Questiomycins, Algicidal Compounds Produced by the Marine Bacterium Alteromonas sp. D and Their Production Cue
Abstract
1. Introduction
2. Results and Discussion
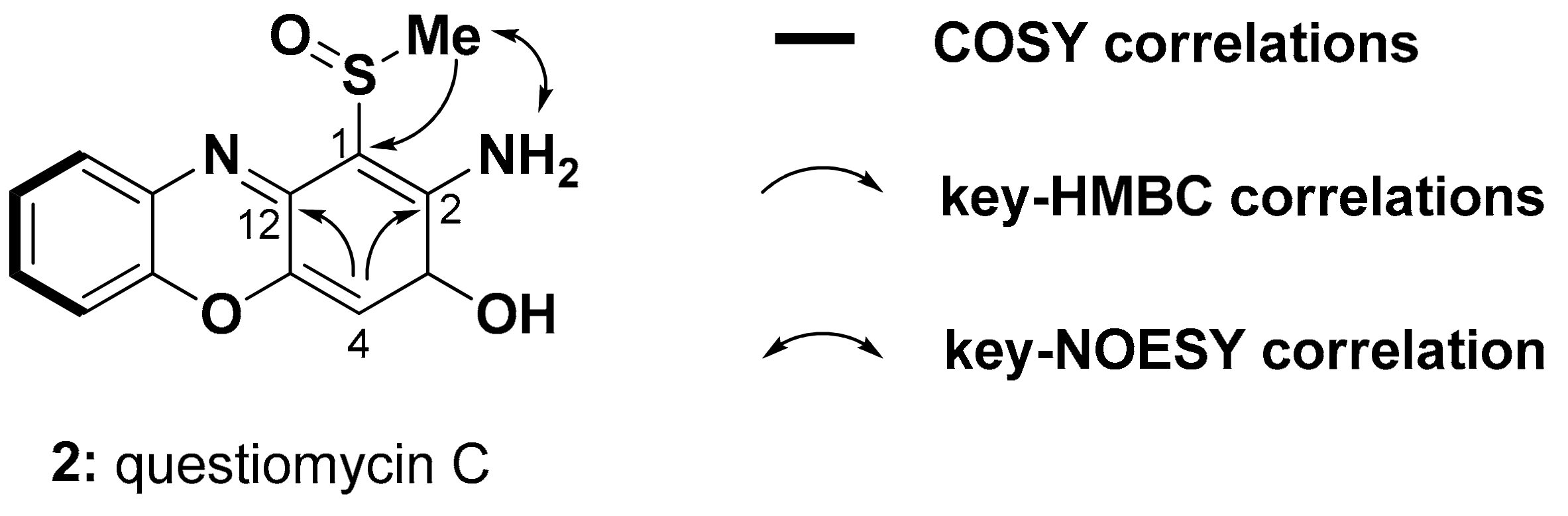
2.1. Purificatin and Structure Elucidation
2.2. Biological Activities
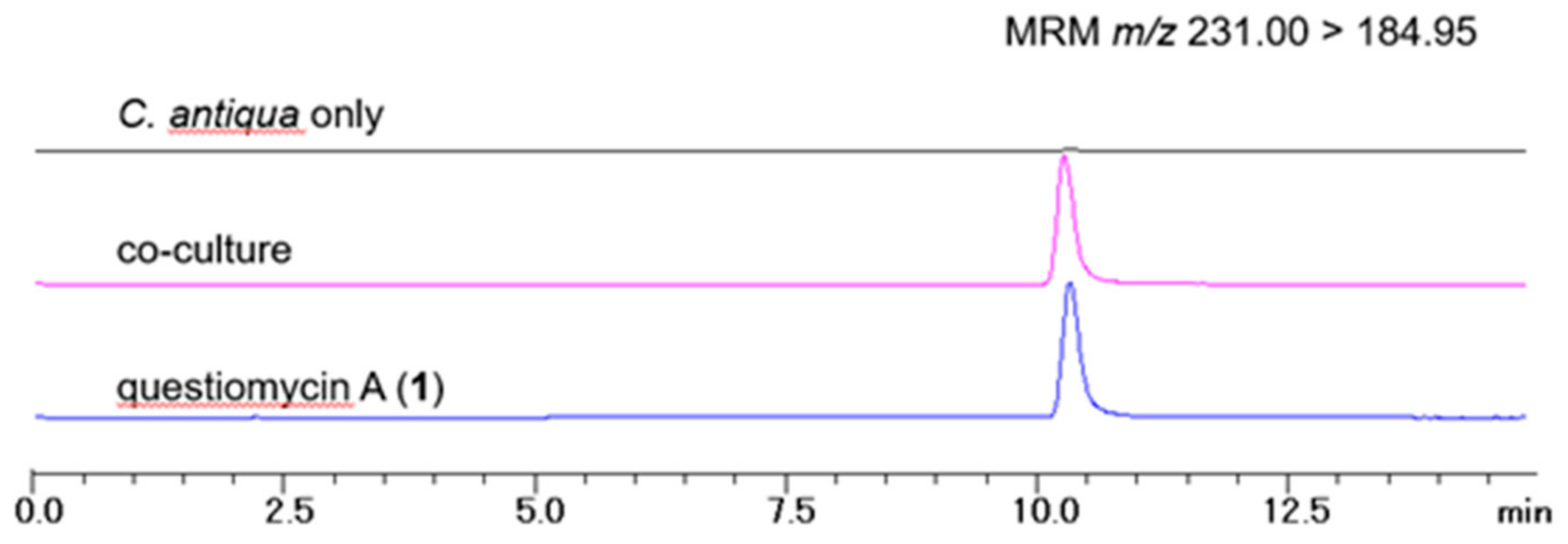
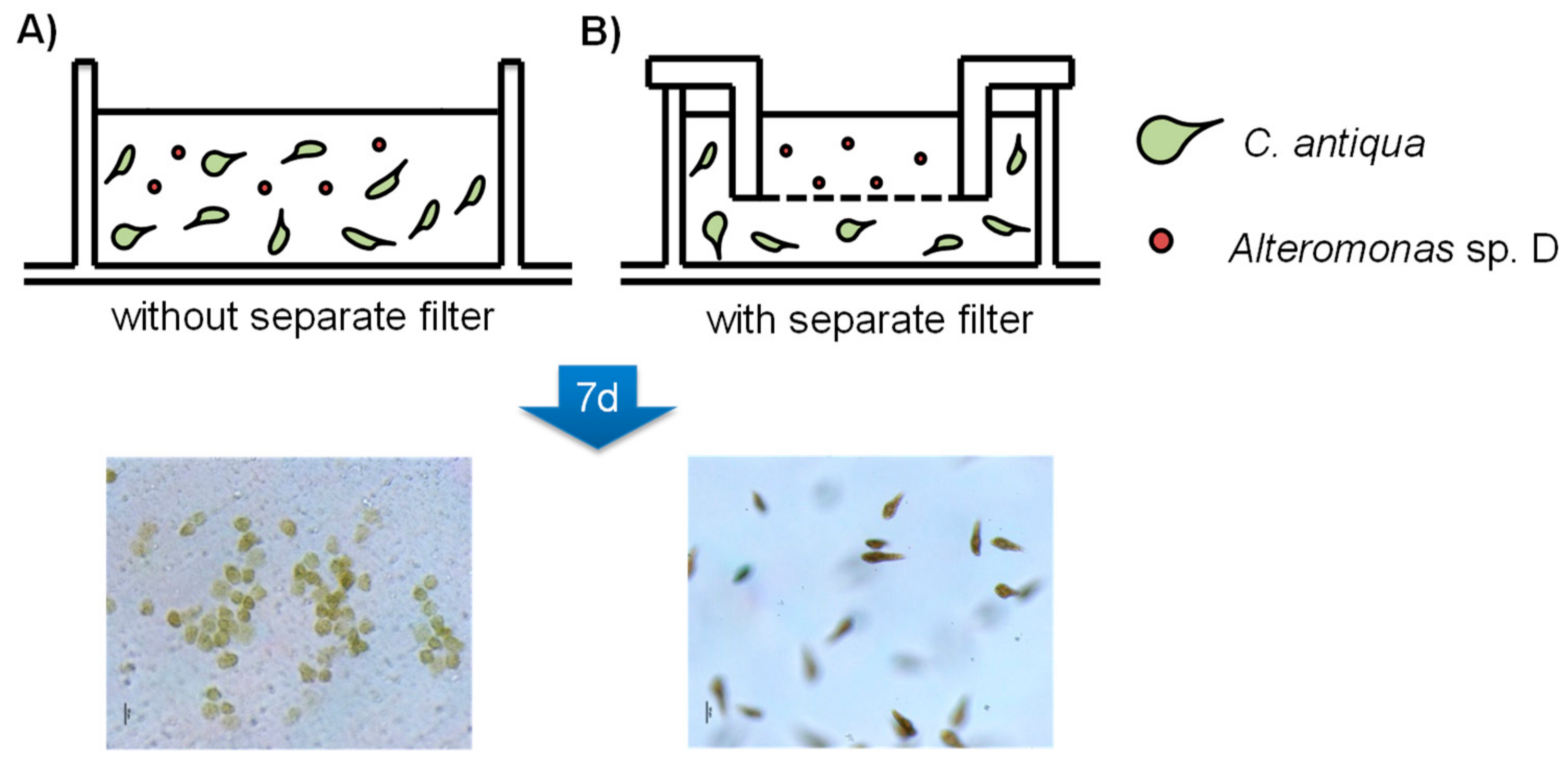
2.3. Co-Cultivation of C. antiqua and Alteromonas sp. D
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Materials
3.3. Cultivation of Bacterium, Extraction, and Isolation of Algicidal Compounds
3.4. Preparation of Questiomycin A (1) and E (4) by Supplemented Fermentation with o-Aminophenol
3.5. Algicidal Assay Using C. antiqua
3.6. Algicidal Assay against Karenia mikimotoi and Chaetoceros didymus
3.7. Artemia salina Assay
3.8. Bangia fuscopurpurea Assay
3.9. Oryzias sp. Assay
3.10. Co-Cultivation Assay
3.11. LC-MS Quantiative Analysis
3.12. Membrane Filter Separated Cultivation Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trainer, V.L.; Bates, S.S.; Lundholm, N.; Thessen, A.E.; Cochlan, W.P.; Adams, N.G.; Trick, C.G. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 2012, 14, 271–300. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.O.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Kouakou, C.R.C.; Poder, T.G. Economic impact of harmful algal blooms on human health: A systematic review. J. Water Health 2019, 17, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Grattan, L.M.; Holobaugh, S.; Morris, J.G., Jr. Harmful algal blooms and public health. Harmful Algae 2016, 57, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Inaba, N.; Imai, I.; Trainer Vera, L.; Onishi, Y.; Ishii, K.-I.; Wyllie-Echeverria, S. Algicidal and growth-inhibiting bacteria associated with seagrass and macroalgae beds in Puget Sound, WA, USA. Harmful Algae 2017, 62, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, R.I.; Estrada, M.; Garces, E. Life histories of microalgal species causing harmful blooms: Haploids, diploids and the relevance of benthic stages. Harmful Algae 2018, 73, 44–57. [Google Scholar] [CrossRef]
- Anderson Donald, M. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean. Coast. Manag. 2009, 52, 342–354. [Google Scholar] [CrossRef]
- Nakano, K.; Lee, T.J.; Matsumura, M. In situ algal bloom control by the integration of ultrasonic radiation and jet circulation to flushing. Environ. Sci. Technol. 2001, 35, 4941–4946. [Google Scholar] [CrossRef]
- Sun, R.; Sun, P.; Zhang, J.; Esquivel-Elizondo, S.; Wu, Y. Microorganisms-based methods for harmful algal blooms control: A review. Bioresour. Technol. 2018, 248, 12–20. [Google Scholar] [CrossRef]
- Imai, I.; Ishida, Y.; Sakaguchi, K.; Hata, Y. Algicidal marine bacteria isolated from northern Hiroshima Bay, Japan. Fish. Sci. 1995, 61, 628–636. [Google Scholar] [CrossRef]
- Demuez, M.; Gonzalez-Fernandez, C.; Ballesteros, M. Algicidal microorganisms and secreted algicides: New tools to induce microalgal cell disruption. Biotechnol. Adv. 2015, 33, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Bigalke, A.; Kaulfuss, A.; Pohnert, G. Strategies and ecological roles of algicidal bacteria. FEMS Microbiol. Rev. 2017, 41, 880–899. [Google Scholar] [CrossRef] [PubMed]
- Mayali, X.; Azam, F. Algicidal bacteria in the sea and their impact on algal blooms. J. Eukaryot. Microbiol. 2004, 51, 139–144. [Google Scholar] [CrossRef]
- Park, J.-H.; Yoshinaga, I.; Nishikawa, T.; Imai, I. Algicidal bacteria in particle-associated form and in free-living form during a diatom bloom in the Seto Inland Sea, Japan. Aquat. Microb. Ecol. 2010, 60, 151–161. [Google Scholar] [CrossRef][Green Version]
- Inaba, N.; Nagai, S.; Sakami, T.; Watanabe, T.; Araki, K.; Kawasaki, S.; Imai, I. Temporal variability of algicidal and growth-inhibiting bacteria at an eelgrass bed in the Ariake Sea, Japan. Biorem. J. 2018, 22, 112–125. [Google Scholar] [CrossRef]
- Onishi, Y.; Mohri, Y.; Tuji, A.; Ohgi, K.; Yamaguchi, A.; Imai, I. The seagrass Zostera marina harbors growth-inhibiting bacteria against the toxic dinoflagellate Alexandrium tamarense. Fish. Sci. 2014, 80, 353–362. [Google Scholar] [CrossRef]
- Paul, C.; Pohnert, G. Interactions of the algicidal bacterium Kordia algicida with diatoms: Regulated protease excretion for specific algal lysis. PLoS ONE 2011, 6, e21032. [Google Scholar] [CrossRef]
- Lee, S.-O.; Kato, J.; Takiguchi, N.; Kuroda, A.; Ikeda, T.; Mitsutani, A.; Ohtake, H. Involvement of an extracellular protease in algicidal activity of the marine bacterium Pseudoalteromonas sp. Strain A28. Appl. Environ. Microbiol. 2000, 66, 4334–4339. [Google Scholar] [CrossRef]
- Kim, J.-D.; Kim, J.-Y.; Park, J.-K.; Lee, C.-G. Selective control of the Prorocentrum minimum harmful algal blooms by a novel algal-lytic bacterium Pseudoalteromonas haloplanktis AFMB-008041. Mar. Biotechnol. 2009, 11, 463–472. [Google Scholar] [CrossRef]
- Li, Y.; Lei, X.; Zhu, H.; Zhang, H.; Guan, C.; Chen, Z.; Zheng, W.; Fu, L.; Zheng, T. Chitinase producing bacteria with direct algicidal activity on marine diatoms. Sci. Rep. 2016, 6, 21984. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, J.F.; Yim, J.H.; Kwon, S.-K.; Lee, C.H.; Lee, H.K. Red to red—The marine bacterium Hahella chejuensis and its product prodigiosin for mitigation of harmful algal blooms. J. Microbiol. Biotechnol. 2008, 18, 1621–1629. [Google Scholar] [PubMed]
- Jeong, S.-Y.; Ishida, K.; Ito, Y.; Okada, S.; Murakami, M. Bacillamide, a novel algicide from the marine bacterium, Bacillus sp. SY-1, against the harmful dinoflagellate, Cochlodinium polykrikoides. Tetrahedron Lett. 2003, 44, 8005–8007. [Google Scholar] [CrossRef]
- Cho, J.Y. Algicidal activity of marine Alteromonas sp. KNS-16 and isolation of active compounds. Biosci. Biotechnol. Biochem. 2012, 76, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Anzai, K.; Isono, K.; Okuma, K.; Suzuki, S. The new antibiotics, questiomycins A and B. J. Antibiot. 1960, 13, 125–132. [Google Scholar] [PubMed]
- Maskey, R.P.; Li, F.C.; Qin, S.; Fiebig, H.H.; Laatsch, H. Chandrananimycins A–C: Production of novel anticancer antibiotics from a marine Actinomadura sp. Isolate M048 by variation of medium composition and growth conditions. J. Antibiot. 2003, 56, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Bitzer, J.; Grosse, T.; Wang, L.; Lang, S.; Beil, W.; Zeeck, A. New aminophenoxazinones from a marine Halomonas sp.: Fermentation, structure elucidation, and biological activity. J. Antibiot. 2006, 59, 86–92. [Google Scholar] [CrossRef]
- Gents, M.B.; Nielsen, S.T.; Mortensen, A.G.; Christophersen, C.; Fomsgaard, I.S. Transformation products of 2-benzoxazolinone (BOA) in soil. Chemosphere 2005, 61, 74–84. [Google Scholar] [CrossRef]
- Understrup, A.G.; Ravnskov, S.; Hansen, H.C.B.; Fomsgaard, I.S. Biotransformation of 2-benzoxazolinone to 2-amino-(3h)-phenoxazin-3-one and 2-acetylamino-(3h)-phenoxazin-3-one in soil. J. Chem. Ecol. 2005, 31, 1205–1222. [Google Scholar] [CrossRef]
- Ohnishi, Y.; Furusho, Y.; Higashi, T.; Chun, H.-K.; Furihata, K.; Sakuda, S.; Horinouchi, S. Structures of grixazone A and B, A-factor-dependent yellow pigments produced under phosphate depletion by Streptomyces griseus. J. Antibiot. 2004, 57, 218–223. [Google Scholar] [CrossRef][Green Version]
- Mauger, A.B.; Lackner, H. The actinomycins. In Anticancer Agents from Natural Products, 2nd ed.; Cragg, G.M., Kingston, D.G.I., Newman, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 363–381. [Google Scholar]
- Suzuki, H.; Furusho, Y.; Higashi, T.; Ohnishi, Y.; Horinouchi, S. A novel o-aminophenol oxidase responsible for formation of the phenoxazinone chromophore of grixazone. J. Biol. Chem. 2006, 281, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Keller, U.; Lang, M.; Crnovcic, I.; Pfennig, F.; Schauwecker, F. The actinomycin biosynthetic gene cluster of Streptomyces chrysomallus: A genetic hall of mirrors for synthesis of a molecule with mirror symmetry. J. Bacteriol. 2010, 192, 2583–2595. [Google Scholar] [CrossRef] [PubMed]
- Washington, C.; Maxwell, J.; Stevenson, J.; Malone, G.; Lowe, E.W.; Zhang, Q.; Wang, G.; McIntyre, N.R. Mechanistic studies of the tyrosinase-catalyzed oxidative cyclocondensation of 2-aminophenol to 2-aminophenoxazin-3-one. Arch. Biochem. Biophys. 2015, 577, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Mausz, M.A.; Pohnert, G. A co-culturing/metabolomics approach to investigate chemically mediated interactions of planktonic organisms reveals influence of bacteria on diatom metabolism. Metabolomics 2013, 9, 349–359. [Google Scholar] [CrossRef]
- Smriga, S.; Fernandez, V.I.; Mitchell, J.G.; Stocker, R. Chemotaxis toward phytoplankton drives organic matter partitioning among marine bacteria. Proc. Natl. Acad. Sci. USA 2016, 113, 1576–1581. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1 and 4 are available from the authors. |
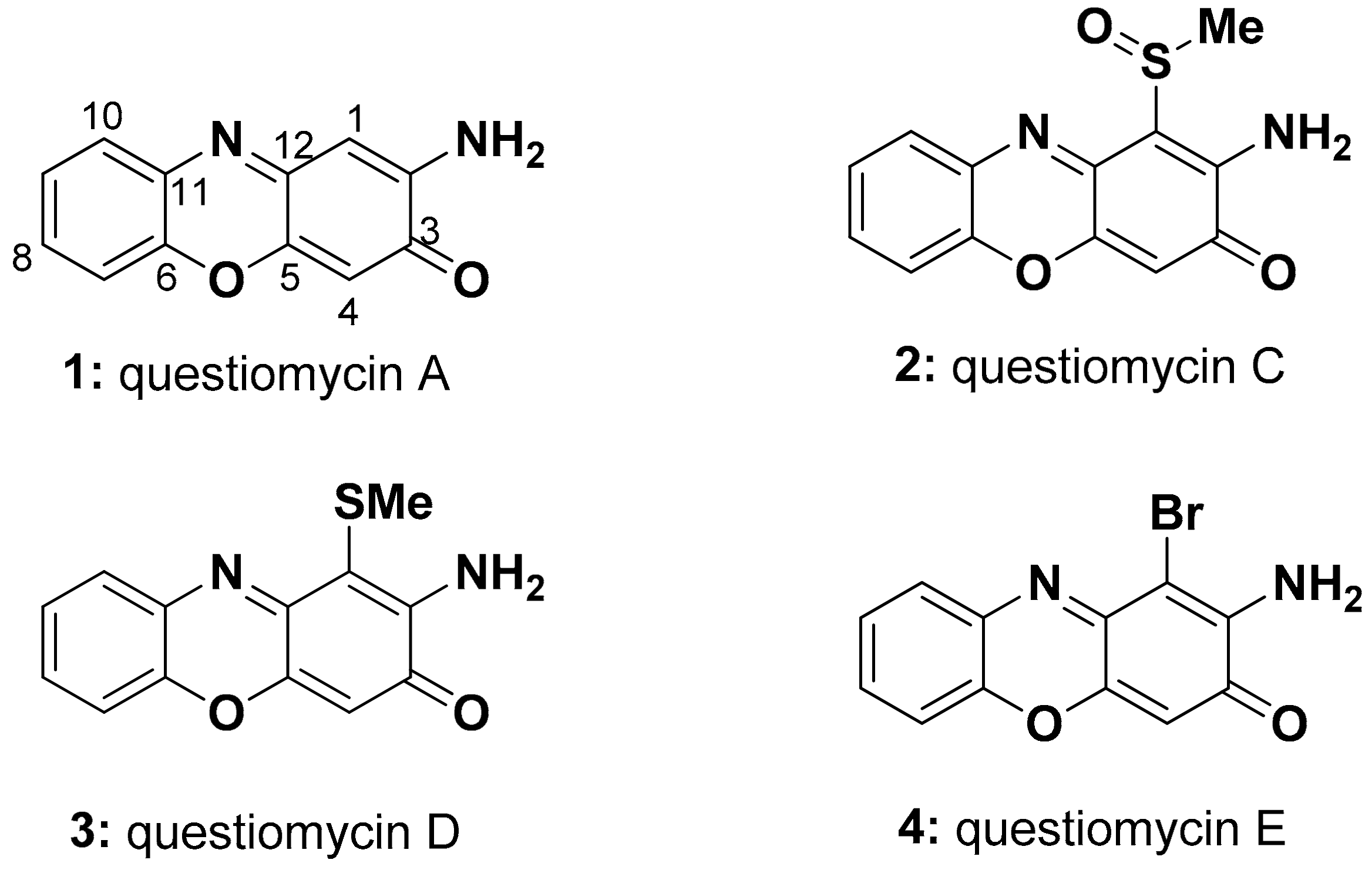
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| 1 | 6.36, s | |||
| 4 | 6.35, s | 6.46, s | 6.39, s | 6.40, s |
| 7 | 7.49, d (7.8) | 7.51, m | 7.51, m | 7.53, m |
| 8 | 7.46, t (7.9) | 7.51, m | 7.51, m | 7.53, m |
| 9 | 7.39, t (7.5) | 7.41, m | 7.43, m | 7.44, m |
| 10 | 7.70, d (7.9) | 7.70, d (7.8) | 7.82, d (7.9) | 7.80, d (7.9) |
| NH2 | 6.60, brs | 7.31, brs | 6.95, brs | 6.98, brs |
| 8.46, brs | ||||
| SOCH3 | 2.98, s | |||
| SCH3 | 2.31, s |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| 1 | 98.3 | 104.5 | 102.1 | 94.7 |
| 2 | 148.8 | 148.8 | 146.5 | 149.5 |
| 3 | 180.2 | 178.2 | 178.4 | 177.4 |
| 4 | 103.4 | 103.6 | 103.3 | 102.4 |
| 5 | 148.2 | 147.4 | 148.8 | 145.6 |
| 6 | 141.9 | 142.1 | 141.8 | 142.1 |
| 7 | 115.9 | 116.0 | 115.7 | 115.8 |
| 8 | 128.8 | 126.7 | 129.4 | 129.9 |
| 9 | 125.2 | 125.5 | 125.4 | 125.5 |
| 10 | 127.9 | 128.0 | 128.5 | 128.4 |
| 11 | 133.7 | 132.5 | 133.3 | 133.1 |
| 12 | 147.3 | 144.5 | 150.1 | 143.9 |
| SOCH3 | 39.0 | |||
| SCH3 | 17.1 |
| Organisms | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Chattonella antiqua | 0.64 ± 0.19 a | 0.18 ± 0.25 a | 6.37 b | 0.20 ± 0.04 a |
| Karenia mikimotoi | 1.18 c | |||
| Chaetoceros didymus | 0.59 c | |||
| Bangia fuscopurpurea | 25.9 ± 1.32 a | |||
| Artemia salina | 91.7 b | > 68.7 d | ||
| Oryzias sp. | 0.75-7.5 e |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umetsu, S.; Kanda, M.; Imai, I.; Sakai, R.; Fujita, M.J. Questiomycins, Algicidal Compounds Produced by the Marine Bacterium Alteromonas sp. D and Their Production Cue. Molecules 2019, 24, 4522. https://doi.org/10.3390/molecules24244522
Umetsu S, Kanda M, Imai I, Sakai R, Fujita MJ. Questiomycins, Algicidal Compounds Produced by the Marine Bacterium Alteromonas sp. D and Their Production Cue. Molecules. 2019; 24(24):4522. https://doi.org/10.3390/molecules24244522
Chicago/Turabian StyleUmetsu, Saki, Mamoru Kanda, Ichiro Imai, Ryuichi Sakai, and Masaki J. Fujita. 2019. "Questiomycins, Algicidal Compounds Produced by the Marine Bacterium Alteromonas sp. D and Their Production Cue" Molecules 24, no. 24: 4522. https://doi.org/10.3390/molecules24244522
APA StyleUmetsu, S., Kanda, M., Imai, I., Sakai, R., & Fujita, M. J. (2019). Questiomycins, Algicidal Compounds Produced by the Marine Bacterium Alteromonas sp. D and Their Production Cue. Molecules, 24(24), 4522. https://doi.org/10.3390/molecules24244522








