.; Meitzner, E.F.; Oline, J.A.; Fisher, S.A.; Frisch, N. Characterization of amberlyst 15 macroreticular sulfonic acid cation exchange resin. Ind. Eng. Chem. Prod. Res. Dev. 1962, 1, 140–144. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds DAF and AMF are available from the authors. |

| Entry | Solvent | DAF yield (%) | ||
|---|---|---|---|---|
| Lipozyme TL IM | Lipozyme RM IM | Novozym 435 | ||
| 1 | H2O | 0.0 | 0.0 | 0.0 |
| 2 | MeOH | 0.0 | 0.0 | 0.0 |
| 3 | EtOH | 0.0 | 0.0 | 0.0 |
| 4 | Acetone | 7.4 ± 1.2 | 0.0 | 12.7 ± 2.4 |
| 5 | THF | 44.3 ± 2.7 | 30.1 ± 2.3 | 96.2 ± 2.8 |
| 6 | Dioxane | 37.4 ± 3.2 | 22.5 ± 1.8 | 90.4 ± 2.5 |
| 7 | DMSO | 0.0 | 0.0 | 0.0 |
| 8 | Ethyl acetate | 9.1 ± 2.1 | 0.0 | 14.4 ± 2.2 |
| 9 | MIBK | 5.7 ± 1.9 | 0.0 | 9.7 ± 1.8 |
| 10 | Hexane | 0.0 | 0.0 | 0.0 |
| 11 | Dichloromethane | 0.0 | 0.0 | 0.0 |
| 12 b | THF | - | - | 95.5 ± 2.9 |
| 13 b | Dioxane | - | - | 92.2 ± 2.3 |
| Entry | Solvent | D-Fructose (g) | D-Fructose Conversion (%) | DAF Yield (%) | DAF Selectivity (%) * |
|---|---|---|---|---|---|
| 1 | THF | 0.5 | 100 | 94.6 | 94.6 |
| 2 | THF | 1.0 | 98.3 | 90.1 | 91.6 |
| 3 | THF | 1.5 | 90.1 | 82.8 | 91.9 |
| 4 | THF | 2.0 | 83.5 | 77.3 | 92.6 |
| 5 | THF | 2.5 | 76.7 | 71.7 | 93.5 |
| 6 | Dioxane | 0.5 | 100 | 91.3 | 91.3 |
| 7 | Dioxane | 1.0 | 95.7 | 86.7 | 90.6 |
| 8 | Dioxane | 1.5 | 88.8 | 80.2 | 90.3 |
| 9 | Dioxane | 2.0 | 79.9 | 75.4 | 94.4 |
| 10 | Dioxane | 2.5 | 73.4 | 69.4 | 94.6 |
| 11 b | THF | 30.0 | 92.0 | 88.0 | 95.6 |
| Solvent | DAF Conversion (%) | AMF Yield (%) | HMF Yield (%) |
|---|---|---|---|
| THF | 12.9 ± 1.0 | 6.1 ± 1.6 | 3.8 ± 0.8 |
| Dioxane | 51.2 ± 1.2 | 29.2 ± 2.1 | 15.6 ± 1.7 |
| DMSO | 59.2 ± 1.4 | 38.7 ± 2.4 | 14.9 ± 1.9 |
| Entry | DAF (g) | DAF Conversion (%) | AMF Yield (%) | HMF Yield (%) |
|---|---|---|---|---|
| 1 | 0.5 | 69.9 | 54.7 | 9.8 |
| 2 | 1.0 | 71.2 | 54.2 | 11.2 |
| 3 | 2.0 | 73.8 | 56.1 | 13.7 |
| 4 | 4.0 | 74.7 | 55.4 | 15.5 |
| 5 | 5.0 | 74.9 | 57.9 | 15.8 |
| Entry | Catalyst - mol% of DAF | Solvent | Time (h) | DAF Conversion (%) | AMF Yield (%) | HMF Yield (%) |
|---|---|---|---|---|---|---|
| 1 | H2SO4-10% | Dioxane | 2 | 69.1 ± 2.3 | 49.9 ± 1.9 | 15.2 ± 2.5 |
| 2 | H2SO4-25% | Dioxane | 2 | 84.6 ± 1.8 | 61.7 ± 1.5 | 17.6 ± 2.2 |
| 3 | H2SO4-50% | Dioxane | 2 | 94.5 ± 2.2 | 59.4 ± 2.8 | 22.7 ± 3.4 |
| 4 | H2SO4-10% | DMSO | 2 | 88.2 ± 1.2 | 73.2 ± 2.7 | 11.7 ± 1.4 |
| 5 | H2SO4-25% | DMSO | 2 | 98.4 ± 1.6 | 86.6 ± 1.7 | 10.4 ± 1.3 |
| 6 | H2SO4-50% | DMSO | 2 | 100 ± 0.0 | 78.1 ± 3.4 | 14.9 ± 3.8 |
| 7 | pTSA-10% | Dioxane | 4 | 66.6 ± 1.1 | 49.3 ± 2.0 | 12.4 ± 1.8 |
| 8 | pTSA-25% | Dioxane | 4 | 85.2 ± 1.3 | 60.9 ± 1.0 | 19.4 ± 2.4 |
| 9 | pTSA-50% | Dioxane | 4 | 97.0 ± 0.9 | 63.7 ± 3.0 | 24.5 ± 3.3 |
| 10 | pTSA-10% | DMSO | 4 | 66.8 ± 2.4 | 52.2 ± 2.5 | 10.3 ± 1.2 |
| 11 | pTSA-25% | DMSO | 4 | 83.4 ± 2.7 | 69.1 ± 2.0 | 10.2 ± 1.2 |
| 12 | pTSA-50% | DMSO | 4 | 100 ± 1.7 | 73.0 ± 2.3 | 19.9 ± 1.6 |
| 13 | Al2O3-10% | Dioxane | 8 | 29.2 ± 1.4 | 22.8 ± 1.1 | 3.9 ± 0.6 |
| 14 | Al2O3-25% | Dioxane | 8 | 30.7 ± 1.7 | 23.9 ± 1.5 | 3.8 ± 1.7 |
| 15 | Al2O3-50% | Dioxane | 8 | 34.7 ± 1.3 | 26.1 ± 1.4 | 4.4 ± 0.8 |
| 16 | Al2O3-100% | Dioxane | 8 | 42.8 ± 2.4 | 31.6 ± 2.0 | 6.9 ± 1.5 |
| 17 | Al2O3-10% | DMSO | 8 | 33.1 ± 1.4 | 24.5 ± 1.5 | 5.2 ± 1.0 |
| 18 | Al2O3-25% | DMSO | 8 | 33.8 ± 0.9 | 24.9 ± 1.3 | 5.0 ± 1.2 |
| 19 | Al2O3-50% | DMSO | 8 | 40.9 ± 1.9 | 28.6 ± 1.5 | 7.0 ± 1.1 |
| 20 | Al2O3-100% | DMSO | 8 | 49.6 ± 1.5 | 39.9 ± 2.3 | 6.2 ± 1.9 |
| 21 | H2SO4-25% | 15%DMSO in dioxane | 2 | 99.1 ± 0.7 | 85.3 ± 0.9 | 11.0 ± 1.3 |
| 22 | pTSA-50% | 15%DMSO in dioxane | 4 | 96.6 ± 1.4 | 76.2 ± 2.9 | 14.8 ± 2.2 |
| 23 | Al2O3-100% | 15%DMSO in dioxane | 8 | 60.9 ± 2.1 | 48.3 ± 1.8 | 6.6 ± 1.5 |
| 24* | H2SO4-25% | DMSO | 2 | - | 95.7 | 3.9 |
| 25* | H2SO4-50% | DMSO | 2 | - | 83.4 | 12.3 |
| 26* | pTSA-25% | DMSO | 4 | - | 94.4 | 4.3 |
| 27* | pTSA-50% | DMSO | 4 | - | 82.1 | 13.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huynh, N.T.T.; Lee, K.W.; Cho, J.K.; Kim, Y.J.; Bae, S.W.; Shin, J.S.; Shin, S. Conversion of D-fructose to 5-acetoxymethyl-2-furfural Using Immobilized Lipase and Cation Exchange Resin. Molecules 2019, 24, 4623. https://doi.org/10.3390/molecules24244623
Huynh NTT, Lee KW, Cho JK, Kim YJ, Bae SW, Shin JS, Shin S. Conversion of D-fructose to 5-acetoxymethyl-2-furfural Using Immobilized Lipase and Cation Exchange Resin. Molecules. 2019; 24(24):4623. https://doi.org/10.3390/molecules24244623
Chicago/Turabian StyleHuynh, Nhan Thanh Thien, Kyung Won Lee, Jin Ku Cho, Yong Jin Kim, Se Won Bae, Jong Shik Shin, and Seunghan Shin. 2019. "Conversion of D-fructose to 5-acetoxymethyl-2-furfural Using Immobilized Lipase and Cation Exchange Resin" Molecules 24, no. 24: 4623. https://doi.org/10.3390/molecules24244623
APA StyleHuynh, N. T. T., Lee, K. W., Cho, J. K., Kim, Y. J., Bae, S. W., Shin, J. S., & Shin, S. (2019). Conversion of D-fructose to 5-acetoxymethyl-2-furfural Using Immobilized Lipase and Cation Exchange Resin. Molecules, 24(24), 4623. https://doi.org/10.3390/molecules24244623