Binding of Gold(III) Porphyrin by the Pro-metastatic Regulatory Protein Human Galectin-3
Abstract
1. Introduction
2. Results
2.1. Human Galectin-3 Binds Au3+TTPS, but Not the Anti-Cancer Drug Roscovitine
2.2. Truncated Galectin-3 Binds at Least 5-fold Weaker to gold(III) Porphyrin
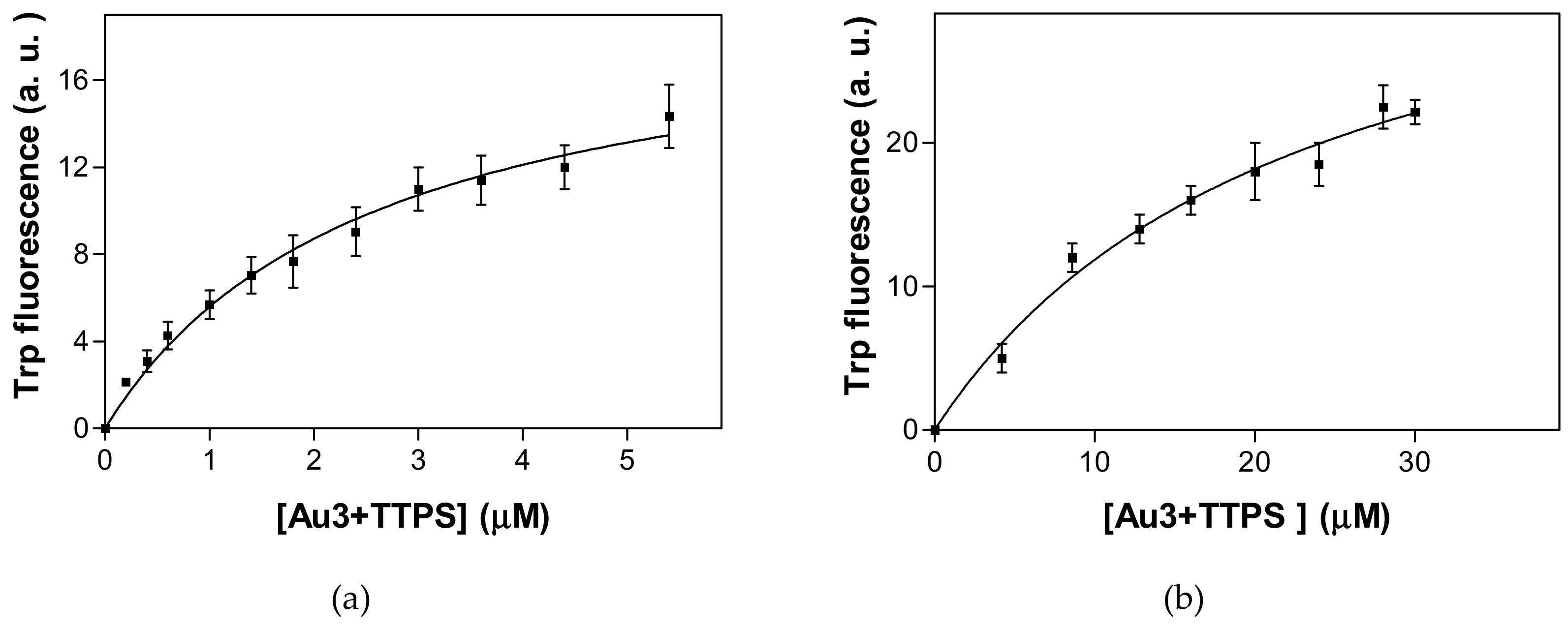
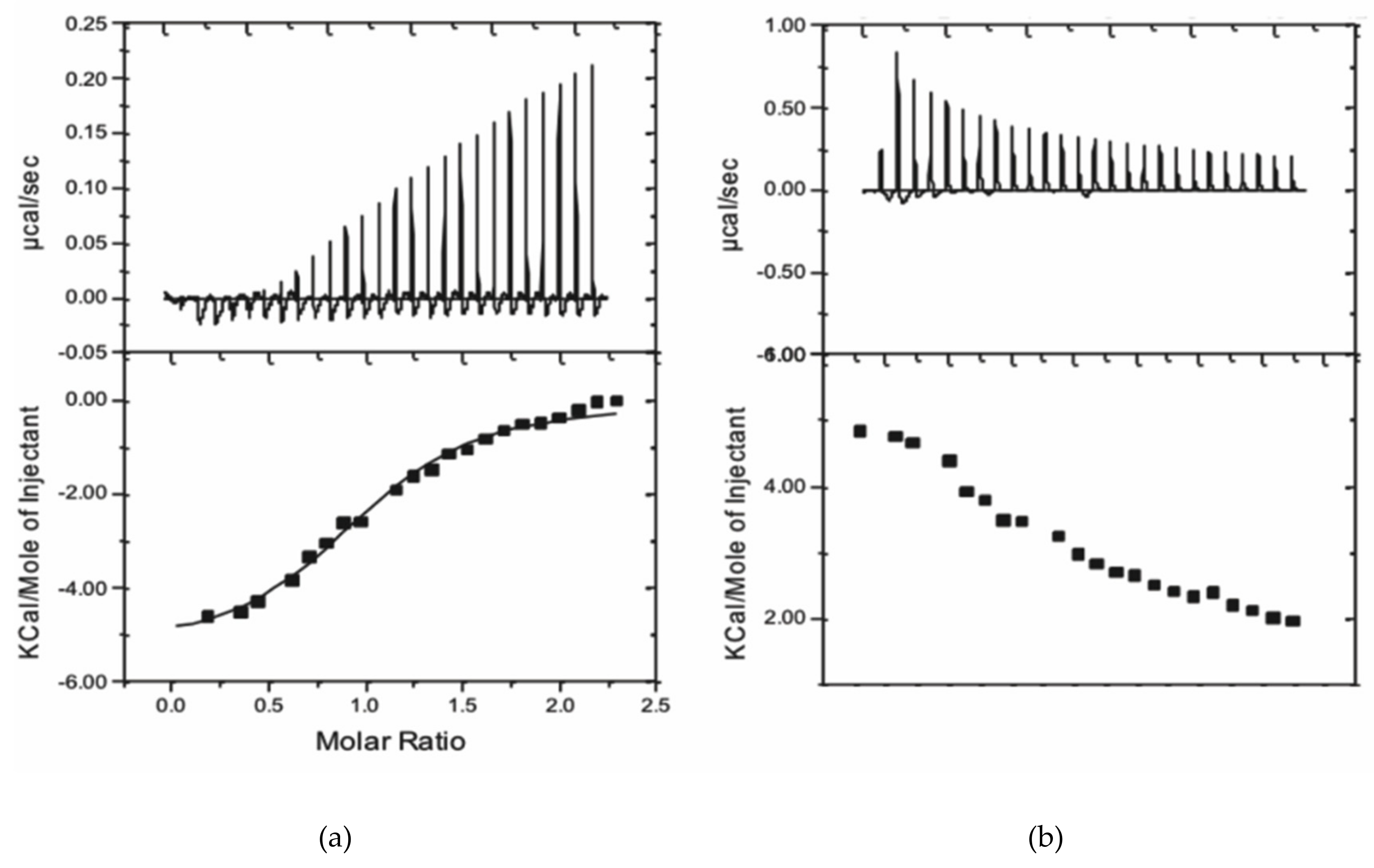
2.3. Tryptophan Fluorescence Spectroscopy and Isothermal Titration Calorimetry Confirm the Low Micromolar Affinity of Au3+TTPS for Gal3, with a Molar Ratio of 1:1
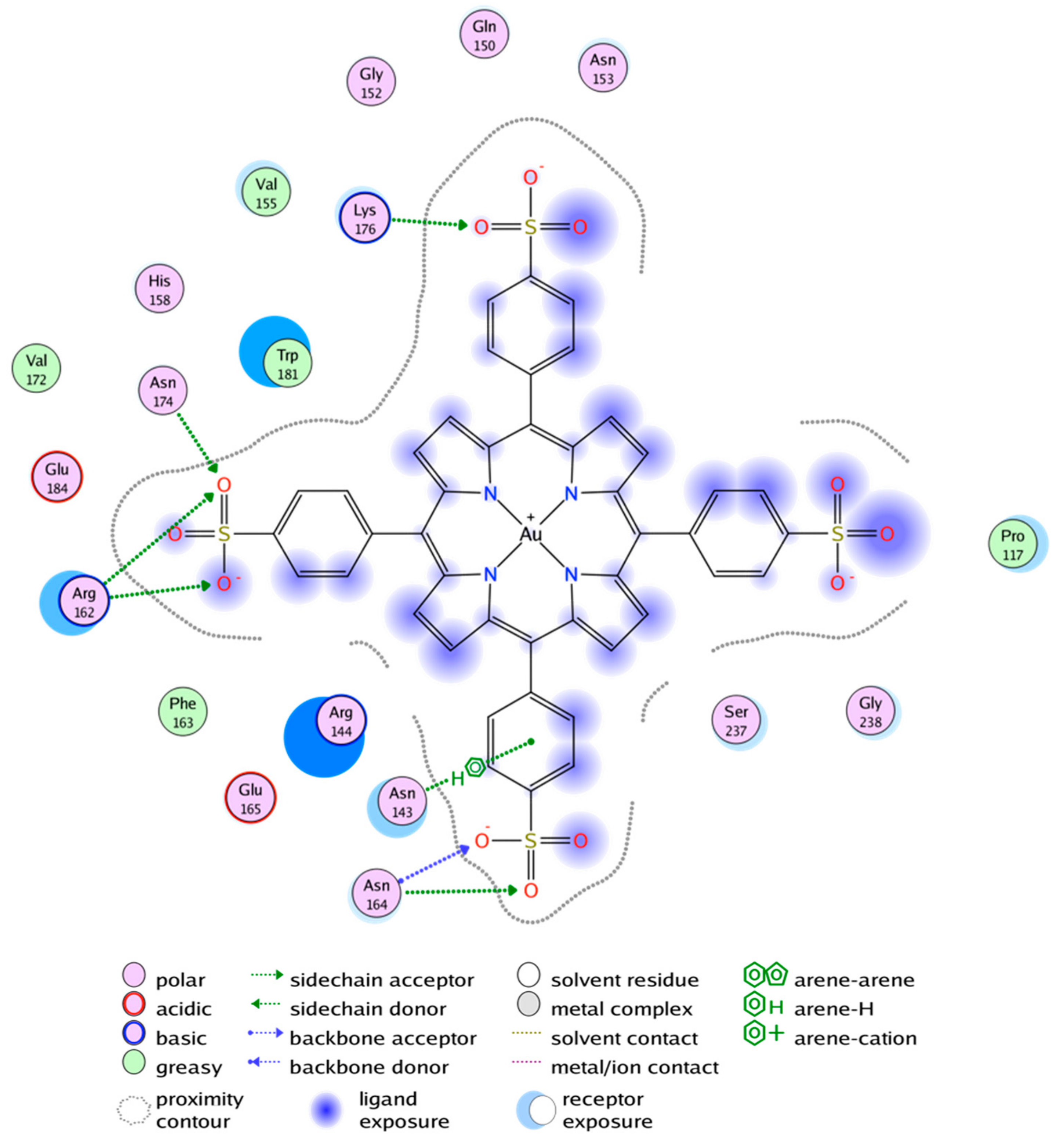
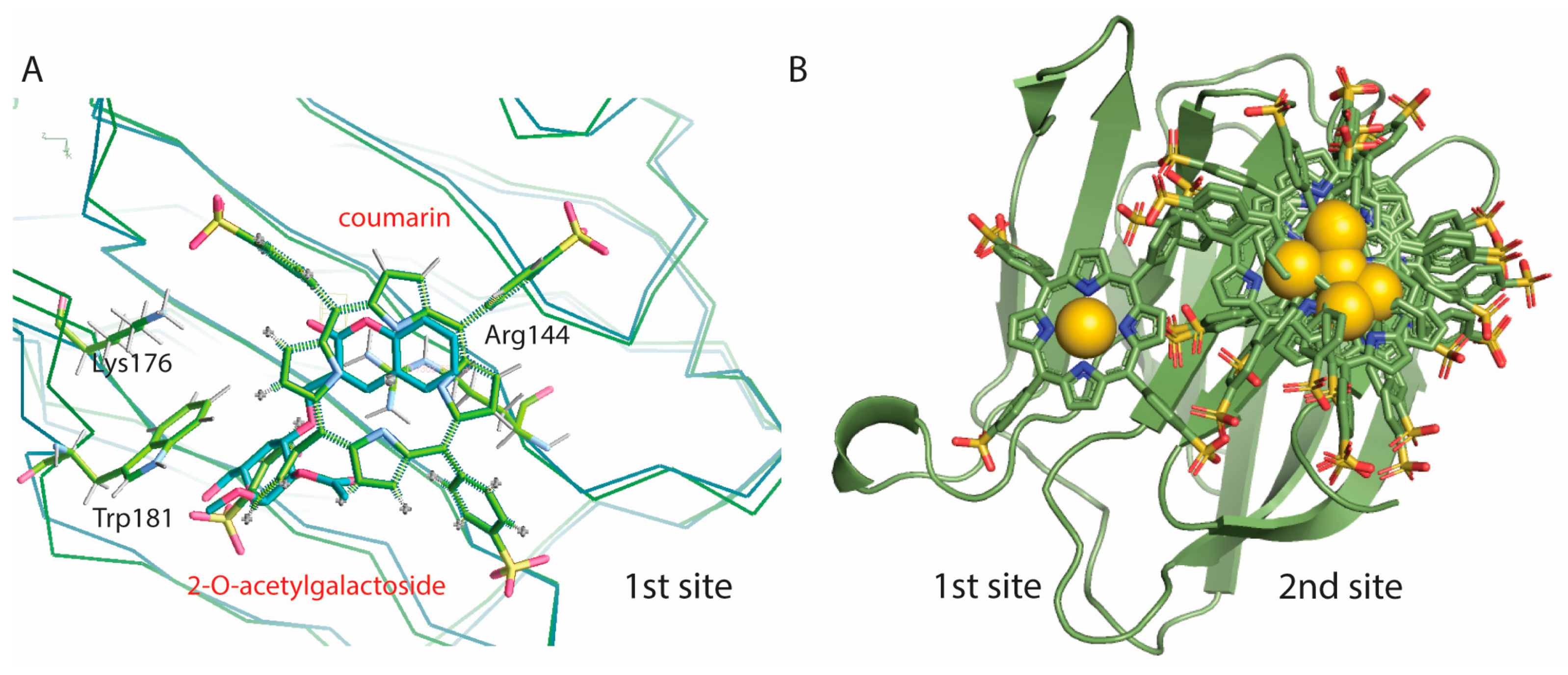
2.4. Molecular Dynamics Relaxation of the Gal3 CRD Structure and Docking of Au3+TPPS
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Microscale Thermophoresis
4.2.2. Intrinsic or Tryptophan Fluorescence Spectroscopy
4.2.3. Isothermal Titration Calorimetry
4.2.4. Co-Crystallization Trials of Gal3 with Gold(III) Porphyrin
4.2.5. Molecular Dynamics Relaxations of Gal3 CRD and Docking of Porphyrins
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barondes, S.H.; Cooper, D.N.; Gitt, M.A.; Leffler, H. Galectins. Structure and function of a large family of animal lectins. J. Biol. Chem. 1994, 269, 20807–20810. [Google Scholar] [PubMed]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell. Sc.i 2018, 131. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.C. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim. Biophys. Acta. 1999, 1473, 172–185. [Google Scholar] [CrossRef]
- Atmanene, C.; Ronin, C.; Teletchea, S.; Gautier, F.M.; Djedaini-Pilard, F.; Ciesielski, F.; Vivat, V.; Grandjean, C. Biophysical and structural characterization of mono/di-arylated lactosamine derivatives interaction with human galectin-3. Biochem. Biophys. Res. Commun. 2017, 489, 281–286. [Google Scholar] [CrossRef]
- Dion, J.; Advedissian, T.; Storozhylova, N.; Dahbi, S.; Lambert, A.; Deshayes, F.; Viguier, M.; Tellier, C.; Poirier, F.; Teletchea, S.; et al. Development of a sensitive microarray platform for the ranking of galectin inhibitors: Identification of a selective galectin-3 inhibitor. Chembiochem 2017, 18, 2428–2440. [Google Scholar] [CrossRef]
- Dion, J.; Deshayes, F.; Storozhylova, N.; Advedissian, T.; Lambert, A.; Viguier, M.; Tellier, C.; Dussouy, C.; Poirier, F.; Grandjean, C. Lactosamine-based derivatives as tools to delineate the biological functions of galectins: Application to skin tissue repair. Chembiochem 2017, 18, 782–789. [Google Scholar] [CrossRef]
- Gouin, S.G.; Garcia Fernandez, J.M.; Vanquelef, E.; Dupradeau, F.Y.; Salomonsson, E.; Leffler, H.; Ortega-Munoz, M.; Nilsson, U.J.; Kovensky, J. Multimeric lactoside “click clusters” as tools to investigate the effect of linker length in specific interactions with peanut lectin, galectin-1, and -3. Chembiochem. 2010, 11, 1430–1442. [Google Scholar] [CrossRef]
- Newlaczyl, A.U.; Yu, L.G. Galectin-3—A jack-of-all-trades in cancer. Cancer Lett. 2011, 313, 123–128. [Google Scholar] [CrossRef]
- Ahmad, N.; Gabius, H.J.; Andre, S.; Kaltner, H.; Sabesan, S.; Roy, R.; Liu, B.; Macaluso, F.; Brewer, C.F. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 2004, 279, 10841–10847. [Google Scholar] [CrossRef]
- Nieminen, J.; St-Pierre, C.; Bhaumik, P.; Poirier, F.; Sato, S. Role of galectin-3 in leukocyte recruitment in a murine model of lung infection by streptococcus pneumoniae. J. Immunol. 2008, 180, 2466–2473. [Google Scholar] [CrossRef]
- Dumic, J.; Dabelic, S.; Flogel, M. Galectin-3: An open-ended story. Biochim. Biophys. Acta. 2006, 1760, 616–635. [Google Scholar] [CrossRef]
- Gong, H.C.; Honjo, Y.; Nangia-Makker, P.; Hogan, V.; Mazurak, N.; Bresalier, R.S.; Raz, A. The nh2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 1999, 59, 6239–6245. [Google Scholar]
- Szabo, P.; Dam, T.K.; Smetana, K., Jr.; Dvorankova, B.; Kubler, D.; Brewer, C.F.; Gabius, H.J. Phosphorylated human lectin galectin-3: Analysis of ligand binding by histochemical monitoring of normal/malignant squamous epithelia and by isothermal titration calorimetry. Anat. Histol. Embryol. 2009, 38, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Birdsall, B.; Feeney, J.; Burdett, I.D.; Bawumia, S.; Barboni, E.A.; Hughes, R.C. Nmr solution studies of hamster galectin-3 and electron microscopic visualization of surface-adsorbed complexes: Evidence for interactions between the n- and c-terminal domains. Biochemistry 2001, 40, 4859–4866. [Google Scholar] [CrossRef]
- Brewer, C.F.; Miceli, M.C.; Baum, L.G. Clusters, bundles, arrays and lattices: Novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr. Opin. Struct. Biol. 2002, 12, 616–623. [Google Scholar] [CrossRef]
- Lepur, A.; Salomonsson, E.; Nilsson, U.J.; Leffler, H. Ligand induced galectin-3 protein self-association. J. Biol. Chem. 2012, 287, 21751–21756. [Google Scholar] [CrossRef] [PubMed]
- Flores-Ibarra, A.; Vertesy, S.; Medrano, F.J.; Gabius, H.J.; Romero, A. Crystallization of a human galectin-3 variant with two ordered segments in the shortened n-terminal tail. Sci. Rep. 2018, 8, 9835. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Leffler, H.; Sakakura, Y.; Kasai, K.; Barondes, S.H. Human breast carcinoma cdna encoding a galactoside-binding lectin homologous to mouse mac-2 antigen. Gene 1991, 99, 279–283. [Google Scholar]
- Hughes, R.C. Galectins as modulators of cell adhesion. Biochimie 2001, 83, 667–676. [Google Scholar] [CrossRef]
- Rapoport, E.M.; Matveeva, V.K.; Kaltner, H.; Andre, S.; Vokhmyanina, O.A.; Pazynina, G.V.; Severov, V.V.; Ryzhov, I.M.; Korchagina, E.Y.; Belyanchikov, I.M.; et al. Comparative lectinology: Delineating glycan-specificity profiles of the chicken galectins using neoglycoconjugates in a cell assay. Glycobiology 2015, 25, 726–734. [Google Scholar] [CrossRef][Green Version]
- Collins, P.M.; Bum-Erdene, K.; Yu, X.; Blanchard, H. Galectin-3 interactions with glycosphingolipids. J. Mol. Biol. 2014, 426, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, E.M.; Andre, S.; Kurmyshkina, O.V.; Pochechueva, T.V.; Severov, V.V.; Pazynina, G.V.; Gabius, H.J.; Bovin, N.V. Galectin-loaded cells as a platform for the profiling of lectin specificity by fluorescent neoglycoconjugates: A case study on galectins-1 and -3 and the impact of assay setting. Glycobiology 2008, 18, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, M.; Granovsky, M.; Quaggin, S.; Dennis, J.W. Negative regulation of t-cell activation and autoimmunity by mgat5 n-glycosylation. Nature 2001, 409, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Hughes, R.C. Macrophage surface glycoproteins binding to galectin-3 (mac-2-antigen). Glycoconj. J. 1997, 14, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Feuk-Lagerstedt, E.; Jordan, E.T.; Leffler, H.; Dahlgren, C.; Karlsson, A. Identification of cd66a and cd66b as the major galectin-3 receptor candidates in human neutrophils. J. Immunol. 1999, 163, 5592–5598. [Google Scholar]
- Grigorian, A.; Torossian, S.; Demetriou, M. T-cell growth, cell surface organization, and the galectin-glycoprotein lattice. Immunol.Rev. 2009, 230, 232–246. [Google Scholar] [CrossRef]
- Markowska, A.I.; Jefferies, K.C.; Panjwani, N. Galectin-3 protein modulates cell surface expression and activation of vascular endothelial growth factor receptor 2 in human endothelial cells. J. Biol. Chem. 2011, 286, 29913–29921. [Google Scholar] [CrossRef]
- Sano, H.; Hsu, D.K.; Yu, L.; Apgar, J.R.; Kuwabara, I.; Yamanaka, T.; Hirashima, M.; Liu, F.T. Human galectin-3 is a novel chemoattractant for monocytes and macrophages. J. Immunol. 2000, 165, 2156–2164. [Google Scholar] [CrossRef]
- Sundqvist, M.; Welin, A.; Elmwall, J.; Osla, V.; Nilsson, U.J.; Leffler, H.; Bylund, J.; Karlsson, A. Galectin-3 type-c self-association on neutrophil surfaces; the carbohydrate recognition domain regulates cell function. J. Leukoc. Biol. 2018, 103, 341–353. [Google Scholar] [CrossRef]
- Moret, F.R.E. Strategies for optimizing the delivery to tumors of macrocyclic photosensitizers used in photodynamic therapy (pdt). J. Porphyr. Phthalocya. 2017, 21, 239–256. [Google Scholar] [CrossRef]
- Obaid, G.; Chambrier, I.; Cook, M.J.; Russell, D.A. Cancer targeting with biomolecules: A comparative study of photodynamic therapy efficacy using antibody or lectin conjugated phthalocyanine-peg gold nanoparticles. Photochem. Photobiol. Sci. 2015, 14, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, A.B.; Day, N.U.; Mani, T.; Rudine, A.B.; Thomas, K.E.; Gederaas, O.A.; Vinogradov, S.A.; Wamser, C.C.; Ghosh, A. Gold tris(carboxyphenyl)corroles as multifunctional materials: Room temperature near-ir phosphorescence and applications to photodynamic therapy and dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2016, 8, 18935–18942. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Hogan, V.; Raz, A. Galectin-3 and cancer stemness. Glycobiology 2018, 28, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, E.; Khaidukov, S.; Baidina, O.; Bojenko, V.; Moiseeva, E.; Pasynina, G.; Karsten, U.; Nifant’ev, N.; LePendue, J.; Bovin, N. Involvement of the galbeta1 - 3galnacbeta structure in the recognition of apoptotic bodies by thp-1 cells. Eur. J. Cell. Biol. 2003, 82, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Farhad, M.; Rolig, A.S.; Redmond, W.L. The role of galectin-3 in modulating tumor growth and immunosuppression within the tumor microenvironment. Oncoimmunology 2018, 7, e1434467. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L. Shiga toxin-a model for glycolipid-dependent and lectin-driven endocytosis. Toxins 2017, 9, 340. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-S.L.; Li, X.-T.; Yu, L.-G.; Wang, L.; Shi, Z.-Y.; Guo, X.-L. Roles of galectin-3 in metabolic disorders and tumor cell metabolism. Int. J. Biol. Macromol. 2019. [Google Scholar]
- Bogoeva, V.P.L.; Bouckaert, J.; Yordanova, A.; Ivanov, I.; Vanderessee, R.; Frochot, C. Dual function of lectins — new perspectives in targeted photodynamic therapy. J. Porphyr. Phthalocya 2019, 23, 1–10. [Google Scholar] [CrossRef]
- Goel, M.; Jain, D.; Kaur, K.J.; Kenoth, R.; Maiya, B.G.; Swamy, M.J.; Salunke, D.M. Functional equality in the absence of structural similarity: An added dimension to molecular mimicry. J. Biol. Chem. 2001, 276, 39277–39281. [Google Scholar] [CrossRef]
- Goel, M.; Damai, R.S.; Sethi, D.K.; Kaur, K.J.; Maiya, B.G.; Swamy, M.J.; Salunke, D.M. Crystal structures of the pna-porphyrin complex in the presence and absence of lactose: Mapping the conformational changes on lactose binding, interacting surfaces, and supramolecular aggregations. Biochemistry 2005, 44, 5588–5596. [Google Scholar] [CrossRef]
- Goel, M.; Anuradha, P.; Kaur, K.J.; Maiya, B.G.; Swamy, M.J.; Salunke, D.M. Porphyrin binding to jacalin is facilitated by the inherent plasticity of the carbohydrate-binding site: Novel mode of lectin-ligand interaction. Acta. Crystallogr. D Biol. Crystallogr. 2004, 60, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Bogoeva, V.P.; Varriale, A.; John, C.M.; D’Auria, S. Human galectin-3 interacts with two anticancer drugs. Proteomics 2010, 10, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Sindrewicz, P.; Li, X.; Yates, E.A.; Turnbull, J.E.; Lian, L.Y.; Yu, L.G. Intrinsic tryptophan fluorescence spectroscopy reliably determines galectin-ligand interactions. Sci. Rep. 2019, 9, 11851. [Google Scholar] [CrossRef] [PubMed]
- Guha, P.; Kaptan, E.; Bandyopadhyaya, G.; Kaczanowska, S.; Davila, E.; Thompson, K.; Martin, S.S.; Kalvakolanu, D.V.; Vasta, G.R.; Ahmed, H. Cod glycopeptide with picomolar affinity to galectin-3 suppresses t-cell apoptosis and prostate cancer metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 5052–5057. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, A.; Delgado, S.; Valverde, P.; Bertuzzi, S.; Berbis, M.A.; Echavarren, J.; Lacetera, A.; Martin-Santamaria, S.; Surolia, A.; Canada, F.J.; et al. Minimizing the entropy penalty for ligand binding: Lessons from the molecular recognition of the histo blood-group antigens by human galectin-3. Angew. Chem. Int. Ed. Engl. 2019, 58, 7268–7272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Miller, M.C.; Xu, X.; Song, C.; Zhang, F.; Zheng, Y.; Zhou, Y.; Tai, G.; Mayo, K.H. Nmr-based insight into galectin-3 binding to endothelial cell adhesion molecule cd146: Evidence for noncanonical interactions with the lectin’s crd beta-sandwich f-face. Glycobiology 2019, 29, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Diehl, C.; Engstrom, O.; Delaine, T.; Hakansson, M.; Genheden, S.; Modig, K.; Leffler, H.; Ryde, U.; Nilsson, U.J.; Akke, M. Protein flexibility and conformational entropy in ligand design targeting the carbohydrate recognition domain of galectin-3. J. Am. Chem. Soc. 2010, 132, 14577–14589. [Google Scholar] [CrossRef]
- Cicenas, J.; Kalyan, K.; Sorokinas, A.; Stankunas, E.; Levy, J.; Meskinyte, I.; Stankevicius, V.; Kaupinis, A.; Valius, M. Roscovitine in cancer and other diseases. Ann. Transl. Med. 2015, 3, 135. [Google Scholar]
- D’Auria, S.; Petrova, L.; John, C.; Russev, G.; Varriale, A.; Bogoeva, V. Tumor-specific protein human galectin-1 interacts with anticancer agents. Molecular bioSystems 2009, 5, 1331–1336. [Google Scholar] [CrossRef]
- Corbeil, C.R.; Williams, C.I.; Labute, P. Variability in docking success rates due to dataset preparation. J. Comput. Aided. Mol. Des. 2012, 26, 775–786. [Google Scholar] [CrossRef]
- Rajput, V.K.; MacKinnon, A.; Mandal, S.; Collins, P.; Blanchard, H.; Leffler, H.; Sethi, T.; Schambye, H.; Mukhopadhyay, B.; Nilsson, U.J. A selective galactose-coumarin-derived galectin-3 inhibitor demonstrates involvement of galectin-3-glycan interactions in a pulmonary fibrosis model. J. Med. Chem. 2016, 59, 8141–8147. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.H.; Sun, R.W.; Lam, J.B.; Li, C.K.; Xu, A.; Ma, D.L.; Abagyan, R.; Wang, Y.; Che, C.M. A gold(iii) porphyrin complex with antitumor properties targets the wnt/beta-catenin pathway. Cancer Res. 2010, 70, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Lum, C.T.; Liu, X.; Sun, R.W.; Li, X.P.; Peng, Y.; He, M.L.; Kung, H.F.; Che, C.M.; Lin, M.C. Gold(iii) porphyrin 1a inhibited nasopharyngeal carcinoma metastasis in vivo and inhibited cell migration and invasion in vitro. Cancer Lett. 2010, 294, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lum, C.T.; Huo, L.; Sun, R.W.; Li, M.; Kung, H.F.; Che, C.M.; Lin, M.C. Gold(iii) porphyrin 1a prolongs the survival of melanoma-bearing mice and inhibits angiogenesis. Acta. Oncol. 2011, 50, 719–726. [Google Scholar] [CrossRef]
- Messori, L.; Marcon, G.; Orioli, P. Gold(iii) compounds as new family of anticancer drugs. Bioinorg. Chem. Appl. 2003, 177–187. [Google Scholar] [CrossRef]
- Dandash, F.; Leger, D.Y.; Fidanzi-Dugas, C.; Nasri, S.; Bregier, F.; Granet, R.; Karam, W.; Diab-Assaf, M.; Sol, V.; Liagre, B. In vitro anticancer activity of new gold(iii) porphyrin complexes in colon cancer cells. J. Inorg. Biochem. 2017, 177, 27–38. [Google Scholar] [CrossRef]
- Dam, T.K.; Gabius, H.J.; Andre, S.; Kaltner, H.; Lensch, M.; Brewer, C.F. Galectins bind to the multivalent glycoprotein asialofetuin with enhanced affinities and a gradient of decreasing binding constants. Biochemistry 2005, 44, 12564–12571. [Google Scholar] [CrossRef]
- Kenoth, R.; Raghunath Reddy, D.; Maiya, B.G.; Swamy, M.J. Thermodynamic and kinetic analysis of porphyrin binding to trichosanthes cucumerina seed lectin. Eur. J. Biochem. 2001, 268, 5541–5549. [Google Scholar] [CrossRef]
- Komath, S.S.; Bhanu, K.; Maiya, B.G.; Swamy, M.J. Binding of porphyrins by the tumor-specific lectin, jacalin [jack fruit (artocarpus integrifolia) agglutinin]. Biosci. Rep. 2000, 20, 265–276. [Google Scholar] [CrossRef]
- Gehlken, C.; Suthahar, N.; Meijers, W.C.; de Boer, R.A. Galectin-3 in heart failure: An update of the last 3 years. Heart Fail. Clin. 2018, 14, 75–92. [Google Scholar] [CrossRef]
- De Boer, R.A.; Voors, A.A.; Muntendam, P.; van Gilst, W.H.; van Veldhuisen, D.J. Galectin-3: A novel mediator of heart failure development and progression. Eur. J. Heart Fail. 2009, 11, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Dings, R.P.M.; Miller, M.C.; Griffin, R.J.; Mayo, K.H. Galectins as molecular targets for therapeutic intervention. Int. J. Mol. Sci. 2018, 19, 905. [Google Scholar] [CrossRef] [PubMed]
- Haketa, Y.; Bando, Y.; Sasano, Y.; Tanaka, H.; Yasuda, N.; Hisaki, I.; Maeda, H. Liquid crystals comprising pi-electronic ions from porphyrin-au(iii) complexes. iScience 2019, 14, 241–256. [Google Scholar] [CrossRef] [PubMed]
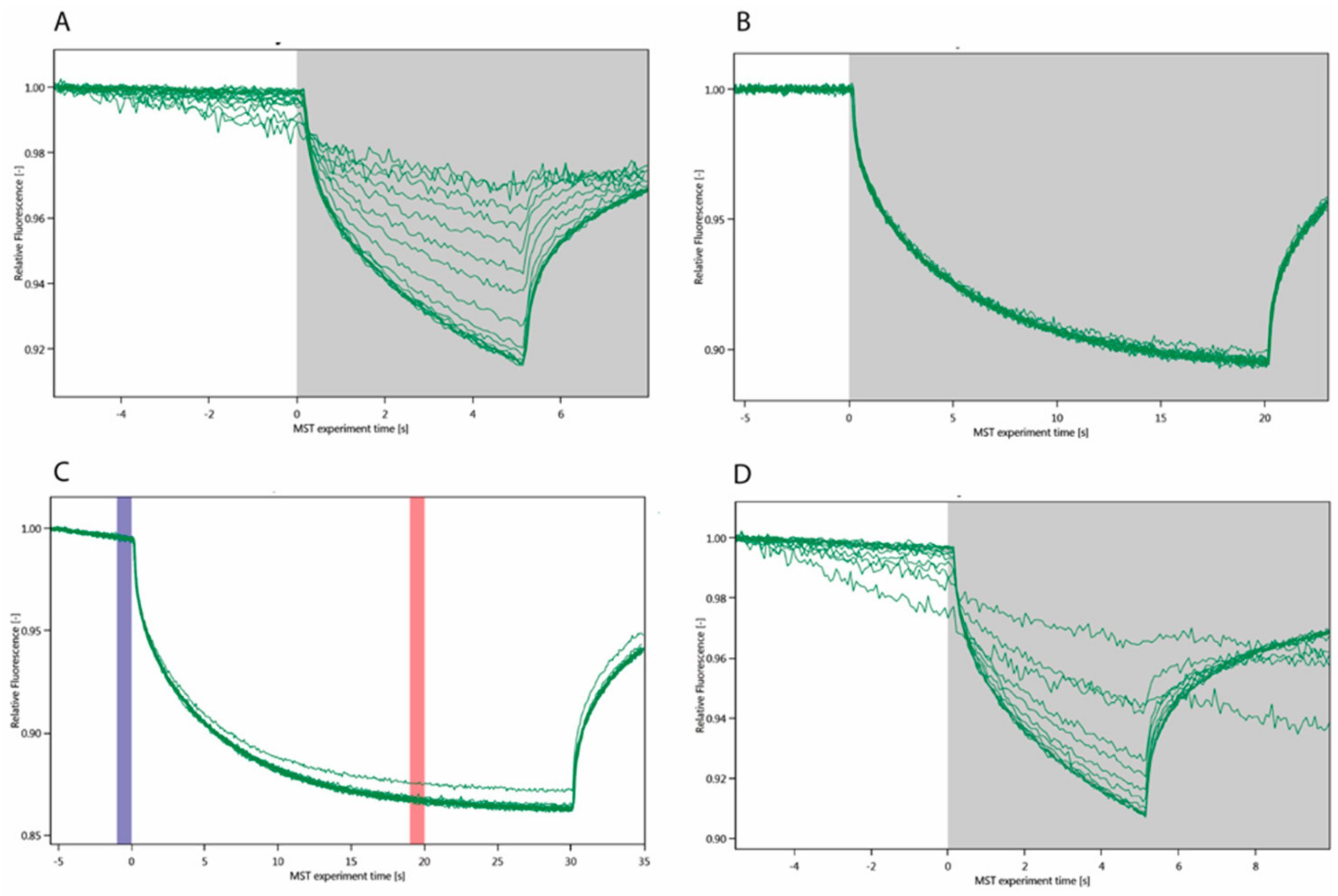
 ) with KD = 25.90 ± 0.65 μM (outlier marked by
) with KD = 25.90 ± 0.65 μM (outlier marked by  ) and Gal3 FL (
) and Gal3 FL ( ) with KD = 5.83 ± 0.65 μM.
) with KD = 5.83 ± 0.65 μM.
 ) with KD = 25.90 ± 0.65 μM (outlier marked by
) with KD = 25.90 ± 0.65 μM (outlier marked by  ) and Gal3 FL (
) and Gal3 FL ( ) with KD = 5.83 ± 0.65 μM.
) with KD = 5.83 ± 0.65 μM.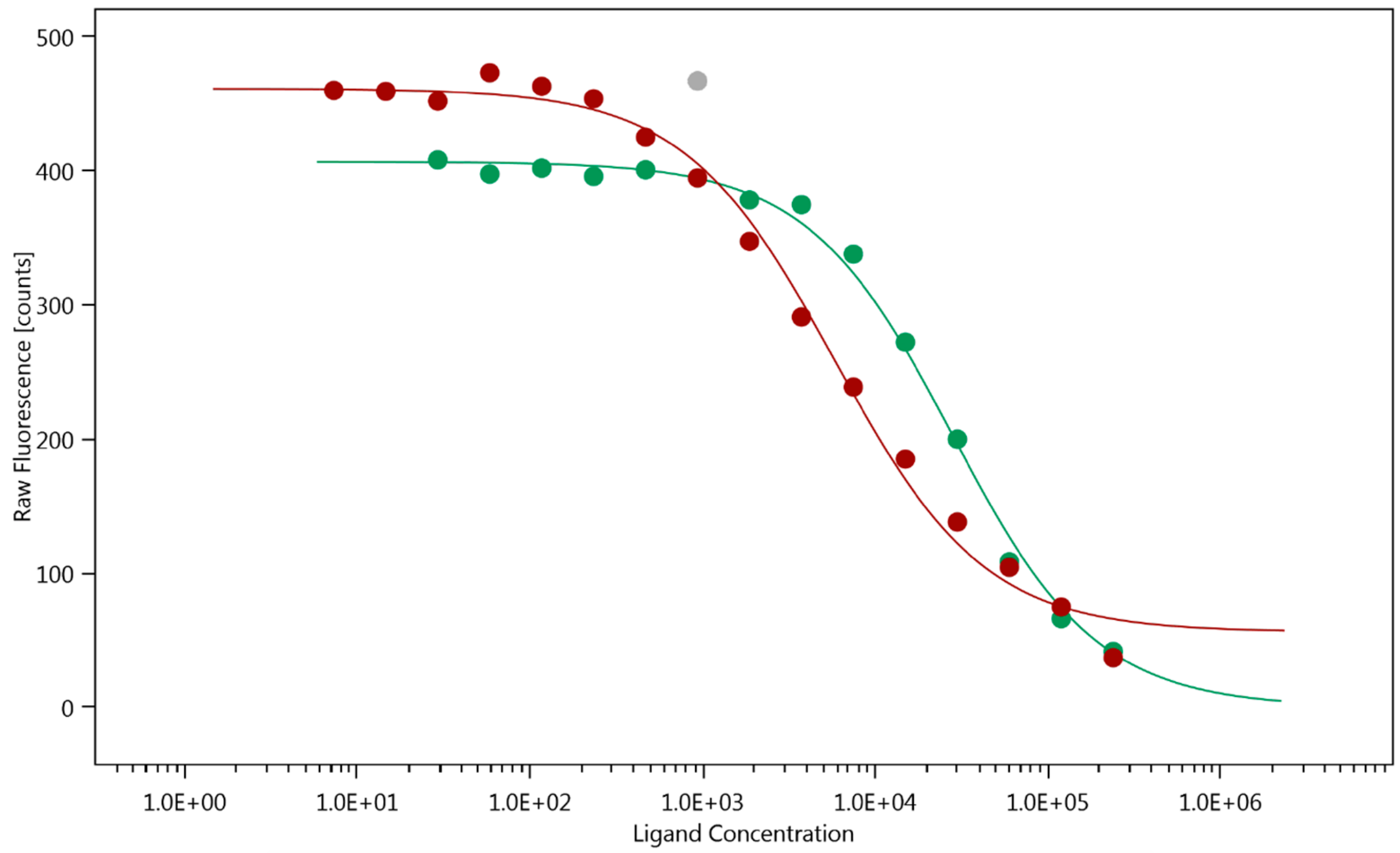
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogoeva, V.; Rangelov, M.; Todorova, N.; Lambert, A.; Bridot, C.; Yordanova, A.; Roos, G.; Grandjean, C.; Bouckaert, J. Binding of Gold(III) Porphyrin by the Pro-metastatic Regulatory Protein Human Galectin-3. Molecules 2019, 24, 4561. https://doi.org/10.3390/molecules24244561
Bogoeva V, Rangelov M, Todorova N, Lambert A, Bridot C, Yordanova A, Roos G, Grandjean C, Bouckaert J. Binding of Gold(III) Porphyrin by the Pro-metastatic Regulatory Protein Human Galectin-3. Molecules. 2019; 24(24):4561. https://doi.org/10.3390/molecules24244561
Chicago/Turabian StyleBogoeva, Vanya, Miroslav Rangelov, Nadezhda Todorova, Annie Lambert, Clarisse Bridot, Anna Yordanova, Goedele Roos, Cyrille Grandjean, and Julie Bouckaert. 2019. "Binding of Gold(III) Porphyrin by the Pro-metastatic Regulatory Protein Human Galectin-3" Molecules 24, no. 24: 4561. https://doi.org/10.3390/molecules24244561
APA StyleBogoeva, V., Rangelov, M., Todorova, N., Lambert, A., Bridot, C., Yordanova, A., Roos, G., Grandjean, C., & Bouckaert, J. (2019). Binding of Gold(III) Porphyrin by the Pro-metastatic Regulatory Protein Human Galectin-3. Molecules, 24(24), 4561. https://doi.org/10.3390/molecules24244561






