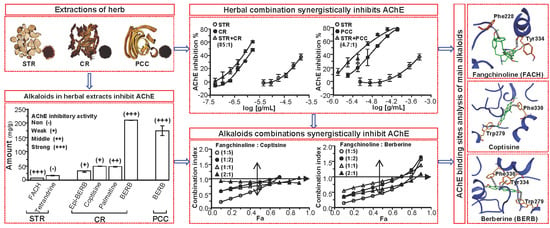
Synergistic Inhibition of Acetylcholinesterase by Alkaloids Derived from Stephaniae Tetrandrae Radix, Coptidis Rhizoma and Phellodendri Chinensis Cortex
Abstract
:1. Introduction
2. Results
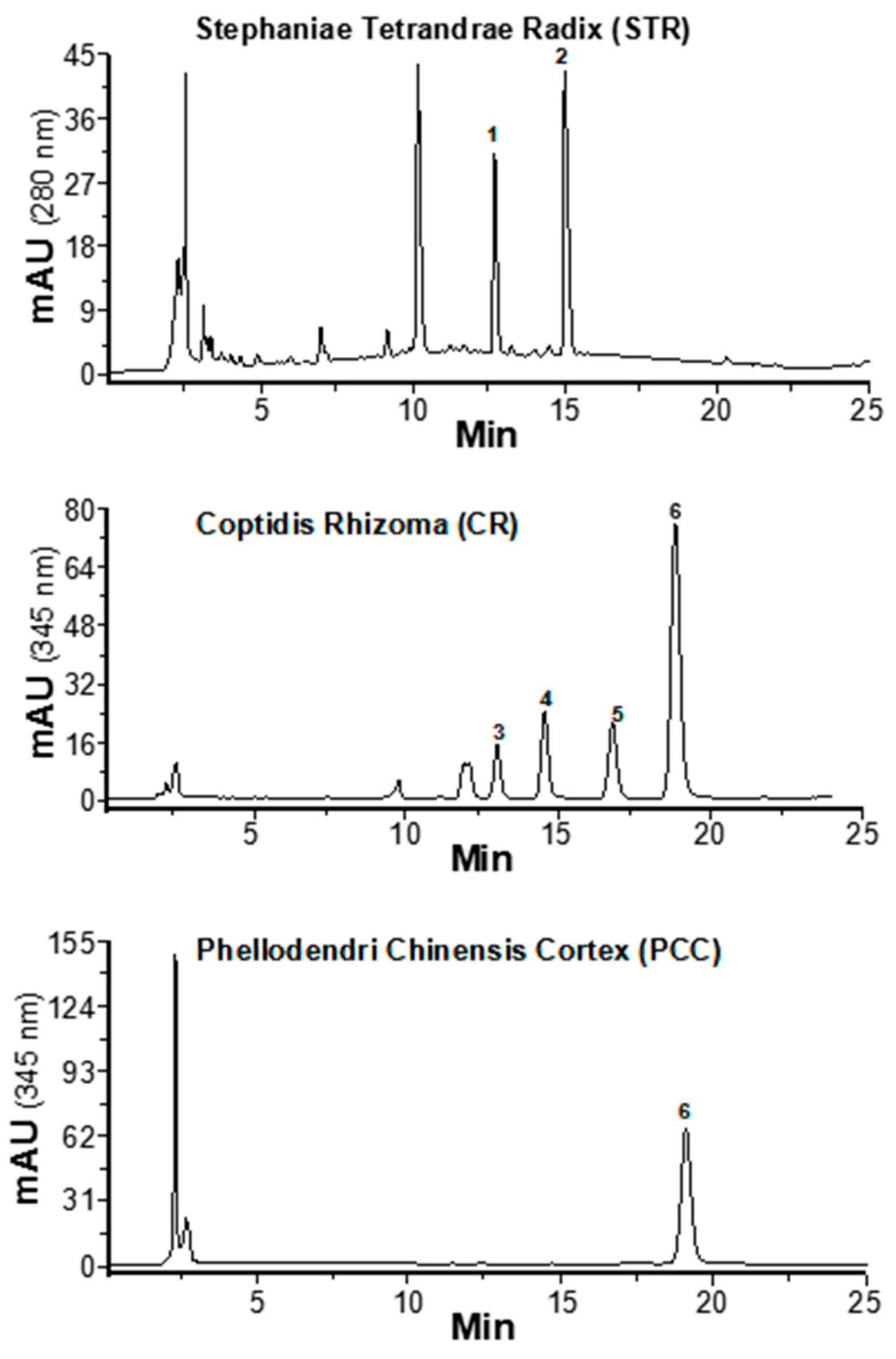
2.1. Determination of Alkaloids in Herbal Extracts
2.2. Herbal Extracts and Their Alkaloids Inhibit AChE
2.3. Herbal Combination Synergistically Inhibits AChE
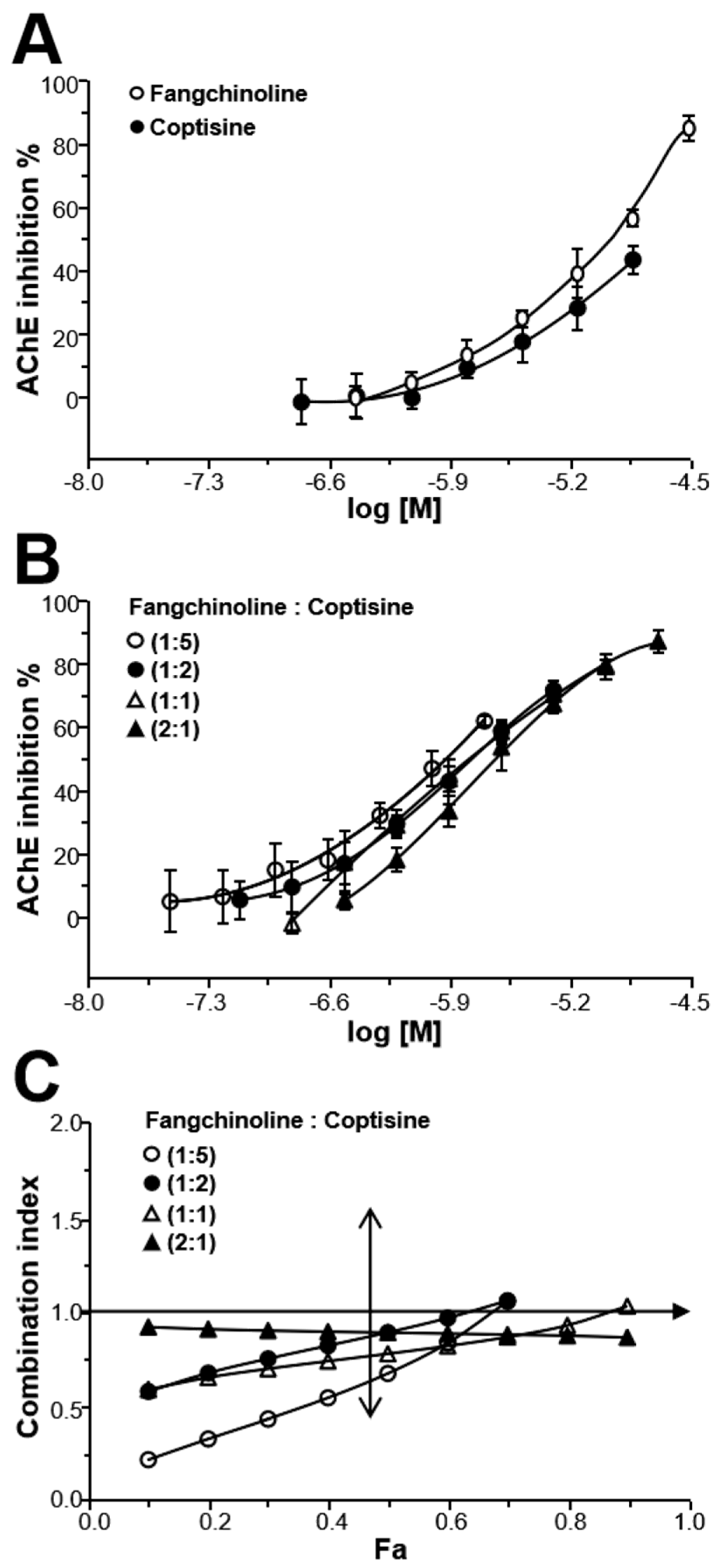
2.4. Fangchinoline and Coptisine Synergistically Inhibit AChE
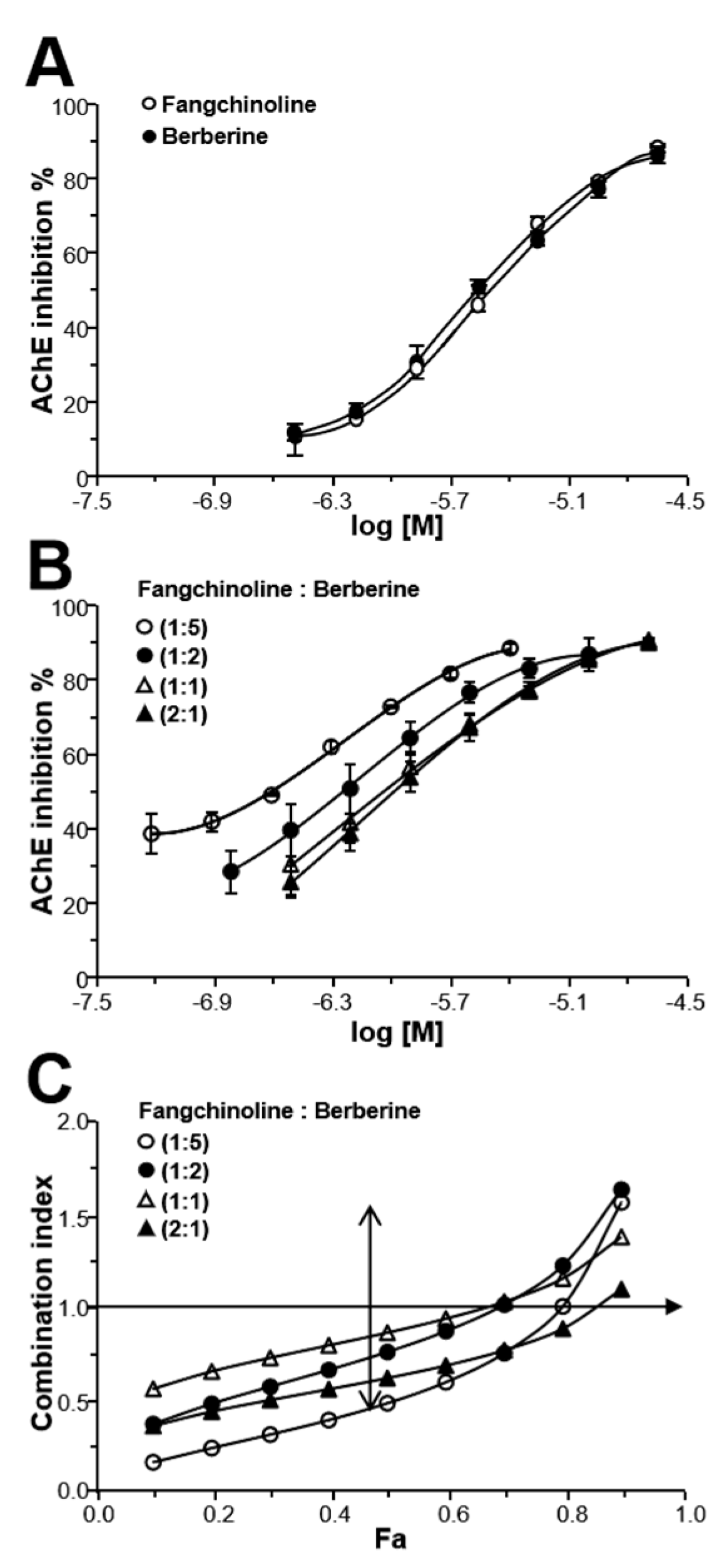
2.5. Fangchinoline and Berberine Synergistically Inhibit AChE
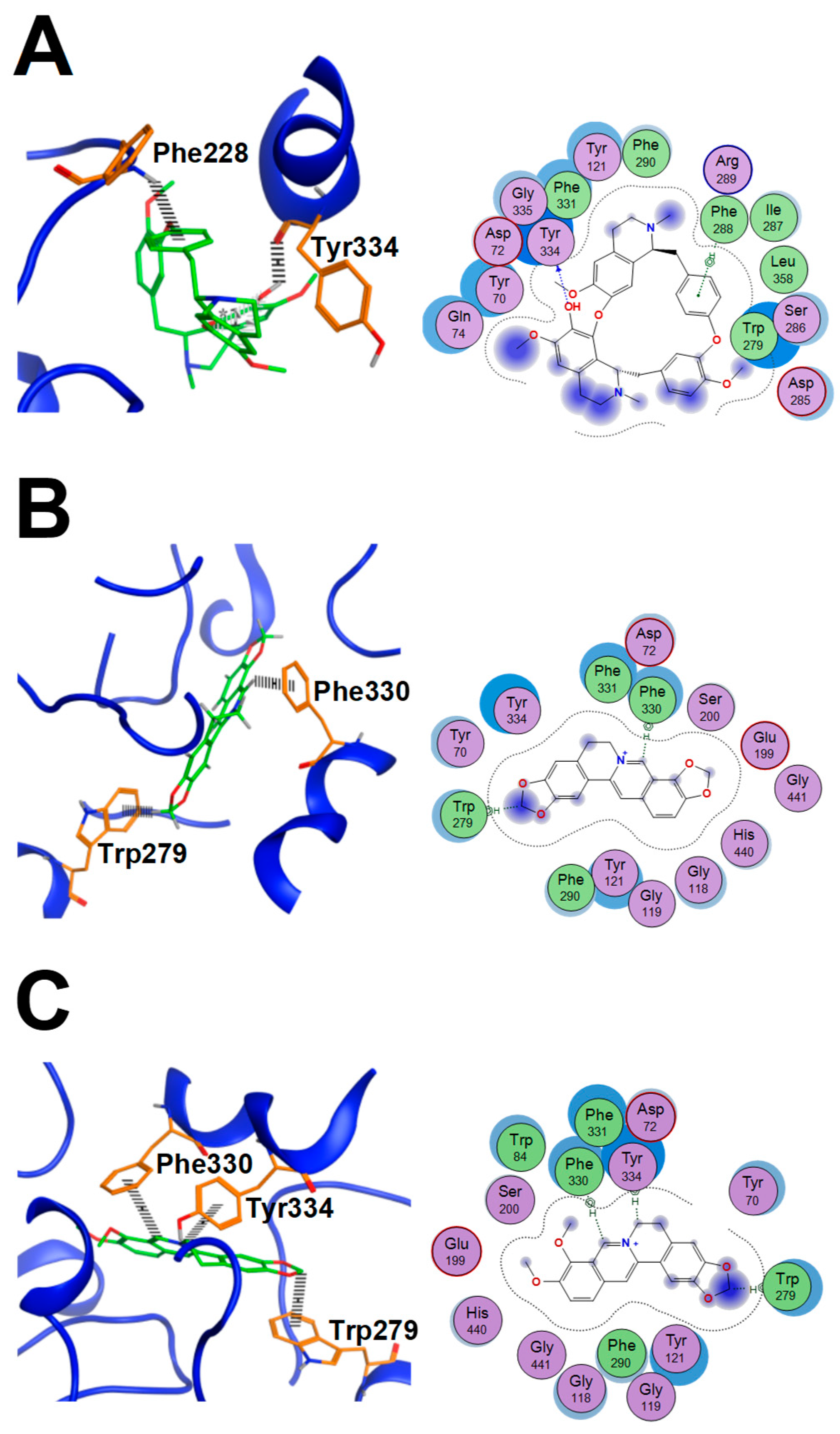
2.6. AChE Binding Sites Analysis of Fangchinoline, Coptisine and Berberine
3. Discussion
4. Materials and Methods
4.1. Chemicals and Preparation of Herb Extracts for HPLC Determination
4.2. Determination of Alkaloids in Herbal Extracts
4.3. Preparation of Herbal Extracts and Alkaloids for AChE Assay
4.4. Ellman Assay
4.5. Synergism Calculation by the Median-Effect Principle
4.6. Molecular Docking
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tsim, K.W.; Soreq, H. Acetylcholinesterase: Old questions and new developments. Front. Mol. Neurosci. 2012, 5, 101. [Google Scholar] [PubMed] [Green Version]
- Campanari, M.L.; García-Ayllón, M.S.; Blazquez-Llorca, L.; Luk, W.K.; Tsim, K.W.; Sáez-Valero, J. Acetylcholinesterase protein level is preserved in the Alzheimer’s brain. J. Mol. Neurosci. 2014, 53, 446–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dierckx, R.I.; Vandewoude, M.F. Donepezil-related toxic hepatitis. Acta Clin. Belg. 2008, 63, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Castellino, S.M.; Tooze, J.A.; Flowers, L.; Hill, D.F.; McMullen, K.P.; Shaw, E.G.; Parsons, S.K. Toxicity and efficacy of the acetylcholinesterase (AChE) inhibitor donepezil in childhood brain tumor survivors: A pilot study. Pediatr. Blood Cancer 2012, 59, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.D.; Zhang, D.B.; Ren, J.; Yang, M.J.; Li, S. Acetylcholinesterase inhibitory activity of the total alkaloid from traditional Chinese herbal medicine for treating Alzheimer’s disease. Med. Chem. Res. 2012, 21, 734–738. [Google Scholar] [CrossRef]
- Murray, A.P.; Faraoni, M.B.; Castro, M.J.; Alza, N.P.; Cavallaro, V. Natural AChE Inhibitors from Plants and their Contribution to Alzheimer’s Disease Therapy. Curr. Neuropharmacol. 2013, 11, 388–413. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, D.; Kaur Dogra, A.; Tahrani, A.; Herrmann, F.; Wink, M. Extracts from traditional Chinese medicinal plants inhibit acetylcholinesterase, a known Alzheimer’s disease target. Molecules 2016, 21, 1161. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Li, J.; Cheung, J.K.; Duan, J.; Ding, A.; Cheung, A.W.; Zhao, K.; Li, W.Z.; Dong, T.T.; Tsim, K.W. Verification of the formulation and efficacy of Danggui Buxue Tang (a decoction of Radix Astragali and Radix Angelicae Sinensis): An exemplifying systematic approach to revealing the complexity of Chinese herbal medicine formulae. Chin. Med. 2007, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.T.; Cheung, J.K.; Li, J.; Chu, G.K.; Duan, R.; Cheung, A.W.; Zhao, K.J.; Dong, T.T.; Tsim, K.W. A Chinese herbal decoction, Danggui Buxue Tang, prepared from Radix Astragali and Radix Angelicae Sinensis stimulates the immune responses. Planta Med. 2006, 72, 1227–1231. [Google Scholar] [CrossRef]
- Choi, R.C.; Gao, Q.T.; Cheung, A.W.; Zhu, J.T.; Lau, F.T.; Li, J.; Li, W.Z.; Chu, G.K.; Duan, R.; Cheung, J.K.; et al. A chinese herbal decoction, danggui buxue tang, stimulates proliferation, differentiation and gene expression of cultured osteosarcoma cells: Genomic approach to reveal specific gene activation. Evid. Based Complement. Altern. Med. 2011, 2011, 307548. [Google Scholar] [CrossRef]
- Zheng, K.Y.; Choi, R.C.; Guo, A.J.; Bi, C.W.; Zhu, K.Y.; Du, C.Y.; Zhang, Z.X.; Lau, D.T.; Dong, T.T.; Tsim, K.W. The membrane permeability of Astragali Radix-derived formononetin and calycosin is increased by Angelicae Sinensis Radix in Caco-2 cells: A synergistic action of an ancient herbal decoction Danggui Buxue Tang. J. Pharm. Biomed. Anal. 2012, 70, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.M.; Li, Z. Study of Fangji-Huangbai gel on analgesic and anti-inflammatory effects. J. Liaoning Univ. Tradit. Chin. Med. 2006, 3, 7–8. [Google Scholar]
- Liu, Y.; Deng, A.J.; Li, X.F.; Li, Z.H.; Zhang, J.L.; Du, G.H.; Qin, H.L. Exclusive control substance of radix Stephaniae tetrandrae. Chin. J. Chin. Mater. Med. 2009, 34, 1943–1948. [Google Scholar]
- Chen, M.L.; Xian, Y.F.; Ip, S.P.; Tsai, S.H.; Yang, J.Y.; Che, C.T. Chemical and biological differentiation of Cortex Phellodendri Chinensis and Cortex Phellodendri Amurensis. Planta Med. 2010, 76, 1530–1535. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, H.S.; Min, K.R.; Kim, Y.; Lim, H.K.; Chang, Y.K.; Chung, M.W. Anti-inflammatory effects of fangchinoline and tetrandrine. J. Ethnopharmacol. 2000, 69, 173–179. [Google Scholar] [CrossRef]
- Sun, Y.F.; Wink, M. Tetrandrine and fangchinoline, bisbenzylisoquinoline alkaloids from Stephania tetrandra can reverse multidrug resistance by inhibiting P-glycoprotein activity in multidrug resistant human cancer cells. Phytomedicine 2014, 21, 1110–1119. [Google Scholar] [CrossRef]
- Jung, H.A.; Min, B.S.; Yokozawa, T.; Lee, J.H.; Kim, Y.S.; Choi, J.S. Anti-Alzheimer and antioxidant activities of Coptidis Rhizoma alkaloids. Biol. Pharm. Bull. 2009, 32, 1433–1438. [Google Scholar] [CrossRef] [Green Version]
- Mak, S.; Luk, W.W.; Cui, W.; Hu, S.Q.; Tsim, K.W.; Han, Y.F. Synergistic inhibition on acetylcholinesterase by the combination of berberine and palmatine originally isolated from Chinese medicinal herbs. J. Mol. Neurosci. 2014, 53, 511–516. [Google Scholar] [CrossRef]
- Kryger, G.; Silman, I.; Sussman, J.L. Structure of acetylcholinesterase complexed with E2020 (Aricept): Implications for the design of new anti-Alzheimer drugs. Structure 1999, 7, 297–307. [Google Scholar] [CrossRef] [Green Version]
- Checler, F.; Turner, A.J. Special issue on Alzheimer’s disease: ‘amyloid cascade hypothesis-20 years on’. J. Neurochem. 2012, 120, iii–iv. [Google Scholar] [CrossRef]
- Schliebs, R.; Arendt, T. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J. Neural Transm. 2006, 113, 1625–1644. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Tang, Y.; Li, W.; Wang, X.; Qiu, Z. Investigation of the binding mode of (−)-meptazinol and bis-meptazinol derivatives on acetylcholinesterase using a molecular docking method. J. Mol. Model. 2006, 12, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Ding, H.L. Alzheimer’s disease: Epidemiology, genetics, and beyond. Neurosci. Bull. 2008, 24, 105–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churcher, I. Tau therapeutic strategies for the treatment of Alzheimer’s disease. Curr. Top. Med. Chem. 2006, 6, 579–595. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Colombres, M.; Nuñez, M.T.; Inestrosa, N.C. Structure and function of amyloid in Alzheimer’s disease. Prog. Neurobiol. 2004, 74, 323–349. [Google Scholar] [CrossRef] [PubMed]
- Giacobini, E. Cholinesterases: New roles in brain function and in Alzheimer’s disease. Neurochem. Res. 2003, 28, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Slowikowski, S.P.; Westaway, D.; Mount, H.T. Interactions between beta-amyloid and central cholinergic neurons: Implications for Alzheimer’s disease. J. Psychiatry Neurosci. 2004, 29, 427–441. [Google Scholar]
- Das, A.; Dikshit, M.; Nath, C. Role of molecular isoforms of acetylcholinesterase in learning and memory functions. Pharmacol. Biochem. Behav. 2005, 81, 89–99. [Google Scholar] [CrossRef]
- Descarries, L.; Aznavour, N.; Hamel, E. The acetylcholine innervation of cerebral cortex: New data on its normal development and its fate in the hAPP (SW, IND) mouse model of Alzheimer’s disease. J. Neural Transm. 2005, 112, 149–162. [Google Scholar] [CrossRef]
- Martini, F.; Pesarico, A.P.; Brüning, C.A.; Zeni, G.; Nogueira, C.W. Ebselen inhibits the activity of acetylcholinesterase globular isoform G4 in vitro and attenuates scopolamine-induced amnesia in mice. J. Cell. Biochem. 2018, 119, 5598–5608. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Alvarez, A.; Pérez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer’s fibrils: Possible role of the peripheral site of the enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef] [Green Version]
- Rees, T.; Hammond, P.I.; Soreq, H.; Younkin, S.; Brimijoin, S. Acetylcholinesterase promotes beta-amyloid plaques in cerebral cortex. Neurobiol. Aging 2003, 24, 777–787. [Google Scholar] [CrossRef]
- Alvarez, A.; Bronfman, F.; Pérez, C.A.; Vicente, M.; Garrido, J.; Inestrosa, N.C. Acetylcholinesterase, a senile plaque component, affects the fibrillogenesis of amyloid-beta-peptides. Neurosci. Lett. 1995, 201, 49–52. [Google Scholar] [CrossRef]
- Alvarez, A.; Opazo, C.; Alarcón, R.; Garrido, J.; Inestrosa, N.C. Acetylcholinesterase promotes the aggregation of amyloid-beta-peptide fragments by forming a complex with the growing fibrils. J. Mol. Biol. 1997, 272, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, V.A.; Parrish, W.R.; Rosas-Ballina, M.; Ochani, M.; Puerta, M.; Ochani, K.; Chavan, S.; Al-Abed, Y.; Tracey, K.J. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav. Immun. 2009, 23, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Shaked, I.; Meerson, A.; Wolf, Y.; Avni, R.; Greenberg, D.; Gilboa-Geffen, A.; Soreq, H. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity 2009, 31, 965–973. [Google Scholar] [CrossRef] [Green Version]
- Durairajan, S.S.; Huang, Y.Y.; Yuen, P.Y.; Chen, L.L.; Kwok, K.Y.; Liu, L.F.; Song, J.X.; Han, Q.B.; Xue, L.; Chung, S.K.; et al. Effects of Huanglian-Jie-Du-Tang and its modified formula on the modulation of amyloid-β precursor protein processing in Alzheimer’s disease models. PLoS ONE 2014, 9, e92954. [Google Scholar] [CrossRef] [Green Version]
- Sangha, J.S.; Sun, X.; Wally, O.S.; Zhang, K.; Ji, X.; Wang, Z.; Wang, Y.; Zidichouski, J.; Prithiviraj, B.; Zhang, J. Liuwei Dihuang (LWDH), a traditional Chinese medicinal formula, protects against β-amyloid toxicity in transgenic Caenorhabditis elegans. PLoS ONE 2012, 7, e43990. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.K.; Yang, H.Q.; Chen, S.D. Traditional Chinese medicine: A promising candidate for the treatment of Alzheimer’s disease. Transl. Neurodegener. 2013, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Wang, C.; Yang, W. Role of berberine in Alzheimer’s disease. Neuropsychiatr. Dis. Treat. 2016, 12, 2509–2520. [Google Scholar] [CrossRef] [Green Version]
- Naaz, H.; Singh, S.; Pandey, V.P.; Singh, P.; Dwivedi, U.N. Anti-cholinergic alkaloids as potential therapeutic agents for Alzheimer’s disease: An in silico approach. Indian J. Biochem. Biophys. 2013, 50, 120–125. [Google Scholar] [PubMed]
- Ng, Y.P.; Or, T.C.; Ip, N.Y. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochem. Int. 2015, 89, 260–270. [Google Scholar] [CrossRef] [PubMed]
- He, L.N.; Liu, Y.W.; Yan, Z.G.; Huang, S.P. Clinical study on improving cognition of patients with vascular dementia by Fangji Dihuang Decoction. J. Sichuan Tradit. Chin. Med. 2011, 29, 65–66. [Google Scholar]
- Liu, N. Observe on the curative effect of Huanglian Jiedu decoction combined with Tianwang Buxin Dan in the treatment of heart and liver Yin deficiency type senile dementia. Mod. J. Integr. Tradit. Chin. West. Med. 2016, 25, 1271–1273. [Google Scholar]
- Xiong, X.J. Fangji Dihuang Decoction formula syndrome and Fengyin decoction formula syndrome: Application in stroke and mental disorders. China J. Chin. Mater. Med. 2019, 44, 602–607. [Google Scholar]
- Khan, T.; Ahmad, R.; Azad, I.; Raza, S.; Joshi, S.; Khan, A.R. Computer-aided drug design and virtual screening of targeted combinatorial libraries of mixed-ligand transition metal complexes of 2-butanone thiosemicarbazone. Comput. Biol. Chem. 2018, 75, 178–195. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, C.; He, W.; Chen, Y. Berberine Alleviates amyloid-beta pathology in the brain of APP/PS1 transgenic mice via inhibiting β/γ-secretases activity and enhancing α-secretases. Curr. Alzheimer Res. 2018, 15, 1045–1052. [Google Scholar] [CrossRef]
- Lu, D.Y.; Tang, C.H.; Chen, Y.H.; Wei, I.H. Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J. Cell. Biochem. 2010, 110, 697–705. [Google Scholar] [CrossRef]
- Lin, T.Y.; Lu, C.W.; Tien, L.T.; Chuang, S.H.; Wang, Y.R.; Chang, W.H.; Wang, S.J. Fangchinoline inhibits glutamate release from rat cerebral cortex nerve terminals (synaptosomes). Neurochem. Int. 2009, 54, 506–512. [Google Scholar] [CrossRef]
- Cho, S.O.; Seong, Y.H. Protective effect of fangchinoline on cyanide-induced neurotoxicity in cultured rat cerebellar granule cells. Arch. Pharm. Res. 2002, 25, 349–356. [Google Scholar] [CrossRef]
- Yu, D.; Tao, B.B.; Yang, Y.Y.; Du, L.S.; Yang, S.S.; He, X.J.; Zhu, Y.W.; Yan, J.K.; Yang, Q. The IDO inhibitor coptisine ameliorates cognitive impairment in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2015, 43, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.F.; Shen, L. Berberine: A potential multipotent natural product to combat Alzheimer’s disease. Molecules 2011, 16, 6732–6740. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Tsim, K.W.; Randall, W.R.; Barnard, E.A. An asymmetric form of muscle acetylcholinesterase contains three subunit types and two enzymic activities in one molecule. Proc. Natl. Acad. Sci. USA 1988, 85, 1262–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Q.Y.; Zhang, C.X. Data Processing System (DPS) software with experimental design, statistical analysis and data mining developed for use in entomological research. Insect Sci. 2013, 20, 254–260. [Google Scholar] [CrossRef]
Sample Availability: Samples of the herbal extracts and alkaloids in this work are available from the authors. |
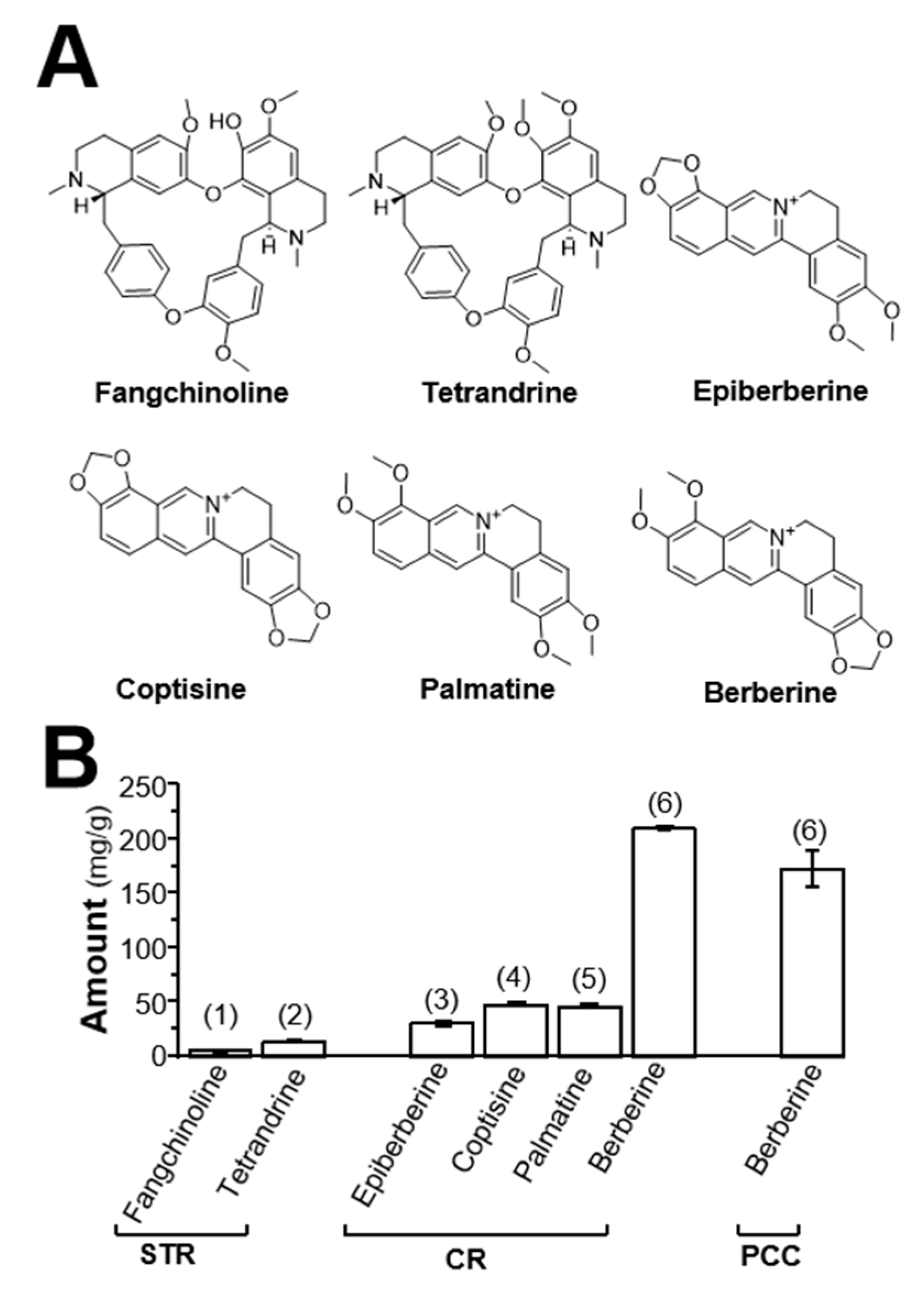

| Alkaloids | Linear Equation | R2 | Linear Range (μg/mL) |
|---|---|---|---|
| Fangchinoline | y = 7541.7x − 5907.9 | 0.9996 | 3.125–200 |
| Tetrandrine | y = 6713x − 21313 | 0.9999 | 6.25–400 |
| Coptisine | y = 37028x − 14419 | 0.9999 | 1.171875–75 |
| Berberine | y = 46365x − 54509 | 0.9998 | 4.6875–300 |
| Palmatine | y = 40443x − 19298 | 0.9998 | 1.171875–75 |
| Epiberberine | y = 32555x − 8332.1 | 0.9998 | 0.625–40 |
| Name | IC50 | AChE Inhibitory Activity 1 |
|---|---|---|
| STR extract | 570.11 ± 10.41 μg/mL | + |
| CR extract | 4.11 ± 0.15 μg/mL | +++ |
| PCC extract | 22.19 ± 3.12 μg/mL | ++ |
| Fangchinoline | 2.17 ± 0.05 μM | +++ |
| Tetrandrine | − | − |
| Coptisine | 13.50 ± 1.48 μM | + |
| Berberine | 2.33 ± 0.16 μM | +++ |
| Palmatine | 6.52 ± 0.84 μM | ++ |
| Epiberberine | 18.70 ± 0.83 μM | + |
| Tacrine 2 | 0.44 ± 0.12 μM | +++ |
| Name | Alkaloids’ Binding Interactions with AChE | Scores 1 | |||
|---|---|---|---|---|---|
| Ligand | Receptor | Interaction | Distance Å | ||
| Fangchinoline | O 4 | O TYR334 (A) | H-donor | 2.59 | −7.11 ± 0.32 |
| 6-ring | N PHE288 (A) | π–H | 3.56 | ||
| Coptisine | C 9 | 6-ring PHE330 (A) | H–π | 3.99 | −6.76 ± 0.02 |
| C 30 | 6-ring TRP279 (A) | H–π | 3.90 | ||
| Berberine | C 9 | 6-ring PHE330 (A) | H–π | 4.03 | −7.00 ± 0.01 |
| C 11 | 6-ring TYR334 (A) | H–π | 3.57 | ||
| C 13 | 6-ring TRP279 (A) | H–π | 3.67 | ||
| Donepezil 2 | C 2 | 6-ring TYR334 (A) | H–π | 4.13 | −7.99 ± 0.02 |
| C 18 | 6-ring TRP279 (A) | H–π | 3.45 | ||
| C 20 | 5-ring TRP279 (A) | H–π | 4.03 | ||
| C 28 | 6-ring TRP84 (A) | H–π | 4.06 | ||
| 6-ring | 6-ring PHE330 (A) | π–π | 3.54 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, X.-P.; Liu, E.Y.L.; Chen, Z.-C.; Xu, M.L.; Yu, A.X.D.; Wu, Q.-Y.; Xia, Y.-J.; Duan, R.; Dong, T.T.X.; Tsim, K.W.K. Synergistic Inhibition of Acetylcholinesterase by Alkaloids Derived from Stephaniae Tetrandrae Radix, Coptidis Rhizoma and Phellodendri Chinensis Cortex. Molecules 2019, 24, 4567. https://doi.org/10.3390/molecules24244567
Kong X-P, Liu EYL, Chen Z-C, Xu ML, Yu AXD, Wu Q-Y, Xia Y-J, Duan R, Dong TTX, Tsim KWK. Synergistic Inhibition of Acetylcholinesterase by Alkaloids Derived from Stephaniae Tetrandrae Radix, Coptidis Rhizoma and Phellodendri Chinensis Cortex. Molecules. 2019; 24(24):4567. https://doi.org/10.3390/molecules24244567
Chicago/Turabian StyleKong, Xiang-Peng, Etta Y.L. Liu, Zhi-Cong Chen, Miranda Li Xu, Anna X.D. Yu, Qi-Yun Wu, Ying-Jie Xia, Ran Duan, Tina T.X. Dong, and Karl W.K. Tsim. 2019. "Synergistic Inhibition of Acetylcholinesterase by Alkaloids Derived from Stephaniae Tetrandrae Radix, Coptidis Rhizoma and Phellodendri Chinensis Cortex" Molecules 24, no. 24: 4567. https://doi.org/10.3390/molecules24244567
APA StyleKong, X.-P., Liu, E. Y. L., Chen, Z.-C., Xu, M. L., Yu, A. X. D., Wu, Q.-Y., Xia, Y.-J., Duan, R., Dong, T. T. X., & Tsim, K. W. K. (2019). Synergistic Inhibition of Acetylcholinesterase by Alkaloids Derived from Stephaniae Tetrandrae Radix, Coptidis Rhizoma and Phellodendri Chinensis Cortex. Molecules, 24(24), 4567. https://doi.org/10.3390/molecules24244567