Effect of High-Pressure Processing (HPP) on the Fatty Acid Profile of Different Sized Ragworms (Hediste diversicolor) Cultured in an Integrated Multi-Trophic Aquaculture (IMTA) System
Abstract
1. Introduction
2. Results
2.1. Fatty Acid Profiles
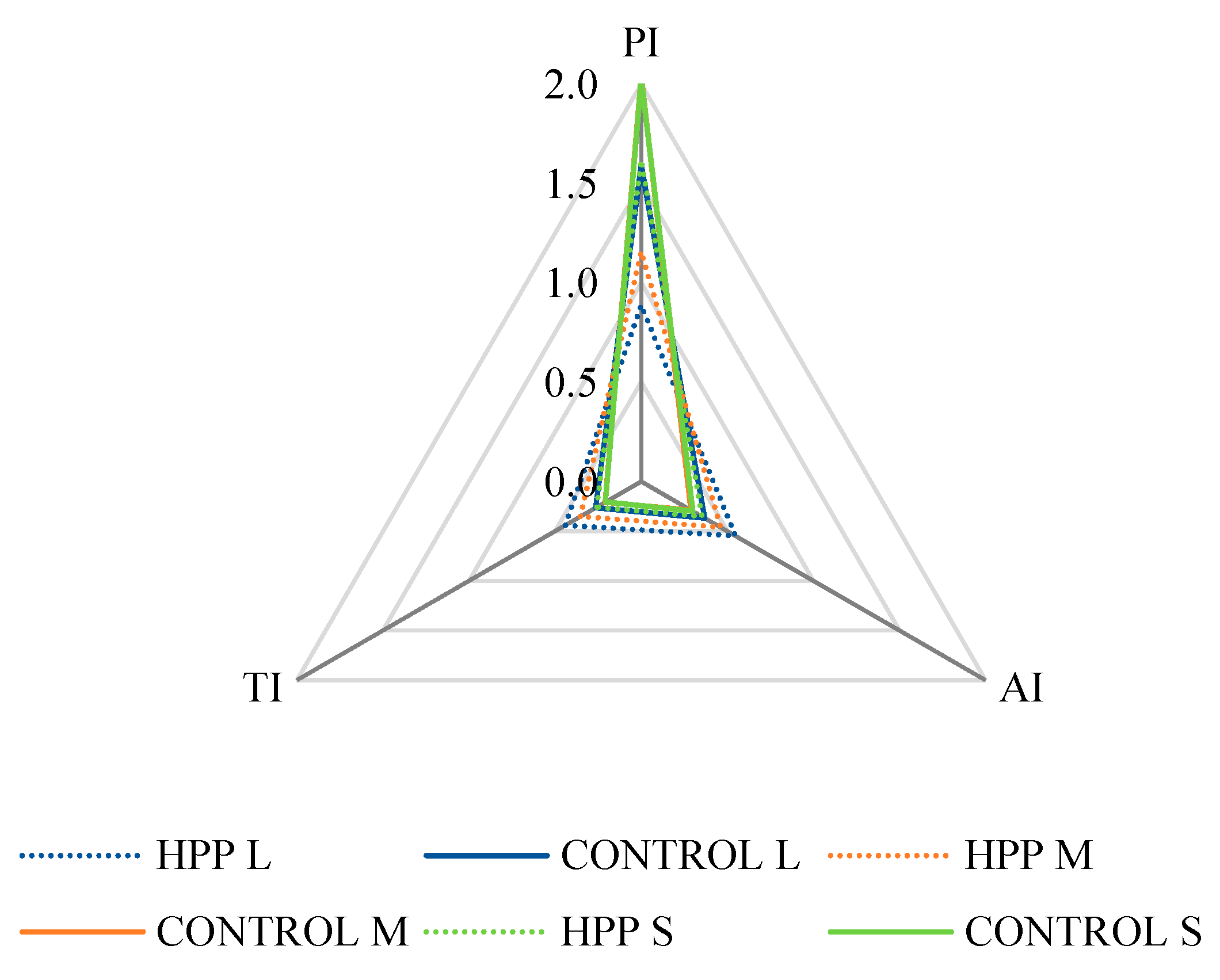
2.2. Lipid Quality Indexes
3. Discussion
4. Materials and Methods
4.1. Sampling and Processing of Ragworms H. Diversicolor Cultured in IMTA System
4.2. High-Pressure Processing Treatments
4.3. Fatty Acids Analysis
4.4. Lipid Quality Indexes
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Available online: http://www.fao.org/3/a-i5555e.pdf (accessed on 4 November 2019).
- Chopin, T.; Robinson, S.M.C.; Troell, M.; Neori, A.; Buschmann, A.H. Multitrophic Integration for Sustainable Marine Aquaculture. In Ecological Engineering, Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 3, pp. 2463–2475. [Google Scholar] [CrossRef]
- Alexander, K.A.; Angel, D.; Freeman, S.; Israel, D.; Johansen, J.; Kletou, D.; Meland, M.; Pecorino, D.; Rebours, C.; Rousou, M.; et al. Improving sustainability of aquaculture in Europe: Stakeholder dialogues on Integrated Multi-trophic Aquaculture (IMTA). Env. Sci. Policy 2016, 55, 96–106. [Google Scholar] [CrossRef]
- Stevens, J.R.; Newton, R.W.; Tlusty, M.; Little, D.C. The rise of aquaculture by-products: Increasing food production, value, and sustainability through strategic utilization. Mar. Policy 2018, 90, 115–124. [Google Scholar] [CrossRef]
- Bischoff, A.A.; Fink, P.; Waller, U. The fatty acid composition of Nereis diversicolor cultured in an integrated recirculated system: Possible implications for aquaculture. Aquaculture 2009, 296, 271–276. [Google Scholar] [CrossRef]
- Palmer, P.J. Polychaete-assisted sand filters. Aquaculture 2010, 306, 369–377. [Google Scholar] [CrossRef]
- Fang, J.; Jiang, Z.; Jansen, H.M.; Hu, F.; Fang, J.; Liu, Y.; Gao, Y.; Du, M. Applicability of Perinereis aibuhitensis Grube for fish waste removal from fish cages in Sanggou Bay, P.R. China. J. Ocean Univ. China 2017, 16, 294–304. [Google Scholar] [CrossRef]
- Marques, B.; Calado, R.; Lillebø, A.I. New species for the biomitigation of a super-intensive marine fish farm effluent: Combined use of polychaete-assisted sand filters and halophyte aquaponics. Sci. Total Env. 2017, 599, 1922–1928. [Google Scholar] [CrossRef]
- García-Alonso, J.; Müller, C.T.; Hardege, J.D. Influence of food regimes and seasonality on fatty acid composition in the ragworm. Aquat. Biol. 2008, 4, 7–13. [Google Scholar] [CrossRef]
- Marques, B.; Lillebo, A.I.; Ricardo, F.; Nunes, C.; Coimbra, M.A.; Calado, R. Adding value to ragworms (Hediste diversicolor) through the bioremediation of a super-intensive marine fish farm. Aquac. Environ. Interact. 2018, 10, 79–88. [Google Scholar] [CrossRef]
- Soudant, P.; Marty, Y.; Moal, J.; Robert, R.; Quéré, C.; Le Coz, J.R.; Samain, J.F. Effect of food fatty acid and sterol quality on Pecten maximus gonad composition and reproduction process. Aquaculture 1996, 143, 361–378. [Google Scholar] [CrossRef]
- Suloma, A.; Ogata, H.Y. Arachidonic acid is a Major Component in Gonadal Fatty acids of Tropical Coral Reef fish in the Philippines and Japan. J. Aquac. Res. Dev. 2011, 2, 1–7. [Google Scholar] [CrossRef]
- Meunpol, O.; Meejing, P.; Piyatiratitivorakul, S. Maturation diet based on fatty acid content for male Penaeus monodon (Fabricius) broodstock. Aquac. Res. 2005, 36, 1216–1225. [Google Scholar] [CrossRef]
- Cardinaletti, G.; Mosconi, G.; Salvatori, R.; Lanari, D.; Tomassoni, D.; Carnevali, O.; Polzonetti-Magni, A.M. Effect of dietary supplements of mussel and polychaetes on spawning performance of captive sole, Solea solea (Linnaeus, 1758). Anim. Reprod. Sci. 2009, 113, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Itoh, A.; Murakami, A.; Tsukashima, Y.; Kitajima, C.; Fujita, S. Effect of nutritional quality of diets given to broodstock on the Verge of Spawning on reproduction of Red-Sea Bream. Bull. Jpn. Soc. Sci. Fish 1984, 50, 1023–1028. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Fernández-Palacios, H.; Tacon, A.G.J. Effect of broodstock nutrition on reproductive performance of fish. Aquaculture 2001, 197, 25–42. [Google Scholar] [CrossRef]
- Sargent, J.; Bell, G.; McEvoy, L.; Tocher, D.; Estevez, A. Recent developments in the essential fatty acid nutrition of fish. Aquaculture 1999, 177, 191–199. [Google Scholar] [CrossRef]
- Bell, J.G.G.; Sargent, J.R. Arachidonic acid in aquaculture feeds: Current status and future opportunities. Aquaculture 2003, 218, 491–499. [Google Scholar] [CrossRef]
- Khajeh, M.; Rahbarghazi, R.; Nouri, M.; Darabi, M. Potential role of polyunsaturated fatty acids, with particular regard to the signaling pathways of arachidonic acid and its derivatives in the process of maturation of the oocytes: Contemporary review. Biomed. Pharmacother. 2017, 94, 458–467. [Google Scholar] [CrossRef]
- Bell, J.G.; Farndale, B.M.; Bruce, M.P.; Navas, J.M.; Carillo, M. Effects of broodstock dietary lipid on fatty acid compositions of eggs from sea bass (Dicentrarchus labrax). Aquaculture 1997, 149, 107–119. [Google Scholar] [CrossRef]
- Luis, O.J.; Passos, A.M. Seasonal changes in lipid content and composition of the polychaete Nereis (Hediste) diversicolor. Comp. Biochem. Phys. B 1995, 111, 579–586. [Google Scholar] [CrossRef]
- Barba, F.J.; Terefe, N.S.; Buckow, R.; Knorr, D.; Orlien, V. New opportunities and perspectives of high-pressure treatment to improve health and safety attributes of foods. A review. Food Res. Int. 2015, 77, 725–742. [Google Scholar] [CrossRef]
- Moreirinha, C.; Almeida, A.; Saraiva, J.A.; Delgadillo, I. High-pressure processing effects on foodborne bacteria by mid-infrared spectroscopy analysis. LWT-Food Sci. Technol. 2016, 73, 212–218. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Balasubramaniam, V.M.; Niranjan, K.; Knorr, D. Opportunities and Challenges in High Pressure Processing of Foods. Crit. Rev. Food Sci. Nutr. 2007, 47, 69–112. [Google Scholar] [CrossRef] [PubMed]
- Heinz, V.; Buckow, R. Food preservation by high pressure. J. Consum. Prot. Food Saf. 2010, 5, 73–81. [Google Scholar] [CrossRef]
- Mengden, R.; Röhner, A.; Sudhaus, N.; Klein, G. High-pressure processing of mild smoked rainbow trout fillets (Oncorhynchus mykiss) and fresh European catfish fillets (Silurus glanis). Innov. Food Sci. Emerg. Technol. 2015, 32, 9–15. [Google Scholar] [CrossRef]
- Ma, L.; Su, Y.C. Validation of high-pressure processing for inactivating Vibrio parahaemolyticus in Pacific oysters (Crassostrea gigas). Int. J. Food Microbiol. 2011, 144, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Aubourg, S.P.; Tabilo-Munizaga, G.; Reyes, J.E.; Rodríguez, A.; Pérez-Won, M. Effect of high-pressure treatment on microbial activity and lipid oxidation in chilled coho salmon. Eur. J. Lipid Sci. Technol. 2010, 112, 362–372. [Google Scholar] [CrossRef]
- Yagiz, Y.; Kristinsson, H.G.; Balaban, M.O.; Welt, B.A.; Ralat, M.; Marshall, M.R. Effect of high-pressure processing and cooking treatment on the quality of Atlantic salmon. Food Chem. 2009, 116, 828–835. [Google Scholar] [CrossRef]
- Yagiz, Y.; Kristinsson, H.G.; Balaban, M.O.; Marshall, M.R. Effect of high-pressure treatment on the quality of rainbow trout (Oncorhynchus mykiss) and mahi mahi (Coryphaena hippurus). J. Food Sci. 2007, 72, 509–515. [Google Scholar] [CrossRef]
- Ramirez-Suarez, J.C.; Morrissey, M.T. Effect of high-pressure processing (HPP) on shelf life of albacore tuna (Thunnus alalunga) minced muscle. Innov. Food Sci. Emerg. Technol. 2006, 7, 19–27. [Google Scholar] [CrossRef]
- Ohshima, T.; Ushio, H.; Koizumi, C. High-pressure processing of fish and fish products. Trends Food Sci. Technol. 1993, 4, 370–375. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kerry, J.P.; Kelly, A.L. Changes in the microbiological and physicochemical quality of high-pressure-treated oysters (Crassostrea gigas) during chilled storage. Food Control 2008, 19, 1139–1147. [Google Scholar] [CrossRef]
- Thakur, B.R.; Nelson, P.E. High-pressure processing and preservation of food. Food Rev. Int. 1998, 14, 427–447. [Google Scholar] [CrossRef]
- Shimidzu, N.; Goto, M.; & Miki, W. Carotenoids as singlet oxygen quenchers in marine organisms. Fish. Sci. 1996, 62, 134–137. [Google Scholar] [CrossRef]
- He, Z.; Huang, Y.; Li, H.; Qin, G.; Wang, T.; Yang, J. Effect of high-pressure treatment on the fatty acid composition of intramuscular lipid in pork. Meat Sci. 2012, 90, 170–175. [Google Scholar] [CrossRef]
- Vázquez, M.; Torres, J.A.; Gallardo, J.M.; Saraiva, J.; Aubourg, S.P. Lipid hydrolysis and oxidation development in frozen mackerel (Scomber scombrus): Effect of a high hydrostatic pressure pre-treatment. Innov. Food Sci. Emerg. Technol. 2013, 18, 24–30. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, X.; Ren, Y.; Fan, E.; Chang, H.; Wu, H. Effects of high-pressure treatment and temperature on lipid oxidation and fatty acid composition of yak (Poephagus grunniens) body fat. Meat Sci. 2013, 94, 489–494. [Google Scholar] [CrossRef]
- Canto, A.C.V.C.S.; Suman, S.P.; Nair, M.N.; Li, S.; Rentfrow, G.; Beach, C.M.; Silva, T.J.P.; Wheeler, T.L.; Shackelford, S.D.; Grayson, A.; et al. Differential abundance of sarcoplasmic proteome explains animal effect on beef Longissimus lumborum color stability. Meat Sci. 2015, 102, 90–98. [Google Scholar] [CrossRef]
- Telahigue, K.; Hajji, T.; Rabeh, I.; Cafsi, M. El The changes of fatty acid composition in sun dried, oven dried and frozen hake (Merluccius merluccius) and sardinella (Sardinella aurita). Afr. J. Biochem. Res. 2013, 7, 158–164. [Google Scholar]
- Aued-Pimentel, S.; Lago, J.H.G.; Chaves, M.H.; Kumagai, E.E. Evaluation of a methylation procedure to determine cyclopropenoids fatty acids from Sterculia striata St. Hil. Et Nauds seed oil. J. Chromatogr. A 2004, 1054, 235–239. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Lubis, Z.; Buckle, K.A. Rancidity and lipid oxidation of dried-salted sardines. Int. J. Food Sci. Technol. 1990, 25, 295–303. [Google Scholar] [CrossRef]
- Anderson, M.J. Animal-sediment relationships revisited: Characterising species’ distributions along an environmental gradient using canonical analysis and quantile regression splines. J. Exp. Mar. Biol. Ecol. 2008, 366, 16–27. [Google Scholar] [CrossRef]
- Clarke, K.; Gorley, R. PRIMER v6: User manual/tutorial. PRIMER-E, Plymouth; Plymouth Marine Laboratory: Plymouth, UK, 2006. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
| Pathogens | Isolated From: | Pressure Applied/ Duration of HPP | References |
|---|---|---|---|
| Photobacterium damselae | Hake (Merluccius merluccius) and dried salted cod (Gadus morhua) | 300 MPa (15 min) | [23] |
| Vibrio anguillarum | |||
| Aeromonas | |||
| Salmonella sp. | |||
| Escherichia coli | |||
| Listeria monocytogenes | Smoked rainbow trout fillets (Oncorhynchus mykiss) and fresh catfish fillets (Silurus glanis) | 400/600 MPa (1/5 min) | [26] |
| Escherichia coli | |||
| Vibrio parahaemolyticus | Oysters (Crassostrea gigas) | 293 MPa (2 min) | [27] |
| Pseudomonas spp. | Coho salmon (Oncorhynchus kisutch) | 135/170/200 MPa (30 s) | [28] |
| Shewanella spp. | |||
| ND † | Atlantic salmon (Salmo salar) | 150/300 MPa (15 min) | [29] |
| ND † | Rainbow trout (Oncorhynchus mykiss) and Mahi Mahi (Coryphaena hippurus) | 150/300/450/600 MPa (15 min) | [30] |
| Psycrophiles | Albacore tuna (Thunnus alalunga) | 310 MPa (6 min) | [31] |
| FA | Control Small | Control Medium | Control Large | HPP Small | HPP Medium | HPP Large |
|---|---|---|---|---|---|---|
| 14:0 | 0.29 ± 0.01 | 0.24 ± 0.01 | 0.35 ± 0.00 | 0.35 ± 0.00 | 0.41 ± 0.02 | 0.54 ± 0.02 |
| 15:0 | 0.25 ± 0.00 | 0.21 ± 0.01 | 0.25 ± 0.00 | 0.27 ± 0.00 | 0.28 ± 0.00 | 0.33 ± 0.02 |
| 16:0 | 4.09 ± 0.06 | 3.46 ± 0.18 | 4.57 ± 0.11 | 4.58 ± 0.06 | 4.77 ± 0.07 | 6.57 ± 0.18 |
| 17:0 | 0.45 ± 0.01 | 0.35 ± 0.02 | 0.42 ± 0.01 | 0.51 ± 0.01 | 0.44 ± 0.01 | 0.55 ± 0.02 |
| 18:0 | 1.92 ± 0.02 | 1.63 ± 0.08 | 1.98 ± 0.03 | 2.09 ± 0.03 | 1.95 ± 0.02 | 2.34 ± 0.05 |
| ∑ SFA 1 | 7.01 ± 0.10 | 5.89 ± 0.30 | 7.58 ± 0.16 | 7.81 ± 0.11 | 7.85 ± 0.12 | 10.32 ± 0.29 |
| 16:1n-7 | 0.78 ± 0.01 | 0.72 ± 0.02 | 0.98 ± 0.04 | 0.93 ± 0.01 | 1.00 ± 0.01 | 1.44 ± 0.03 |
| 18:1n-9 | 1.93 ± 0.03 | 1.65 ± 0.04 | 1.84 ± 0.02 | 2.04 ± 0.05 | 1.85 ± 0.02 | 1.88 ± 0.07 |
| 18:1n-7 | 1.00 ± 0.01 | 0.91 ± 0.03 | 0.99 ± 0.01 | 1.04 ± 0.04 | 1.04 ± 0.01 | 1.23 ± 0.06 |
| 18:1n-5 | 2.08 ± 0.02 | 1.69 ± 0.03 | 1.98 ± 0.06 | 2.35 ± 0.05 | 1.99 ± 0.02 | 2.86 ± 0.08 |
| 20:1n-9 | 2.20 ± 0.03 | 1.91 ± 0.07 | 2.06 ± 0.02 | 2.34 ± 0.05 | 1.94 ± 0.02 | 2.18 ± 0.04 |
| 22:1n-9 | 0.84 ± 0.01 | 1.05 ± 0.04 | 1.07 ± 0.01 | 0.76 ± 0.02 | 0.85 ± 0.01 | 0.82 ± 0.02 |
| ∑ MUFA 2 | 8.83 ± 0.11 | 7.92 ± 0.23 | 8.92 ± 0.15 | 9.46 ± 0.23 | 8.67 ± 0.08 | 10.55 ± 0.32 |
| 18:2n-6 | 0.85 ± 0.01 | 0.73 ± 0.03 | 0.78 ± 0.01 | 0.81 ± 0.01 | 0.64 ± 0.01 | 0.69 ± 0.03 |
| 18:3n-6 | 0.25 ± 0.00 | 0.24 ± 0.01 | 0.32 ± 0.02 | 0.25 ± 0.00 | 0.22 ± 0.00 | 0.30 ± 0.02 |
| 20:2n-6 | 0.63 ± 0.01 | 0.61 ± 0.02 | 0.44 ± 0.00 | 0.64 ± 0.01 | 0.56 ± 0.00 | 0.82 ± 0.02 |
| ∑ PUFA 3 | 2.11 ± 0.02 | 1.99 ± 0.07 | 2.32 ± 0.03 | 2.04 ± 0.03 | 1.73 ± 0.10 | 2.24 ± 0.10 |
| 20:4n-6 (AA) | 0.79 ± 0.01 | 0.83 ± 0.04 | 0.82 ± 0.00 | 0.68 ± 0.01 | 0.63 ± 0.01 | 0.67 ± 0.03 |
| 20:5n-3 (EPA) | 7.53 ± 0.11 | 6.32 ± 0.02 | 6.68 ± 0.17 | 6.77 ± 0.11 | 5.07 ± 0.05 | 5.33 ± 0.17 |
| 22:4n-6 | 0.90 ± 0.01 | 1.08 ± 0.05 | 1.01 ± 0.01 | 0.78 ± 0.01 | 0.77 ± 0.01 | 0.85 ± 0.04 |
| 22:5n-3 | 1.49 ± 0.02 | 1.40 ± 0.06 | 1.27 ± 0.04 | 1.38 ± 0.03 | 1.04 ± 0.01 | 1.09 ± 0.04 |
| 22:6n-3 (DHA) | 0.76 ± 0.01 | 0.71 ± 0.02 | 0.59 ± 0.01 | 0.63 ± 0.01 | 0.44 ± 0.02 | 0.50 ± 0.03 |
| ∑ HUFA 4 | 11.47 ± 0.16 | 10.35 ± 0.16 | 10.37 ± 0.23 | 10.25 ± 0.18 | 7.97 ± 0.08 | 8.44 ± 0.30 |
| ∑ Others 5 | 0.78 ± 0.01 | 0.62 ± 0.03 | 0.65 ± 0.02 | 0.93 ± 0.01 | 0.82 ± 0.1 | 1.05 ± 0.05 |
| ∑ Total | 30.20 ± 0.04 | 26.77 ± 0.79 | 29.84 ± 0.59 | 30.49 ± 0.56 | 27.03 ± 0.39 | 32.60 ± 1.05 |
| Control vs. HPP Small | Control vs. HPP Medium | Control vs. HPP Large | ||||||
|---|---|---|---|---|---|---|---|---|
| FA | Contr.% | Cum.% | FA | Contr.% | Cum.% | FA | Contr.% | Cum.% |
| 20:5n-3 | 8.7 | 8.7 | 16:0 | 11.43 | 11.43 | 16:0 | 13.4 | 13.4 |
| 16:0 | 8.53 | 17.23 | 20:5n-3 | 8.27 | 19.7 | 18:1n-7 | 11.37 | 24.77 |
| 18:1n-7 | 8.09 | 25.32 | 22:6n-3 | 7.65 | 27.35 | 16:1n-7 | 9.2 | 33.98 |
| 16:1n-7 | 7.6 | 32.92 | 22:5n-3 | 7.13 | 34.48 | 20:5n-3 | 8.49 | 42.47 |
| 22:6n-3 | 7.14 | 40.06 | 22:2n-6 | 7.12 | 41.6 | iso16:0 | 7.07 | 49.53 |
| 22:2n-6 | 6.12 | 46.18 | 16:1n-7 | 6.58 | 48.18 | |||
| 20:4n-6 | 5.83 | 52.01 | 14:0 | 5.57 | 53.75 | |||
| Lipid Quality Index | Control | HPP | ||||
|---|---|---|---|---|---|---|
| Small | Medium | Large | Small | Medium | Large | |
| AI | 0.30 ± 0.00 a | 0.29 ± 0.02 a | 0.37 ± 0.00 b | 0.35 ± 0.00 b | 0.46 ± 0.01 c | 0.55 ± 0.00 d |
| TI | 0.21 ± 0.00 a | 0.21 ± 0.01 a | 0.26 ± 0.00 b | 0.26 ± 0.00 b | 0.35 ± 0.00 c | 0.44 ± 0.00 d |
| PI | 2.03 ± 0.01 a | 2.04 ± 0.12 a | 1.59 ± 0.01 b | 1.62 ± 0.02 b | 1.16 ± 0.01 c | 0.89 ± 0.01 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, B.; Lillebø, A.I.; Domingues, M.d.R.M.; Saraiva, J.A.; Calado, R. Effect of High-Pressure Processing (HPP) on the Fatty Acid Profile of Different Sized Ragworms (Hediste diversicolor) Cultured in an Integrated Multi-Trophic Aquaculture (IMTA) System. Molecules 2019, 24, 4503. https://doi.org/10.3390/molecules24244503
Marques B, Lillebø AI, Domingues MdRM, Saraiva JA, Calado R. Effect of High-Pressure Processing (HPP) on the Fatty Acid Profile of Different Sized Ragworms (Hediste diversicolor) Cultured in an Integrated Multi-Trophic Aquaculture (IMTA) System. Molecules. 2019; 24(24):4503. https://doi.org/10.3390/molecules24244503
Chicago/Turabian StyleMarques, Bruna, Ana Isabel Lillebø, Maria do Rosário M. Domingues, Jorge A. Saraiva, and Ricardo Calado. 2019. "Effect of High-Pressure Processing (HPP) on the Fatty Acid Profile of Different Sized Ragworms (Hediste diversicolor) Cultured in an Integrated Multi-Trophic Aquaculture (IMTA) System" Molecules 24, no. 24: 4503. https://doi.org/10.3390/molecules24244503
APA StyleMarques, B., Lillebø, A. I., Domingues, M. d. R. M., Saraiva, J. A., & Calado, R. (2019). Effect of High-Pressure Processing (HPP) on the Fatty Acid Profile of Different Sized Ragworms (Hediste diversicolor) Cultured in an Integrated Multi-Trophic Aquaculture (IMTA) System. Molecules, 24(24), 4503. https://doi.org/10.3390/molecules24244503







