Solvent-Free Process for the Development of Photocatalytic Membranes
Abstract
:1. Introduction
2. Results and Discussion
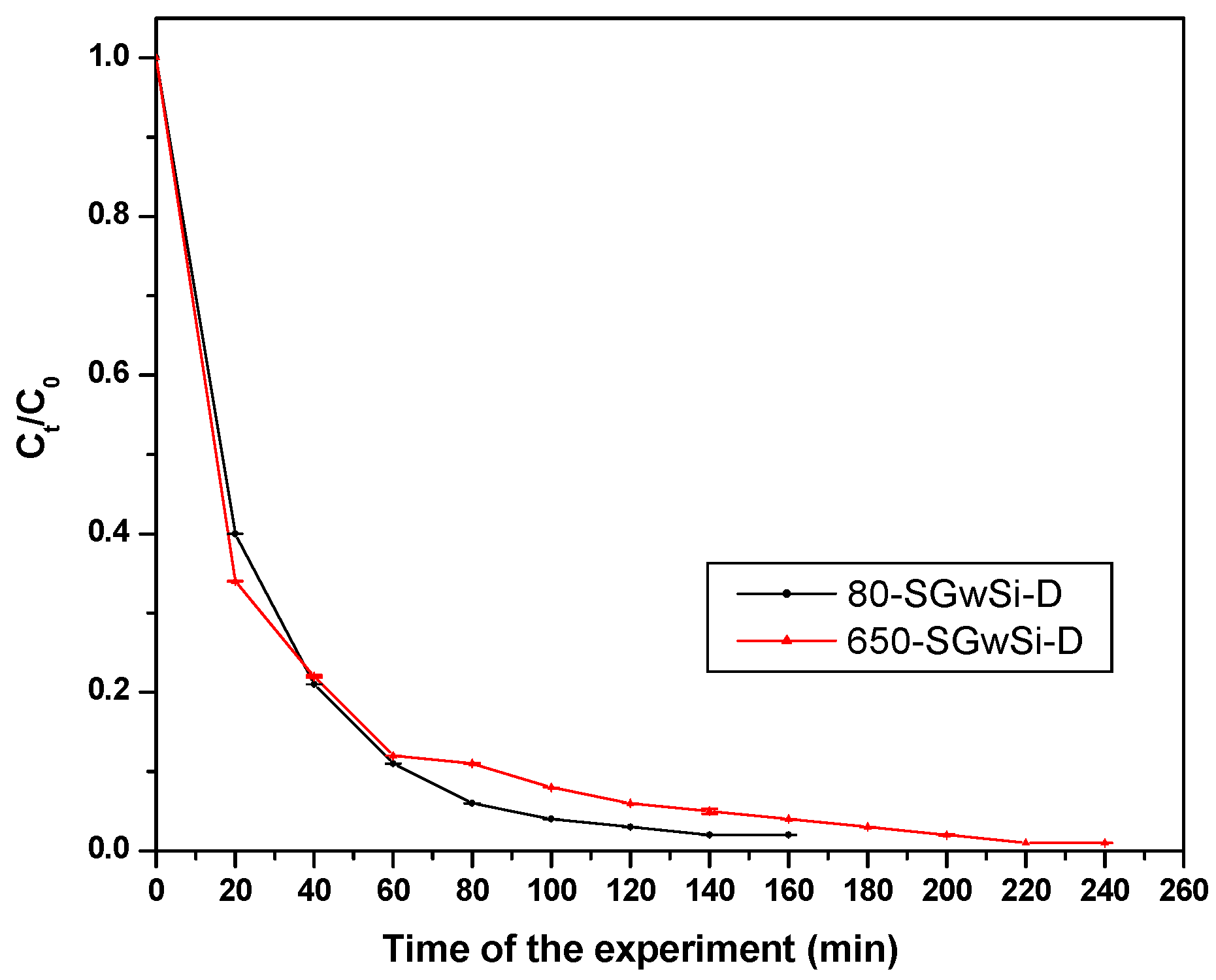
2.1. Photocatalytic Performance
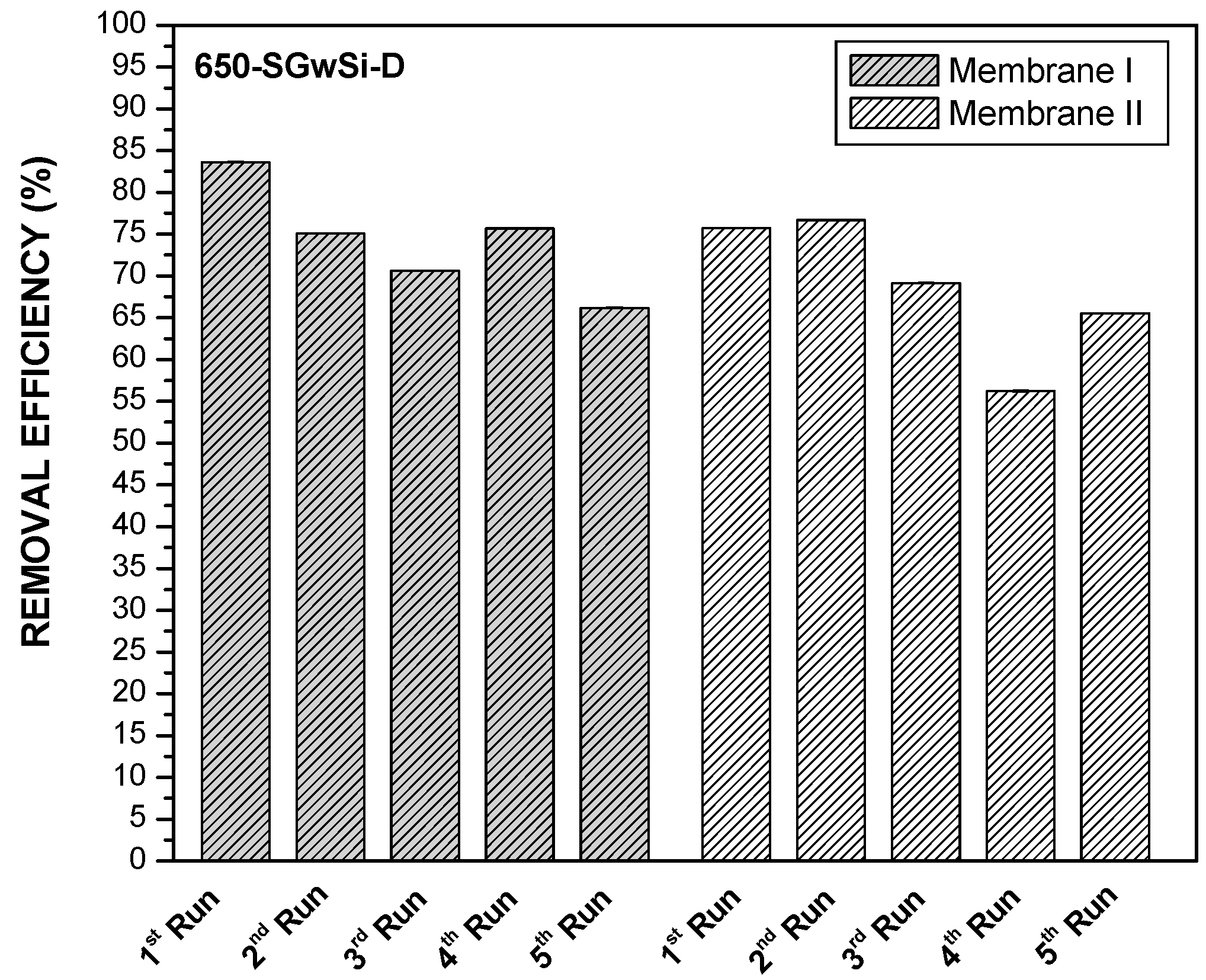
Assessment of the Reusability Potential of the Membranes
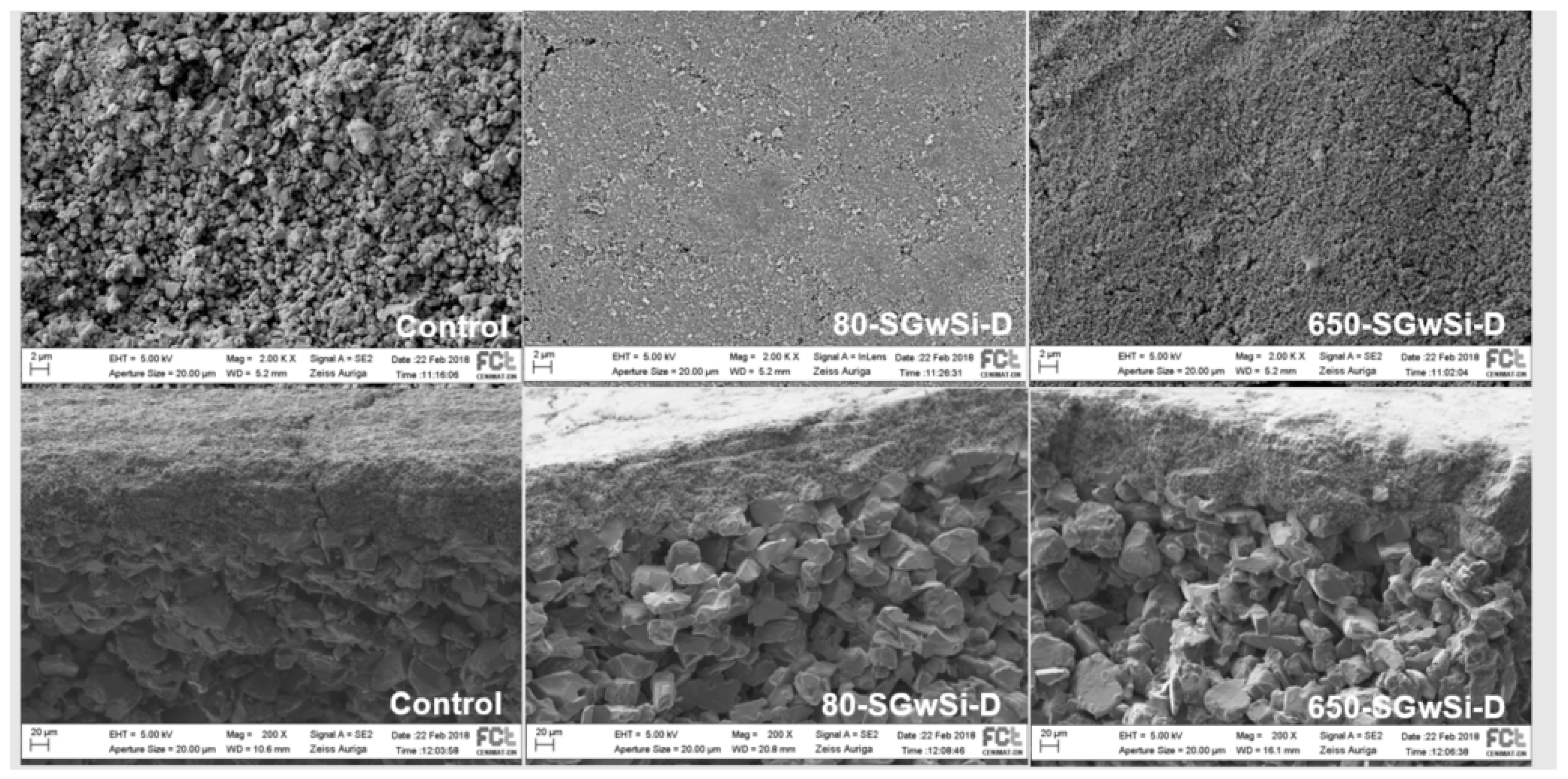
2.2. Morphology Characterization
Porosity of Membranes
2.3. Contact Angle
2.4. Membrane Filtration Performance
2.5. Economic Benefits of using Water Instead of Solvents
3. Materials and Methods
3.1. Modification of Ceramic Membranes
3.1.1. Temperature Effect on Photocatalytic Activity
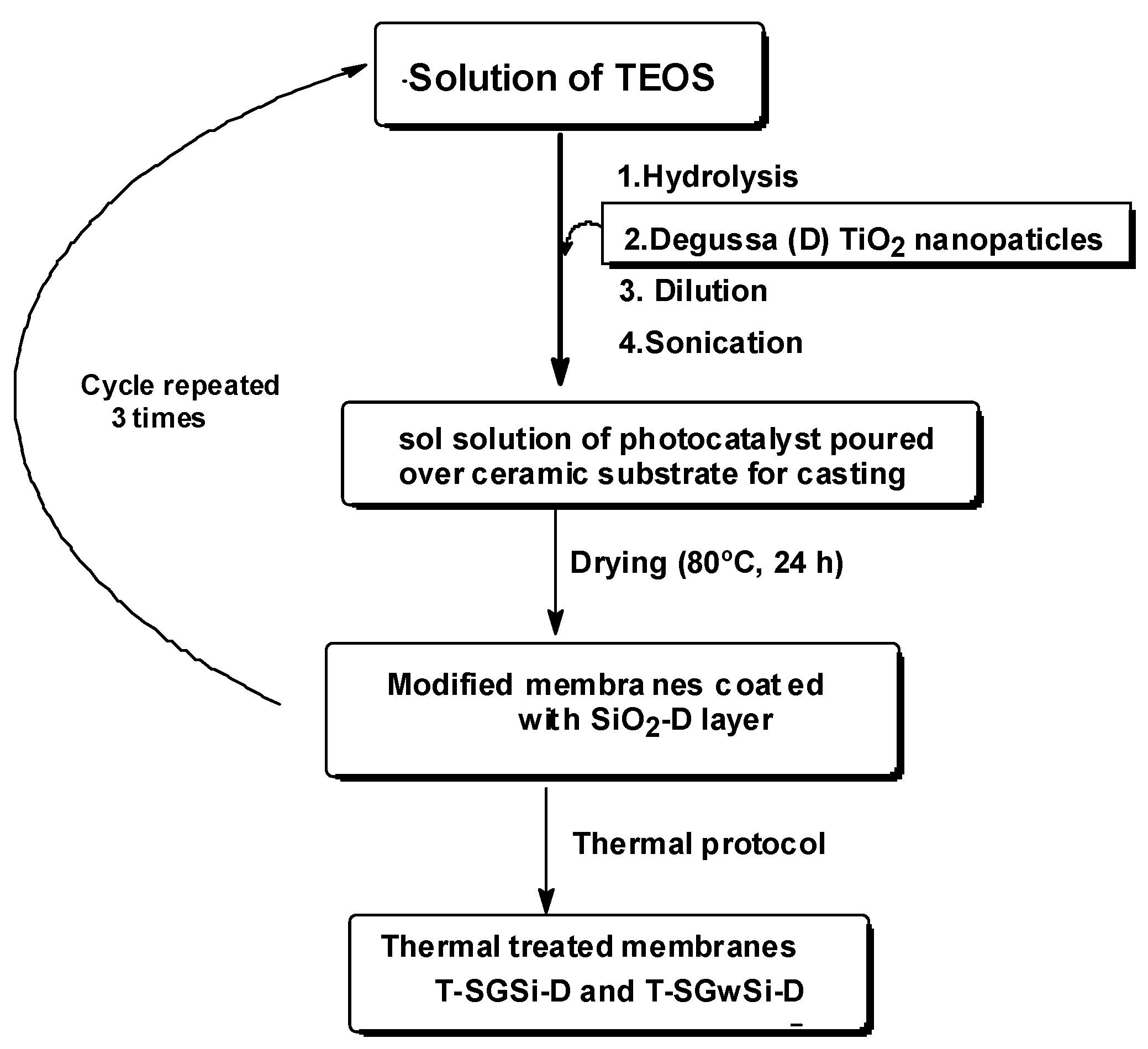
3.1.2. Solvent-Free Process for the Production of Photocatalytic Membranes
3.2. Assessment of the Photocatalytic Membranes and Selection of the Most Promising One
3.2.1. Assays to Test the Photocatalytic Performance
3.2.2. Morphology Characterization
3.2.3. Contact Angle
3.2.4. Membrane Filtration Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Li, C.; Ren, X.; Liu, K.; Yang, J. Fine platinum nanoparticles supported on a porous ceramic membrane as efficient catalysts for the removal of benzene. Sci. Rep. 2017, 7, 16589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antolini, E.; Gonzalez, E.R. Ceramic materials as supports for low-temperature fuel cell catalysts. Solid State Ion. 2009, 180, 746–763. [Google Scholar] [CrossRef]
- Zhou, Y.; Fukushima, M.; Miyazaki, H.; Yoshizawa, Y.I.; Hirao, K.; Iwamoto, Y.; Sato, K. Preparation and characterization of tubular porous silicon carbide membrane supports. J. Memb. Sci. 2011, 369, 112–118. [Google Scholar] [CrossRef]
- Fraga, M.C.; Sanches, S.; Crespo, J.G.; Pereira, V.J. Assessment of a new silicon carbide tubular honeycomb membrane for treatment of olive mill wastewaters. Membranes 2017, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Vaccaro, L.; Lanari, D.; Marrocchi, A.; Strappaveccia, G. Flow approaches towards sustainability. Green Chem. 2014, 16, 3680–3704. [Google Scholar] [CrossRef]
- Fraga, M.C.; Sanches, S.; Pereira, V.J.; Crespo, J.G.; Yuan, L.; Marcher, J.; de Yuso, M.V.M.; Rodríguez-Castellón, E.; Benavente, J. Morphological, chemical surface and filtration characterization of a new silicon carbide membrane. J. Eur. Ceram. Soc. 2017, 37, 899–905. [Google Scholar] [CrossRef]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Sanches, S.; Nunes, C.; Passarinho, P.C.; Ferreira, F.C.; Pereira, V.J.; Crespo, J.G. Development of photocatalytic titanium dioxide membranes for degradation of recalcitrant compounds. J. Chem. Technol. Biot. 2016, 92, 1727–1737. [Google Scholar] [CrossRef]
- Geltmeyer, J.; Teixido, H.; Meire, M.; Van Acker, T.; Deventer, K.; Vanhaecke, F.; Van Hulle, S.; De Buysser, K.; De Clerck, K. TiO2 functionalized nanofibrous membranes for removal of organic (micro) pollutants from water. Sep. Purif. Technol. 2017, 179, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Wan, S.; Yu, Z.; Wang, L. Optimization and modeling of preparation conditions of TiO2 nanoparticles coated on hollow glass microspheres using response surface methodology. Sep. Purif. Technol. 2014, 125, 156–162. [Google Scholar] [CrossRef]
- Rajoriya, S.; Bargole, S.; George, S.; Saharan, V.K.; Gogate, P.R.; Pandit, A.B. Synthesis and characterization of samarium and nitrogen doped TiO2 photocatalysts for photo-degradation of 4-acetamidophenol in combination with hydrodynamic and acoustic cavitation. Sep. Purif. Technol. 2019, 209, 254–269. [Google Scholar] [CrossRef]
- Kim, J.; Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef] [PubMed]
- Brinker, C.J.; Sehgal, R.; Hietala, S.L.; Deshpande, R.; Smith, D.M.; Loy, D.; Ashley, C.S. Sol-gel strategies for controlled porosity inorganic materials. J. Membr. Sci. 1994, 94, 85–102. [Google Scholar] [CrossRef]
- Langlet, M.; Kim, A.; Audier, M.; Herrmann, J.M. Sol-Gel preparation of photocatalytic TiO2 films on polymer substrates. J. Sol.-Gel Sci. Technol. 2002, 25, 223–234. [Google Scholar] [CrossRef]
- Sun, L.; An, T.; Wan, S.; Li, G.; Bao, N.; Hu, X.; Fu, J.; Sheng, G. Effect of synthesis conditions on photocatalytic activities of nanoparticulate TiO2 thin films. Sep. Purif. Technol. 2009, 68, 83–89. [Google Scholar] [CrossRef]
- Fateh, R.; Dillert, R.; Bahnemann, D. Preparation and characterization of transparent hydrophilic photocatalytic TiO2/SiO2 thin films on polycarbonate. Langmuir 2013, 29, 3730–3739. [Google Scholar] [CrossRef]
- Gao, X.; Wachs, I.E. Titania–silica as catalysts: Molecular structural characteristics and physico-chemical properties. Catal. Today 1999, 51, 233–254. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jang, J.-W.; Youn, D.H.; Kim, E.S.; Choi, S.H.; Shin, T.J.; Lee, J.S. Photocatalytic selective oxidation of the terminal methyl group of dodecane with molecular oxygen over atomically dispersed Ti in a mesoporous SiO2 matrix. Green Chem. 2013, 15, 3387–3395. [Google Scholar] [CrossRef]
- Huertas, R.M.; Fraga, M.C.; Crespo, J.G.; Pereira, V.J. Sol-gel membrane modification for enhanced photocatalytic activity. Sep. Purif. Technol. 2017, 180, 69–81. [Google Scholar] [CrossRef]
- Fraga, C.; Huertas, R.; Crespo, J.G.; Pereira, V.J. Novel submerged photocatalytic membrane reactor for treatment of olive mill wastewaters. Catalysts 2019, 9, 769. [Google Scholar] [CrossRef] [Green Version]
- DeVierno Kreuder, A.; House-Knight, T.; Whitford, J.; Ponnusamy, E.; Miller, P.; Jesse, N.; Rodenborn, R.; Sayag, S.; Gebel, M.; Aped, I.; et al. A method for assessing greener alternatives between chemical products following the 12 principles of green chemistry. ACS Sustain. Chem. Eng. 2017, 5, 2927–2935. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Li, X.; Gao, Q.; Yuan, J.; Wen, J.; Fang, Y.; Liu, W.; Zhang, S.; Liu, Y. Metal-free carbon nanotube-SiC nanowire heterostructures with enhanced photocatalytic H2 evolution under visible light irradiation. Catal. Sci. Technol. 2015, 5, 2798–2806. [Google Scholar] [CrossRef]
- Chen, Y.; Dionysiou, D.D. Correlation of structural properties and film thickness to photocatalytic activity of thick TiO2 films coated on stainless steel. Appl. Catal. B 2006, 69, 24–33. [Google Scholar] [CrossRef]
- Wang, X.; Wu, G.; Zhou, B.; Shen, J. thermal annealing effect on optical properties of binary TiO(2)-SiO(2) sol-gel coatings. Materials 2013, 6, 76–84. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Cheng, K.; Weng, W. SiO2/TiO2 Nanocomposite films on polystyrene for light-induced cell detachment application. ACS Appl. Mater. Interfaces 2017, 9, 2130–2137. [Google Scholar]
- Oliveira, B.R.; Sanches, S.; Huertas, R.; Crespo, M.T.B.; Pereira, V.J. Treatment of a real water matrix inoculated with Aspergillus fumigatus using a photocatalytic membrane reactor. 2019. Submitted. [Google Scholar]
- Sanches, S.; Fraga, M.C.; Silva, N.A.; Nunes, P.; Crespo, J.G.; Pereira, V.J. Pilot scale nanofiltration treatment of olive mill wastewater: A technical and economical evaluation. Environ. Sci. Pollut. Res. 2017, 24, 3506–3518. [Google Scholar] [CrossRef]
- Mills, A. An overview of the methylene blue ISO test for assessing the activities of photocatalytic films. Appl. Catal. B 2012, 128, 144–149. [Google Scholar] [CrossRef]
- Mahyar, A.; Behnajady Mohammad, A.; Modirshahla, N. Enhanced Photocatalytic degradation of c.i. basic violet 2 using TiO2–SiO2 composite nanoparticles. Photochem. Photobiol. Sci. 2011, 87, 795–801. [Google Scholar] [CrossRef]
- Kim, D.-K.; Jeong, K.-S.; Kang, Y.-S.; Kang, H.-K.; Cho, S.W.; Kim, S.-O.; Suh, D.; Kim, S.; Cho, M.-H. Controlling the defects and transition layer in SiO2 films grown on 4H-SiC via direct plasma-assisted oxidation. Sci. Rep. 2016, 6, 34945. [Google Scholar] [CrossRef]
- Fardad, M.A.; Yeatman, E.M.; Dawnay, E.J.C.; Green, M.; Horowitz, F. Effects of H2O on structure of acid-catalysed SiO2 sol-gel films. J. Non-Cryst. Solids 1995, 183, 260–267. [Google Scholar] [CrossRef]
- Chen, H.-S.; Huang, S.-H.; Perng, T.-P. Preparation and characterization of molecularly homogeneous silica–titania film by sol–gel process with different synthetic strategies. Appl. Mater. Interfaces 2012, 4, 5188–5195. [Google Scholar] [CrossRef]
- Almarzooqi, F.; Bilad, M.; Mansoor, B.; Arafat, H. A comparative study of image analysis and porometry techniques for characterization of porous membranes. J. Mater. Sci. 2016, 51, 2017–2032. [Google Scholar] [CrossRef]
- Masselin, I.; Durand-Bourlier, L.; Laine, J.-M.; Sizaret, P.-Y.; Chasseray, X.; Lemordant, D. Membrane characterization using microscopic image analysis. J. Membrane Sci. 2001, 186, 85–96. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image Processing with ImageJ. J. Biophotonics 2004, 11, 36–42. [Google Scholar]
Sample Availability: Samples are not available from the authors. |






| Membranes | Methylene Blue Removal (%) | Methylene Blue Adsorption (%) |
|---|---|---|
| Control | 48 | 13 |
| 80-SGSi-D | 72 | 20 |
| 300-SGSi-D | 77 | 27 |
| 500-SGSi-D | 73 | 29 |
| 650-SGSi-D | 82 | 31 |
| 80-SGwSi-D | 77 | 17 |
| 650-SGwSi-D | 74 | 24 |
| Membranes | k (min−1) | t1/2 (min) |
|---|---|---|
| Control | 0.0110 | 63 |
| Methylene blue | 0.0040 | 192 |
| 80-SGSi-D | 0.0220 | 31 |
| 300-SGSi-D | 0.0244 | 28 |
| 500-SGSi-D | 0.0216 | 32 |
| 650-SGSi-D | 0.0285 | 24 |
| 80-SGwSi-D | 0.0275 | 25 |
| 650-SGwSi-D | 0.0225 | 31 |
| Membranes * | Control Z1 × 2000 | Control Z2 × 2000 | 80-SGwSi-D Z1 × 2000 | 80-SGwSi-D Z2 × 2000 | 650-SGwSi-D Z1 × 2000 | 650-SGwSi-D Z2 × 2000 |
|---|---|---|---|---|---|---|
| Pore density (µm−2) | 1.7 | 1.5 | 11.6 | 11.1 | 3.8 | 10.1 |
| Mean pore area (µm2) | 0.05 ± 0.16 | 0.06 ± 0.23 | 0.01 ± 0.02 | 0.01 ± 0.03 | 0.01 ± 0.03 | 0.01 ± 0.01 |
| Minimum pore area (µm2) | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 |
| Maximum pore area (µm2) | 3.409 | 7.136 | 0.79 | 1.00 | 2.15 | 0.19 |
| Porosity (%) | 9.0 | 9.3 | 12.0 | 8.6 | 3.4 | 6.3 |
| Average circularity | 0.77 ± 0.39 | 0.80 ± 0.26 | 0.90 ± 0.19 | 0.93 ± 0.16 | 0.93 ± 0.16 | 0.94 ± 0.15 |
| Average feret diameter (µm) | 0.32 ± 0.26 | 0.33 ± 0.45 | 0.15 ± 0.12 | 0.13 ± 0.11 | 0.13 ± 0.13 | 0.12 ± 0.07 |
| Maximum feret’s diameter (µm) | 1.000 | 6.963 | 3.215 | 2.896 | 5.461 | 1.079 |
| Minimum feret’s diameter (µm) | 0.073 | 0.083 | 0.081 | 0.078 | 0.076 | 0.079 |
| Control | 80-SGwSi-D | 650-SGwSi-D | ||||
|---|---|---|---|---|---|---|
| MF | MF + UV | MF | MF + UV | MF | MF + UV | |
| MB permeability (Lh−1m−2bar−1) during the test | 19,126 ± 950 | 19,857 ± 3490 | 3479 ± 280 | 2645 ± 705 | 1955 ± 135 | 2486 ± 363 |
| Time of filtration (min) | 5 | 5 | 30 | 38 | 50 | 37 |
| % Photocatalytic degradation in the feed | n.a | 0 | n.a | 31 | n.a | 39 |
| % Total removal | 3 | 0 | 12 | 37 | 15 | 45 |
| Samples | Ti (mg/L) | |
|---|---|---|
| Control solutions, no filtration performed | Distilled water | <0.005 |
| Citric acid 2% (w/v) | 0.05 | |
| NaOH 4% (w/v) | 0.05 | |
| Control membrane | Distilled water | 0.03 |
| Citric acid 2% (w/v) | 0.04 | |
| NaOH 4% (w/v) | 0.09 | |
| 80-SGwSi-D membrane | Distilled water | <0.005 |
| Citric acid 2% (w/v) | 0.05 | |
| NaOH 4% (w/v) | 0.05 | |
| 650-SGwSi-D membrane | Distilled water | <0.005 |
| Citric acid 2% (w/v) | 0.05 | |
| NaOH 4% (w/v) | 0.12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huertas, R.M.; Fraga, M.C.; Crespo, J.G.; Pereira, V.J. Solvent-Free Process for the Development of Photocatalytic Membranes. Molecules 2019, 24, 4481. https://doi.org/10.3390/molecules24244481
Huertas RM, Fraga MC, Crespo JG, Pereira VJ. Solvent-Free Process for the Development of Photocatalytic Membranes. Molecules. 2019; 24(24):4481. https://doi.org/10.3390/molecules24244481
Chicago/Turabian StyleHuertas, Rosa M., Maria C. Fraga, João G. Crespo, and Vanessa J. Pereira. 2019. "Solvent-Free Process for the Development of Photocatalytic Membranes" Molecules 24, no. 24: 4481. https://doi.org/10.3390/molecules24244481




