Chemical Composition of Essential Oil from Flower Heads of Arnica Chamissonis Less. under a Nitrogen Impact
Abstract
:1. Introduction
2. Results
2.1. Content and Yield of Essential Oils
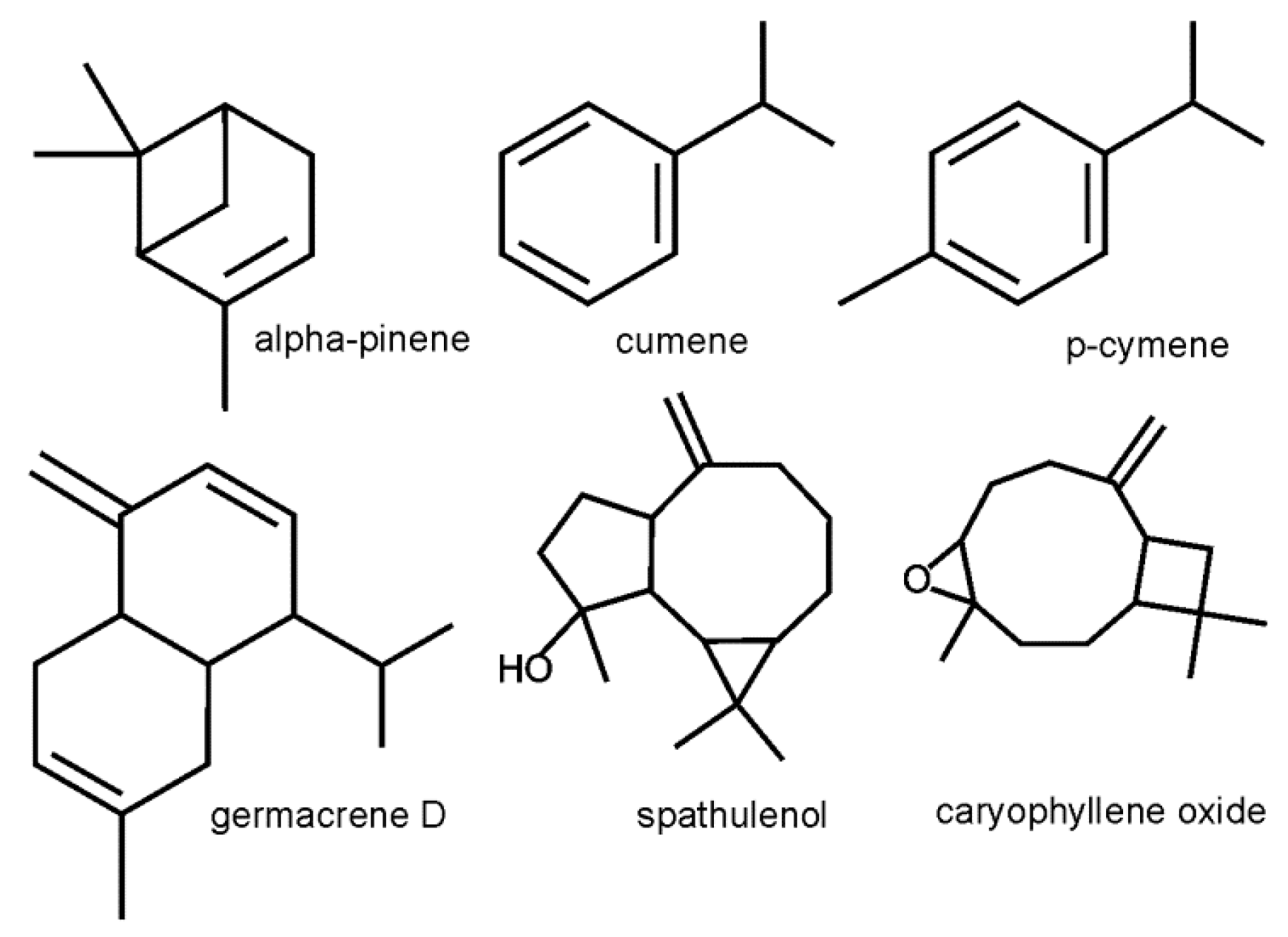
2.2. Chemical Composition and Diversity of Volatile Oils
2.3. Differentiation of the EO Content
2.4. Yield and Diversity of Main EO Components
3. Discussion
3.1. Raw Material and the Concentration and Yield of Essential Oils
3.2. Chemical Composition and Diversity of Volatile Oils
3.3. Yield of Main Components of Volatile Oils
3.4. Role and Value of the Main Components of Essential Oils from A. Chamissonis Flower Heads
4. Materials and Methods
4.1. Plant Material and Soil Conditions
4.2. Raw Material Collection
4.3. Qualitative and Quantitative Analysis of Essential Oil
4.3.1. Assay of the Essential Oil Content
4.3.2. GC-MS Analysis
4.3.3. Qualitative and Quantitative Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rehman, R.; Hanif, M.A.; Mushtaq, Z.; Al-Sadi, A.M. Biosynthesis of essential oils in aromatic plants: A review. Food Rev. Int. 2016, 32, 117–160. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Özek, T.; Krimer, N.; Tümen, G. A comparative study of essential oils of wild and cultivated Satureja hortensis L. J. Essent. Oil Res. 2004, 16, 422–424. [Google Scholar] [CrossRef]
- Omidbaigi, R.; Hejazi, M. Essential oil content and compositions of Satureja hortensis of two different origins. J. Essent. Oil Bear. Plants 2013, 7, 175–178. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Sweet basil essential oil composition: Relationship between cultivar, foliar feeding with nitrogen and oil content. J. Essent. Oil Res. 2012, 24, 217–227. [Google Scholar] [CrossRef]
- Andrzejewska, J.; Woropaj-Janczak, M. German chamomile performance after stubble catch crops and response to nitrogen fertilization. Ind. Crops Prod. 2014, 62, 350–358. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Hussain Sherazi, S.T.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum L.) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Castelo, A.V.M.; Del Menezzi, C.H.S.; Resck, I.S. Seasonal variation in the yield and the chemical composition of essential oils from two Brazilian natives arbustive species. J. Appl. Sci. 2012, 12, 753–760. [Google Scholar] [CrossRef]
- Karamanos, A.J.; Sotiropoulou, D.E.K. Field studies of nitrogen application on Greek oregano (Origanum vulgare ssp. hirtum (Link) Ietswaart) essential oil during two cultivation seasons. Ind. Crops Prod. 2013, 46, 246–252. [Google Scholar]
- Aboukhalid, K.; Al Faiz, C.; Douaik, A.; Bakha, M.; Kursa, K.; Agacka-Mołdoch, M.; Machon, N.; Tomi, F.; Lamiri, A. Influence of environmental factors on essential oil variability in Origanum compactum Benth. Growing wild in Morocco. Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef]
- Zutic, I.; Borosic, J.; Benko, B.; Novak, B.; Dragovic-Uzelac, V. Arnica chamissonis growing in Croatia as affected by climate and pricking. Cereal Res. Comm. 2008, 36, 287–290. [Google Scholar]
- Kowalski, R.; Sugier, D.; Sugier, P.; Kołodziej, B. Evaluation of the chemical composition of essential oils with respect to the maturity of flower heads of Arnica montana L. and Arnica chamissonis Less. cultivated for industry. Ind. Crops Prod. 2015, 76, 857–865. [Google Scholar] [CrossRef]
- Skubij, N.; Dzida, K. Essential oil composition of summer savory (Satureja hortensis L.) cv. Saturn depending on nitrogen nutrition and plant development phases in raw material cultivated for industrial use. Ind. Crops Prod. 2019, 135, 260–270. [Google Scholar] [CrossRef]
- Sahzabi, A.A.; Ashoorabadi, E.S.; Shiranirad, A.H.; Abbaszadeh, B.; Farahani, H.A. The methods of nitrogen application influence on essential oil yield and water use efficiency of summer savory (Satureja hortensis L.). J. Hortic. For. 2010, 2, 52–56. [Google Scholar]
- Ghamarnia, H.; Bashipour, M.; Ghobadi, M.E. Evaluation at different level of irrigation on the seed yield and water use efficiency of coriander medicinal plant in a semi-arid climate. Water Irrig. Manag. 2012, 2, 15–24. [Google Scholar]
- Abbaszadeh, B.; Farahani, H.A.; Valadabadi, S.A.; Darvishi, H.H. Nitrogenous fertilizer influence on quantity and quality values of balm (Melissa officinalis L.). J. Agric. Ext. Rural Dev. 2009, 1, 31–33. [Google Scholar]
- Nurzyńska-Wierdak, R. Does mineral fertilization modify essential oil content and chemical composition in medicinal plants? Acta Sci. Pol. Hortorum Cultus 2013, 12, 3–16. [Google Scholar]
- Sugier, D.; Sugier, P.; Kowalski, R.; Kołodziej, B.; Olesińska, K. Foliar boron fertilization as factor affecting the essential oil content and yield of oil components from flower heads of Arnica montana L. and Arnica chamissonis Less. cultivated for industry. Ind. Crops Prod. 2017, 109, 587–597. [Google Scholar] [CrossRef]
- Baranauskiene, R.; Venskutonis, P.R.; Viskelis, P.; Dambrauskiene, E. Influence of nitrogen fertilizers on the yield and composition of thyme (Thymus vulgaris). J. Agric. Food Chem. 2003, 51, 7751–7758. [Google Scholar] [CrossRef]
- Bufalo, J.; Cantrell, C.L.; Astatkie, T.; Zheljazkov, V.D.; Gawde, A.; Boaro, C.S.F. Organic versus conventional fertilization effects on sweet basil (Ocimum basilicum L.) growth in a greenhouse system. Ind. Crops Prod. 2015, 74, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Nigussie, A.; Chala, M.; Lulie, B.; Adugna, G. Response of spearmint (Mentha spicata L.) to nitrogen and phosphorus fertilizers at Koka, Ethiopia. Acad. Res. J. Agri. Sci. Res. 2017, 5, 414–418. [Google Scholar]
- Niakan, M.R.; Khavarinajad, M.B.; Rezai, M. Effect of N, P and K on fresh and dry weight, leaf area and essential oil Menta piperita L. J. Med. Aromat. Plant Res. Iran 2004, 2, 131–148. [Google Scholar]
- El-Leithy, A.S.; El-Hanafy, S.H.; Khattab, M.E.; Ahmed, S.S.; El-Sayed, A.A.A. Effect of nitrogen fertilization rates, plant spacing and their interaction on essential oil percentage and total flavonoid content of summer savory (Satureja hortensis L.) plant. Egypt. J. Chem. 2017, 60, 805–816. [Google Scholar]
- Sifola, M.I.; Barbieri, G. Growth, yield and essentials oil content of three cultivars of basil grown under different levels of nitrogen in the field. Sci. Hort. 2006, 108, 408–413. [Google Scholar] [CrossRef]
- Omer, E.A. Response of wild Egyptian oregano to nitrogen fertilization in a sandy soil. J. Plant. Nutr. 1999, 22, 103–114. [Google Scholar] [CrossRef]
- Dadkhah, A.; Dahaghi, M.A.; Rasam, G. Effects of different level of nitrogen and phosphorous fertilizers on yield quantity and quality of Matricaria recutita. Planta Med. 2011, 77. [Google Scholar] [CrossRef]
- Maguire, B. A monograph of the genus arnica (Senecioneae, Compositae). Brittonia 1943, 4, 386–510. [Google Scholar]
- Merfort, I.; Wendisch, D. Flavonoid glycosides from Arnica montana and Arnica chamissonis. Planta Med. 1987, 5, 434–437. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Sugier, D.; Cichocka, J. Seeds of Arnica montana and Arnica chamissonis as a potential source of natural antioxidants. Herba Pol. 2009, 55, 60–71. [Google Scholar]
- Roki, D.; Menković, N.; Šavikin-Fodulović, K.; Krivokuća-Dokić, D.; Ristić, M.; Grubišić, D. Flavonoids and essential oil in flower heads of introduced Arnica chamissonis. J. Herbs Spices Med. Plants 2001, 8, 19–27. [Google Scholar]
- Cassell, A.C.; Walsh, C.; Belin, M.; Cambornac, M.; Rohit, J.R.; Lubrano, C. Establishment of plantation from micropropagated Arnica chamissonsis a pharmaceutical substitute for the endangered A. montana. Plant Cell Tiss. Org. Cult. 1999, 156, 139–144. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Świeca, M.; Sugier, D.; Cichocka. Comparison of in vitro lipoxygenase, xanthine oxidase inhibitory and antioxidant activity of Arnica montana and Arnica chamissonis tinctures. Acta Sci. Pol. Hortorum Cultus 2011, 10, 15–27. [Google Scholar]
- Willuhn, G.; Kresken, J.; Wendisch, D. Sesquiterpenelactone aus Arnica chamissonis III: 4-0-acetyl-6-desoxychamissonolid und 6-0-propionyl-11,13-dihydrohelenalin. Planta Med. 1983, 47, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Nichterlein, K. Arnica montana (mountain arnica): In vitro culture and theproduction of sesquiterpene lactones and other metabolites. Biotechnol. Agric. For. 1995, 33, 47–61. [Google Scholar]
- Ganzera, M.; Egger, C.; Zidorn, C.; Stuppner, H. Quantitative analysis of flavonoids and phenolic acids in Arnica montana L. by micellar electrokinetic capillary chromatography. Anal. Chim. Acta 2008, 614, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Merfort, I. Arnika—Aktueller Stand hinsichtlich Wirksamkeit, Pharmakokinetik und Nebenwirkungen. Z. Phytother. 2010, 31, 188–192. [Google Scholar] [CrossRef]
- Nowak, T. Lowland arnica—Culivation in gostyńskoleszczyński region. Wiadomości Zielar. 2002, 1, 18–19. [Google Scholar]
- Falniowski, A.; Bazos, I.; Hodálová, I.; Lansdown, R.; Petrova, A. Arnica montana. In The IUCN Red List of Threatened Species; IUCN: Cambridge, UK, 2011. [Google Scholar] [CrossRef]
- Sugier, P.; Kołos, A.; Wołkowycki, D.; Sugier, D.; Plak, A.; Sozinov, O. Evaluation of species inter-relations and soil conditions in Arnica montana L. habitats: A step towards active protection of endangered and high-valued medicinal plant species in NE Poland. Acta Soc. Bot. Pol. 2018, 87, 1–16. [Google Scholar] [CrossRef]
- Ristić, M.; Krivokuća-Dokić, D.; Radanović, D.; Nastovska, T. Essential oil of Arnica montana and Arnica chamissonis. Hem. Ind. 2007, 61, 272–277. [Google Scholar] [CrossRef] [Green Version]
- Dorman, H.J.D.; Figueiredo, A.C.; Barroso, J.G.; Deans, S.G. In vitro evaluation of antioxidant activity of essential oils and their components. Flavour Frag. J. 2000, 15, 12–16. [Google Scholar] [CrossRef]
- Aydin, E.A.; Türkez, H.B.; Geyikoğlu, F.A. Antioxidative, anticancer and genotoxic properties of α-pinene on N2a neuroblastoma cells. Biologia 2013, 68, 1004–1009. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendez, A.F. Antiinflammatory and chondroprotective activity of (+)-α-pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Amaral, R.G.; Baldissera, M.D.; Grando, T.H.; Couto, J.C.M.; Posser, C.P.; Ramos, A.P.; Sagrillo, M.R.; Vaucher, R.A.; Da Silva, A.S.; Becker, A.P.; et al. Combination of the essential oil constituents α-pinene and β-caryophyllene as a potentiator of trypanocidal action on Trypanosoma evansi. J. Appl. Biomed. 2016, 14, 265–272. [Google Scholar] [CrossRef]
- Kang, E.; Lee, D.H.; Jung, Y.J.; Shin, S.Y.; Koh, D.; Lee, Y.H. α-Pinene inhibits tumor invasion through downregulation of nuclear factor (NF)-jB-regulated matrix metalloproteinase-9 gene expression in MDA-MB-231 human breast cancer cells. Appl. Biol. Chem. 2016, 59, 511–516. [Google Scholar] [CrossRef]
- Judžentienė, A.; Būdienė, J. Analysis of the chemical composition of flower essential oils from Arnica montana of Lithuanian origin. Chemija 2009, 20, 190–194. [Google Scholar]
- Magharri, E.; Razavi, S.M.; Ghorbani, E.; Nahar, L.; Sarker, S.D. Chemical composition, some allelopathic aspects, free-radical scavenging property and antifungal activity of the volatile oil of the flowering tops of Leucanthemum vulgare Lam. Rec. Nat. Prod. 2015, 9, 538–545. [Google Scholar]
- Czaikoski, K.; Mesomo, M.C.; de Paula Scheer, A.; Dalla Santa, O.R.; Queiroga, C.L.; Corazza, M.L. Kinetics, composition and biological activity of Eupatorium intermedium flower extracts obtained from scCO2 and compressed propane. J. Supercrit. Fluids 2015, 97, 145–153. [Google Scholar] [CrossRef]
- Chavan, M.; Wakte, P.; Shinde, D. Analgesic and anti-inflammatory activity of caryophyllene oxide from Annona squamosa L. bark. Phytomedicine 2010, 17, 149–151. [Google Scholar] [CrossRef]
- Pastor, J.; García, M.; Steinbauer, S.; Setzer, W.N.; Scull, R.; Gille, L.; Monzote, L. Combinations of ascaridole, carvacrol, and caryophyllene oxide against Leishmania. Acta Trop. 2015, 145, 31–38. [Google Scholar] [CrossRef]
- Tabanca, N.; Demirci, B.; Ali, A.; Ali, Z.; Blythe, E.K.; Khan, I.A. Essential oils of green and red Perilla frutescens as potential sources of compounds for mosquito management. Ind. Crops Prod. 2015, 65, 36–44. [Google Scholar] [CrossRef]
- Abd El-Gawad, A.M. Chemical constituents, antioxidant and potential allelopathic effect of the essential oil from the aerial parts of Cullen plicata. Ind. Crops Prod. 2016, 80, 36–41. [Google Scholar] [CrossRef]
- Montanari, R.M.; Barbosa, L.C.A.; Demuner, A.J.; Silva, C.J.; Carvalho, L.S.; Andrade, N.J. Chemical composition and antibacterial activity of essential oils from Verbenaceae species: Alternative sources of (E)-caryophyllene and germacrene-D. Química Nova 2011, 34, 1550–1555. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas, J.; Rojas, J.; Rojas-Fermin, L.; Lucena, M.; Buitrago, A. Essential oil composition and antibacterial activity of Monticalia greenmaniana (Asteraceae). Nat. Prod. Commun. 2012, 7, 243–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passos, J.L.; Barbosa, L.C.; Demuner, A.J.; Alvarenga, E.S.; Silva, C.M.; Barreto, R.W. Chemical characterization of volatile compounds of Lantana camara L. and L. radula Sw. and their antifungal activity. Molecules 2012, 17, 11447–11455. [Google Scholar] [CrossRef]
- Da Silva, E.B.P.; Matsuo, A.L.; Figueiredo, C.R.; Chaves, M.H.; Sartorelli, P.; Lago, J.H.G. Chemical constituents and cytotoxic evaluation of essential oils from leaves of Porcelia macrocarpa (Annonaceae). Nat. Prod. Commun. 2013, 8, 277–279. [Google Scholar] [CrossRef] [Green Version]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils’ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Pljevljakušić, D.; Rančić, D.; Ristić, M.; Vujisić, L.; Radanović, D.; Dajić-Stevanović, Z. Rhizome and root yield of the cultivated Arnica montana L.: Chemical composition and histochemical localization of essential oil. Ind. Crops Prod. 2012, 39, 177–189. [Google Scholar] [CrossRef]
- Sugier, D.; Kołodziej, B.; Bielińska, E. The effect of leonardite application on Arnica montana L. yielding and chosen chemical properties and enzymatic activity of the soil. J. Geochem. Explor. 2013, 129, 76–81. [Google Scholar] [CrossRef]
- Sugier, D.; Sugier, P.; Gawlik-Dziki, U. Propagation and introduction of Arnica montana L. into cultivation: A step to reduce the pressure on endangered and high valued medicinal plant species. Sci. World J. 2013, 2013, 414363. [Google Scholar] [CrossRef]
- Pljevljakušić, D.; Janković, T.; Jelačić, S.; Novakovič, M.; Menkovič, N.; Beatovič, D.; Dajić-Stevanović, Z. Morphological and chemical characterization of Arnica montana L. under different cultivation models. Ind. Crops Prod. 2014, 52, 233–244. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, D.J. A generalization of the retention index system including liner temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–467. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Compounds by Gas Chromatography/Quadrupole Mass Spectroscopy; Allured: Carol Stream, IL, USA, 2001. [Google Scholar]
- Balabanova, V.; Vitkova, A. Flower yield of Arnica sp. cultivated in two floristic regions in Bulgaria. J. Agric. Ecol. Res. Int. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Radanović, D.; Marković, T.; Antić-Mladenović, S.; Pljevljakušić, D.; Ristić, M.; KrivokućaĐokić, D. Yield and quality of Arnica (Arnica montana and Arnica chamissonis var. foliosa) cultivated in Serbia. In Proceedings of the 1st International Scientific Conference on Medicinal, Aromatic and Spice Plants, Nitra, Slovakia, 5–6 December 2007; pp. 157–161. [Google Scholar]
- Nenaah, G.E. Chemical composition, toxicity and growth inhibitory activities of essential oils of three Achillea species and their nano-emulsions against Tribolium castaneum (Herbst). Ind. Crops Prod. 2014, 53, 252–260. [Google Scholar] [CrossRef]
- Carrillo-Hormaza, L.; Mora, C.; Alvarez, R.; Alzate, F.; Osorio, E. Chemical composition and antibacterial activity against Enterobacter cloacae of essential oils from Asteraceae species growing in the Páramos of Colombia. Ind. Crops Prod. 2015, 77, 108–115. [Google Scholar] [CrossRef]
- Vidic, D.; Zeljković, S.Ć.; Dizdar, M.; Maksimović, M. Essential oil composition and antioxidant activity of four Asteraceae species from Bosnia. J. Essent. Oil Res. 2016, 28, 445–457. [Google Scholar] [CrossRef]
- Alvarez-Castellanos, P.P.; Pascual-Villalobos, M.J. Effect of fertilizer on yield and composition of flowerhead essential oil of Chrysanthemum coronarium (Asteraceae) cultivated in Spain. Ind. Crops Prod. 2003, 17, 77–81. [Google Scholar] [CrossRef]
- Mirzajani, Z.; Hadavi, E.; Kashi, A. Changes in the essential oil content and selected traits of sweet basil (Ocimum basilicum L.) as induced by foliar sprays of citric acid and salicylic acid. Ind. Crops Prod. 2015, 76, 269–274. [Google Scholar] [CrossRef]
- Goudarzi, T.; Saharkhiz, M.J.; Rowshan, V.; Taban, A. Changes in essential oil content and composition of Tansy (Tanacetum vulgare L.) under foliar application of salicylic and orthophosphoric acids. J. Essent. Oil Res. 2016, 28, 64–70. [Google Scholar] [CrossRef]
- Dordas, C. Foliar application of calcium and magnesium improves growth, yield, and essential oil yield of oregano (Origanum vulgare ssp. hirtum). Ind. Crops Prod. 2009, 29, 599–608. [Google Scholar] [CrossRef]
- Sotiropoulou, D.E.; Karamanos, A.J. Field studies of nitrogen application on growth and yield of Greek oregano (Origanum vulgare ssp. hirtum (Link) Ietswaart). Ind. Crops Prod. 2010, 32, 450–457. [Google Scholar] [CrossRef]
- Heidari, S.; Azizi, M.; Soltani, F.; Hadian, J. Foliar application of Ca(NO3)2 and KNO3 affects growth essential oil content, and oil composition of French tarragon. Ind. Crops Prod. 2014, 62, 526–532. [Google Scholar] [CrossRef]
- Umpiérrez, M.L.; Santos, E.; Mendoza, Y.; Altesor, P.; Rossini, C. Essential oil from Eupatorium buniifolium leaves as potential varroacide. Parasitol. Res. 2013, 112, 3389–3400. [Google Scholar] [CrossRef] [PubMed]
- Bailen, M.; Julio, L.F.; Diaz, C.E.; Sanz, J.; Martínez-Díaz, R.A.; Cabrera, R.; Burillo, J.; Gonzalez-Coloma, A. Chemical composition and biological effects of essential oils from Artemisia absinthium L. cultivated under different environmental conditions. Ind. Crops Prod. 2013, 49, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Boskovic, Z.; Radulovic, N.; Stojanovic, G. Essential oil composition of four Achillea species from the Balkans and its chemotaxonomic significance. Chem. Nat. Compd. 2005, 41, 674–678. [Google Scholar] [CrossRef]
- Giuliani, C.; Lazzaro, L.; Calamassi, R.; Calamai, L.; Romoli, R.; Fico, G.; Foggi, B.; Lippi, M.M. A volatolomic approach for studying plant variability: The case of selected Helichrysum species (Asteraceae). Phytochemistry 2016, 130, 128–143. [Google Scholar] [CrossRef]
- Zhigzhitzhapova, S.V.; Radnaeva, L.D.; Gao, Q.; Chen, S.; Zhang, F. Chemical composition of volatile organic compounds of Artemisia vulgaris L. (Asteraceae) from the Qinghai-Tibet Plateau. Ind. Crops Prod. 2016, 83, 462–469. [Google Scholar] [CrossRef]
- Azadi, B.; Nouri, E. The essential oil composition of Centaurea intricata Boiss. flowering aerial parts. Asian J. Biomed. Pharmaceut. Sci. 2014, 4, 25–27. [Google Scholar] [CrossRef]
- De Almeida, L.F.R.; Portella, R.d.O.; Bufalo, J.; Marques, M.O.M.; Facanali, R.; Frei, F. Non-oxygenated sesquiterpenes in the essential oil of Copaifera langsdorffii Desf. increase during the day in the dry season. PLoS ONE 2016, 11, e0149332. [Google Scholar] [CrossRef]
- Smeriglio, A.; Trombetta, D.; Cornara, L.; Valussi, M.; De Feo, V.; Caputo, L. Characterization and phytotoxicity assessment of essential oils from plant byproducts. Molecules 2019, 24, 2941. [Google Scholar] [CrossRef] [Green Version]
- Chahed, T.; Dhifi, W.; Hosni, K.; Msaada, K.; Kchouk, M.E.; Marzouk, B. Composition of Tunisian pistachio hull essential oil during fruit formation and ripening. J. Essent. Oil Res. 2008, 20, 122–125. [Google Scholar] [CrossRef]
- Malti, C.E.W.; Baccati, C.; Mariani, M.; Hassani, F.; Babali, B.; Atik-Bekkara, F.; Paoli, M.; Maury, J.; Tomi, F.; Bekhechi, C. Biological activities and chemical composition of Santolina africana Jord. et Fourr. aerial part essential oil from Algeria: Occurrence of polyacetylene derivatives. Molecules 2019, 24, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usta, A.; Üçüncü, O.; Cansu, T.B.; Terzioglu, S.; Yayli, N. Chemical composition of the essential oils from flower of Senecio vernalis, and Senecio platyphyllus var. platyphyllus. Asian J. Chem. 2009, 21, 6369–6374. [Google Scholar]
- Mahboubi, M.; Feizabadi, M.M. Antimicrobial activity of Ducrosia anethifolia essential oil and main component, decanal against methicillin-resistant and methicillin susceptible Staphylococcus aureus. J. Essent. Oil Bear. Plants 2009, 12, 574–579. [Google Scholar] [CrossRef]
- Him, A.; Ozbek, H.; Turel, I.; Oner, A.C. Antinociceptive activity of alpha-pinene and fenchone. Pharmacologyonline 2008, 3, 363–369. [Google Scholar]
- Matsuo, A.L.; Figueiredo, C.R.; Arruda, D.C.; Pereira, F.V.; Scutti, J.A.; Massaoka, M.H.; Travassos, L.R.; Sartorelli, P.; Lago, J.H. α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem. Biophys. Res. Commun. 2011, 411, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Chen, Q.; Liu, Y.; Zhou, X.; Wang, X. Isolation and biological activities of decanal, linalool, valencene, and octanal from sweet orange oil. J. Food Sci. 2012, 77, 1156–1161. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals, 2nd ed.; Churchill Livingstone: London, UK, 2014. [Google Scholar]
- Tan, L.T.H.; Lee, L.H.; Yin, W.F.; Chan, C.K.; Kadir, H.A.; Chan, K.G. Traditional Uses, Phytochemistry, and Bioactivities of Cananga Odorata (Ylang-Ylang). Evid. Based Complement. Altern. Med. 2015, 2015, 896314. [Google Scholar] [CrossRef] [Green Version]
- Polish Pharmacopoeia VI; The Republic of Poland, The Minister of Health: Warsaw, Poland, 2002.
- NIST/EPA/NIH. Mass Spectral Library with Search Program: Data Version: NIST08, Software Version 2.0f; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2005.
- Joulain, D.; König, W.A. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; E.B. Verlag: Hamburg, Germany, 1998. [Google Scholar]
- Kowalski, R.; Wawrzykowski, J. Effect of ultrasound-assisted maceration on the quality of oil from the leaves of thyme Thymus vulgaris L. Flavour Fragr. J. 2009, 24, 69–74. [Google Scholar] [CrossRef]
- Kovach, W. MVSP—A Multivariate Statistical Package for Windows. Ver. 3.1; Kovach Computing Services: Wales, UK, 1999. [Google Scholar]
Sample Availability: Samples of the essential oils from the flower heads of A. chamissonis are available from the authors. |



| Nitrogen Rate (kg ha−1) | 0 | 30 | 60 | 90 | 120 |
|---|---|---|---|---|---|
| Crop yield (kg ha−1) | |||||
| L soil | 1210.0b ± 38.3 | 1363.4cd ± 26.2 | 1582.2e ± 34.7 | 1837.2f ± 26.6 | 1941.2g ± 22.0 |
| S soil | 994.6a ± 41.7 | 1177.1b ± 31.1 | 1297.1c ± 42.3 | 1430.0d ± 30.7 | 1563.1e ± 42.4 |
| Two-way ANOVA results: soil (F = 793.19, p < 0.05), N (F = 508.24, p < 0.05), soil × N (F = 17.27, p < 0.05) | |||||
| EO content [%(v/w)] | |||||
| L soil | 0.151c ± 0.001 | 0.157c ± 0.001 | 0.169f ± 0.001 | 0.178g ± 0.001 | 0.180g ± 0.001 |
| S soil | 0.137a ± 0.001 | 0.146b ± 0.001 | 0.156d ± 0.001 | 0.161e ± 0.001 | 0.162e ± 0.001 |
| Two-way ANOVA results: soil (F = 1899.1, p < 0.05), N (F = 927.3, p < 0.05), soil × N (F = 10.8, p < 0.05) | |||||
| EO yield (g ha−1) | |||||
| L soil | 1829.6b ± 58.0 | 2144.7c ± 40.8 | 267868f ± 58.7 | 3264.7g ± 47.4 | 3488.4h ± 41.5 |
| S soil | 1354.9a ± 46.8 | 1713.0b ± 45.2 | 2022.1c ± 65.9 | 2301.5d ± 49.4 | 2529.1e ± 58.5 |
| Two-way ANOVA results: soil (F = 1820.1, p < 0.05), N (F = 1027.5, p < 0.05), soil × N (F = 48.9, p < 0.05) | |||||
| Compounds | RI | RILit | L0 | L30 | L60 | L90 | L120 | S0 | S30 | S60 | S90 | S120 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cumene | 928 | 931 | 11.28 | 8.61 | 11.71 | 9.15 | 10.52 | 10.86 | 6.28 | 9.52 | 10.15 | 9.99 |
| alpha-pinene | 940 | 939 | 26.49 | 24.44 | 24.88 | 27.29 | 26.01 | 14.21 | 18.96 | 21.68 | 21.21 | 21.42 |
| camphene | 955 | 954 | 0.13 | 0.12 | 0.13 | 0.14 | 0.13 | 0.02 | 0.09 | 0.13 | 0.12 | 0.11 |
| thuja-2.4(10)-diene | 961 | 960 | 0.70 | 0.50 | 0.70 | 0.81 | 0.62 | 0.26 | 0.37 | 0.51 | 0.48 | 0.52 |
| benzaldehyde | 963 | 960 | 0.49 | 0.34 | 0.45 | 0.57 | 0.46 | 0.66 | 0.42 | 0.49 | 0.54 | 0.4 |
| sabinene | 978 | 975 | 0.20 | 0.17 | 0.16 | 0.18 | 0.18 | 0.16 | 0.19 | 0.19 | 0.16 | |
| beta-pinene | 983 | 979 | 3.62 | 3.45 | 3.55 | 3.14 | 3.51 | 2.22 | 2.79 | 3.32 | 3.11 | 3.09 |
| 6-methyl-5-hepten-2-one | 986 | 986 | 0.13 | 0.11 | 0.12 | 0.17 | 0.14 | 0.12 | 0.11 | 0.14 | 0.12 | |
| myrcene | 996 | 991 | 1.62 | 1.62 | 1.68 | 1.36 | 1.76 | 1.44 | 1.62 | 1.81 | 1.77 | 1.60 |
| mesitylene | 999 | 996 | 0.10 | 0.08 | 0.11 | 0.12 | 0.09 | 0.06 | 0.08 | 0.09 | 0.09 | |
| n-octanal | 1002 | 999 | 0.23 | 0.19 | 0.24 | 0.31 | 0.21 | 0.21 | 0.28 | 0.24 | 0.24 | |
| alpha-phellandrene | 1012 | 1003 | 0.78 | 0.63 | 0.64 | 0.58 | 0.36 | 0.75 | 0.54 | 0.35 | 0.44 | 0.44 |
| alpha-terpinene | 1016 | 1017 | 0.13 | 0.13 | 0.15 | 0.12 | 0.12 | 0.09 | 0.11 | 0.11 | 0.13 | |
| beta-phellandrene (β-phellandrene) | 1028 | 1030 | 0.33 | 0.31 | 0.38 | 0.31 | 0.29 | 0.29 | 0.25 | 0.28 | 0.32 | |
| p-cymene | 1029 | 1025 | 7.40 | 5.99 | 5.47 | 5.69 | 7.85 | 5.15 | 6.40 | 9.06 | 7.13 | 5.94 |
| limonene | 1032 | 1029 | 0.70 | 0.67 | 0.61 | 0.53 | 0.77 | 0.53 | 0.64 | 0.83 | 0.75 | 0.61 |
| benzene acetaldehyde | 1041 | 1042 | 3.43 | 2.22 | 2.90 | 3.59 | 4.23 | 0.34 | 2.17 | 2.79 | 2.82 | 2.49 |
| gamma-terpinene | 1053 | 1060 | 0.07 | 0.11 | 0.10 | 0.06 | 0.06 | 0.02 | 0.06 | 0.06 | 0.08 | 0.06 |
| alpha-methyl-benzene methanol | 1056 | 1063 | 0.25 | 0.07 | 0.23 | 0.34 | 0.02 | 0.68 | 0.12 | 0.02 | 0.12 | 0.14 |
| otrho-tolualdehyde | 1062 | 1068 | 0.45 | 0.20 | 0.26 | 0.60 | 0.33 | 0.64 | 0.20 | 0.40 | 0.35 | 0.24 |
| para-tolualdehyde | 1064 | 1069 | 0.57 | 0.31 | 0.41 | 0.67 | 0.59 | 0.82 | 0.47 | 0.65 | 0.59 | 0.36 |
| para-tolualdehyde | 1077 | 1082 | 0.42 | 0.28 | 0.33 | 0.51 | 0.39 | 0.26 | 0.36 | 0.27 | 0.30 | |
| 6-camphenone | 1082 | 1097 | 0.20 | 0.18 | 0.21 | 0.24 | 0.17 | |||||
| linalool | 1106 | 1097 | 0.28 | 0.23 | 0.29 | 0.28 | 0.26 | 0.28 | 0.26 | 0.25 | 0.22 | |
| nonanal | 1110 | 1101 | 0.98 | 0.98 | 1.05 | 1.03 | 0.89 | 1.19 | 1.05 | 1.23 | 1.08 | 1.08 |
| cis-para-menth-2-en-1-ol | 1130 | 1122 | 0.27 | 0.18 | 0.18 | 0.26 | 0.20 | 0.20 | 0.21 | 0.20 | 0.17 | |
| alpha-campholenal | 1135 | 1126 | 0.84 | 0.75 | 0.76 | 1.00 | 0.93 | 0.80 | 0.89 | 1.04 | 0.93 | 0.69 |
| trans-pinocarveol | 1147 | 1139 | 0.55 | 0.47 | 0.50 | 0.69 | 0.61 | 0.49 | 0.49 | 0.62 | 0.52 | 0.43 |
| cis-verbenol | 1150 | 1141 | 0.77 | 0.43 | 0.65 | 0.79 | 0.62 | 0.51 | 0.48 | 0.63 | 0.52 | 0.53 |
| trans-verbenol | 1154 | 1145 | 1.64 | 1.21 | 1.29 | 0.95 | 1.60 | 1.32 | 1.29 | 2.16 | 1.69 | 1.26 |
| pinocarvone | 1160 | 1165 | 0.53 | 0.43 | 0.53 | 0.67 | 0.53 | 0.59 | 0.46 | 0.52 | 0.58 | 0.55 |
| para-mentha-1.5-dien-8-ol | 1171 | 1170 | 0.42 | 0.29 | 0.40 | 0.39 | 0.40 | 0.34 | 0.36 | 0.39 | 0.33 | 0.24 |
| terpinen-4-ol | 1188 | 1177 | 0.20 | 0.13 | 0.17 | 0.18 | 0.15 | 0.18 | 0.14 | 0.15 | 0.16 | 0.11 |
| naphthalene | 1185 | 1181 | 0.13 | 0.13 | 0.16 | 0.17 | 0.12 | 0.13 | 0.12 | 0.13 | 0.15 | 0.12 |
| myrtenal | 1207 | 1196 | 0.79 | 0.69 | 0.72 | 0.80 | 0.87 | 0.85 | 0.73 | 0.86 | 0.69 | 0.63 |
| safranal | 1202 | 1197 | 0.28 | 0.24 | 0.29 | 0.35 | 0.29 | 0.45 | 0.26 | 0.30 | 0.32 | 0.26 |
| decanal | 1208 | 1202 | 4.48 | 4.52 | 4.79 | 4.37 | 3.76 | 6.20 | 4.74 | 4.94 | 4.48 | 4.45 |
| trans-carveol | 1223 | 1217 | 0.29 | 0.26 | 0.28 | 0.33 | 0.36 | 0.25 | 0.30 | 0.39 | 0.36 | 0.27 |
| thymol methyl ether | 1234 | 1235 | 0.13 | 0.14 | 0.17 | 0.16 | 0.28 | 0.00 | 0.18 | 0.19 | 0.20 | 0.16 |
| carvacrol methyl ether | 1249 | 1245 | 1.44 | 1.34 | 1.52 | 1.25 | 1.47 | 1.02 | 1.31 | 1.40 | 1.28 | 1.39 |
| bornyl acetate | 1288 | 1289 | 0.24 | 0.25 | 0.24 | 0.25 | 0.28 | 0.25 | 0.28 | 0.25 | 0.26 | |
| thymol | 1304 | 1290 | 0.22 | 0.25 | 0.30 | 0.07 | 0.07 | 0.21 | ||||
| carvacrol | 1313 | 1299 | 0.20 | 0.17 | 0.23 | 0.25 | 0.25 | 0.22 | ||||
| myrtenyl acetate | 1327 | 1327 | 0.14 | 0.27 | ||||||||
| 2E.4E-decadienal | 1327 | 1317 | 0.2 | 0.31 | 0.20 | |||||||
| 7-epi-silphiperfol-5-ene | 1349 | 1348 | 0.12 | 0.13 | 0.08 | 0.14 | 0.10 | |||||
| eugenol | 1383 | 1359 | 0.18 | 0.10 | 0.32 | 0.22 | 0.29 | 0.06 | 0.09 | 0.09 | 0.18 | |
| beta-maaliene | 1388 | 1382 | 0.35 | 0.59 | 0.41 | 0.39 | 0.41 | 0.98 | 0.69 | 0.52 | 0.56 | 0.51 |
| alpha-isocomene | 1394 | 1388 | 0.26 | 0.39 | 0.30 | 0.28 | 0.25 | 0.82 | 0.44 | 0.29 | 0.36 | 0.38 |
| cyperene | 1405 | 1399 | 0.13 | 0.19 | 0.15 | 0.14 | 0.09 | 0.48 | 0.19 | 0.15 | 0.16 | 0.18 |
| E-caryophyllene | 1417 | 1419 | 0.43 | 0.68 | 0.55 | 0.22 | 0.37 | 0.85 | 0.68 | 0.71 | 0.64 | |
| beta-duprezianene< > | 1424 | 1423 | 0.61 | 0.87 | 0.74 | 0.63 | 0.45 | 1.58 | 0.81 | 0.52 | 0.68 | 0.85 |
| (E)-alpha-ionone | 1428 | 1430 | 0.16 | 0.14 | 0.13 | 0.19 | 0.21 | 0.19 | ||||
| beta-copaene | 1435 | 1432 | 0.07 | 0.02 | 0.07 | 0.07 | 0.09 | |||||
| (Z)-beta-farnesene | 1463 | 1443 | 0.10 | 0.22 | 0.17 | 0.13 | 0.17 | 0.21 | 0.16 | 0.19 | 0.26 | |
| alpha-humulene | 1465 | 1455 | 0.33 | 0.53 | 0.43 | 0.36 | 0.29 | 0.91 | 0.51 | 0.33 | 0.44 | 0.53 |
| gamma-muurolene | 1487 | 1480 | 0.15 | 0.12 | 0.09 | 0.16 | ||||||
| germacrene D | 1494 | 1485 | 4.98 | 4.74 | 6.86 | 6.39 | 4.65 | 5.42 | 3.96 | 1.93 | 3.29 | 6.07 |
| (Z)-alpha-bisabolene | 1498 | 1507 | 0.09 | 0.06 | 0.29 | 0.30 | 0.38 | 0.24 | 0.05 | |||
| alpha-bulnesene | 1509 | 1510 | 0.07 | 0.18 | 0.46 | 0.19 | 0.23 | |||||
| delta-amorphene | 1512 | 1512 | 0.27 | 0.10 | 0.10 | |||||||
| gamma-cadinene | 1525 | 1514 | 0.15 | 0.23 | 0.06 | 0.08 | 0.14 | 0.49 | 0.24 | 0.15 | 0.14 | 0.16 |
| delta-cadinene | 1529 | 1523 | 0.44 | 0.74 | 0.62 | 0.40 | 0.43 | 1.06 | 0.64 | 0.45 | 0.56 | 0.72 |
| 10-epi-cubebol | 1546 | 1535 | 0.10 | 0.15 | 0.14 | 0.18 | ||||||
| lippifoli-1(6)-en-5-one | 1560 | 1553 | 1.34 | 2.03 | 2.03 | 2.05 | 1.83 | 3.08 | 2.26 | 2.00 | 2.07 | 2.11 |
| spathulenol | 1582 | 1578 | 4.05 | 5.01 | 4.54 | 5.48 | 4.02 | 7.36 | 6.24 | 4.49 | 4.52 | 5.10 |
| caryophyllene oxide | 1587 | 1583 | 3.98 | 4.90 | 4.55 | 5.17 | 4.45 | 5.64 | 5.10 | 4.99 | 4.93 | 5.01 |
| salvial-4(14)-en-1-one | 1604 | 1595 | 0.62 | 0.92 | 0.81 | 0.64 | 0.65 | 1.11 | 0.97 | 1.08 | ||
| humulene epoxide II | 1623 | 1608 | 2.36 | 2.07 | 1.60 | 1.39 | 1.62 | 2.47 | 2.65 | 1.99 | 2.16 | 2.16 |
| epi-alpha-cadinol | 1655 | 1640 | 1.01 | 2.09 | 1.25 | 1.25 | 1.36 | 2.77 | 2.91 | 1.74 | 2.08 | 2.40 |
| epoxy allo-alloaromadendrene | 1661 | 1641 | 0.33 | 0.66 | 0.36 | 0.38 | 0.40 | 0.76 | 0.80 | 0.44 | 0.53 | 0.79 |
| 14-hydroxy-9-epi-(E)-caryophyllene | 1669 | 1670 | 1.78 | 1.41 | 1.13 | 1.03 | 1.06 | 2.84 | 2.06 | 1.22 | 1.30 | 1.41 |
| valeranone | 1692 | 1685 | 0.60 | 1.00 | 0.78 | 0.66 | 0.94 | 2.48 | 1.33 | 1.06 | 1.06 | 1.19 |
| guaia-3.10(14)-dien-11-ol | 1704 | 1678 | 0.42 | 0.71 | 0.54 | 0.49 | 0.30 | 1.68 | 0.95 | 0.41 | 0.61 | 0.83 |
| khusinol | 1708 | 1680 | 1.08 | 1.67 | 1.36 | 1.51 | 1.13 | 3.01 | 2.68 | 1.31 | 1.52 | 1.80 |
| Monoterpene Hydrocarbons | 53.74 | 47.01 | 50.44 | 49.69 | 52.54 | 35.71 | 38.59 | 48.21 | 46.18 | 44.66 | ||
| Aromatic Aldehydes | 5.36 | 3.35 | 4.35 | 5.94 | 6.00 | 2.46 | 3.52 | 4.69 | 4.57 | 3.79 | ||
| Aliphatic Aldehydes | 5..69 | 5.69 | 6.08 | 5.71 | 4.86 | 7.39 | 6.00 | 6.45 | 5.80 | 5.77 | ||
| Sesquiterpene Hydrocarbons | 11.30 | 13.87 | 14.15 | 12.75 | 10.96 | 19.15 | 14.45 | 9.49 | 11.65 | 15.53 | ||
| Sesquiterpene Alcohols | 6.56 | 9.48 | 7.69 | 8.73 | 6.91 | 14.82 | 12.93 | 8.09 | 8.91 | 10.13 | ||
| Oxygenated Sesquiterpenes | 3.98 | 4.90 | 4.55 | 5.17 | 4.45 | 5.64 | 5.10 | 4.99 | 4.93 | 5.01 | ||
| Others | 13.17 | 12.03 | 12.52 | 11.97 | 12.43 | 12.31 | 13.32 | 13.05 | 13.22 | 12.85 | ||
| Sum of Identified (%) | 99.80 | 96.33 | 99.78 | 99.96 | 98.15 | 97.48 | 93.91 | 94.97 | 95.26 | 97.74 |
| Chemical Variables | Axis 1 | Axis 2 |
|---|---|---|
| (a) | ||
| Eigenvalues | 20.798 | 4.557 |
| Percentage | 70.317 | 15.407 |
| Cumulative percentage | 70.317 | 85.724 |
| (b) | ||
| alpha-pinene | 0.876 | 0.035 |
| germacrene D | 0.072 | 0.655 |
| p-cymene | 0.075 | −0.487 |
| cumene | 0.087 | 0.357 |
| spathulenol | −0.188 | 0.164 |
| Nitrogen rate (kg ha−1) | Soil | 0 | 30 | 60 | 90 | 120 |
|---|---|---|---|---|---|---|
| alpha-pinene | L | 482.65d ± 15.35 | 524.16e ± 5.08 | 665.38f ± 14.61 | 894.17g ± 12.93 | 905.17 g ± 5.60 |
| S | 196.82a ± 8.12 | 325.01b ± 8.58 | 438.40c ± 14.29 | 488.15d ± 10.49 | 542.30e ± 14.68 | |
| Two-way ANOVA results: soil (F = 6583.8, p < 0.05), N (F = 1639.0, p < 0.05), soil × N (F = 112.6, p < 0.05) | ||||||
| cumene | L | 205.52d ± 6.54 | 184.66c ± 1.79 | 313.17h ± 6.88 | 299.80g ± 4.33 | 366.10i ± 2.27 |
| S | 149.37b ± 6.16 | 107.65a ± 2.84 | 192.51c ± 6.28 | 233.61e ± 5.02 | 252.92f ± 6.85 | |
| Two-way ANOVA results: soil (F = 2780.5, p < 0.05), N (F = 1316.89, p < 0.05), soil × N (F = 59.4, p < 0.05) | ||||||
| p-cymene | L | 134.83c ± 4.29 | 128.47c ± 1.24 | 146.29d ± 3.21 | 186.44f ± 2.70 | 273.19g ± 1.69 |
| S | 70.83a ± 2.92 | 109.71b ± 2.90 | 183.20f ± 5.97 | 164.10e ± 3.52 | 150.39d ± 4.07 | |
| Two-way ANOVA results: soil (F = 1222.2, p < 0.05), N (F = 1276.9, p < 0.05), soil × N (F = 586.4, p < 0.05) | ||||||
| germacrene D | L | 90.74d ± 2.89 | 101.66e ± 0.98 | 183.46h ± 4.03 | 209.37i ± 3.03 | 161.82g ± 1.00 |
| S | 74.55bc ± 3.08 | 67.88b ± 1.79 | 39.03a ± 1.27 | 75.72c ± 1.63 | 153.68f ± 4.16 | |
| Two-way ANOVA results: soil (F = 6527.5, p < 0.05), N (F = 1307.7, p < 0.05), soil × N (F = 1237.7, p < 0.05) | ||||||
| spathulenol | L | 73.79a ± 2.35 | 107.45d ± 1.04 | 121.42e ± 2.67 | 179.55h ± 2.60 | 139.90g ± 0.87 |
| S | 101.23c ± 4.18 | 106.97d ± 2.82 | 90.79b ± 2.96 | 104.03cd ± 2.23 | 129.12f ± 3.49 | |
| Two-way ANOVA results: soil (F = 465.4, p < 0.05), N (F = 554.6, p < 0.05), soil × N (F = 389.3, p < 0.05) | ||||||
| decanal | L | 81.63a ± 2.60 | 96.94b ± 0.94 | 128.10e ± 2.81 | 143.19f ± 2.07 | 130.85e ± 0.81 |
| S | 85.27a ± 3.52 | 81.25a ± 2.14 | 99.89bc ± 3.26 | 103.11c ± 2.21 | 112.66d ± 3.05 | |
| Two-way ANOVA results: soil (F = 646.3, p < 0.05), N (F = 455.6, p < 0.05), soil × N (F = 77.0, p < 0.05) | ||||||
| caryophyllene oxide | L | 72.52 a ± 2.60 | 105.09 c ± 0.94 | 121.68 e ± 2.81 | 169.40 g ± 2.07 | 154.86f ± 0.81 |
| S | 77.57a ± 3.20 | 87.42b ± 2.31 | 100.90c ± 3.29 | 113.47d ± 2.44 | 126.84e ± 3.43 | |
| Two-way ANOVA results: soil (F = 877.2, p < 0.05), N (F = 1032.3, p < 0.05), soil × N (F = 140.4, p < 0.05) | ||||||
| beta-pinene | L | 65.96c ± 2.10 | 73.99d ± 0.72 | 94.94f ± 2.09 | 102.88g ± 1.49 | 122.15h ± 0.76 |
| S | 30.53a ± 1.26 | 47.83 b ± 1.26 | 67.13 c ± 2.19 | 71.58 d ± 1.54 | 78.23 e ± 2.12 | |
| Two-way ANOVA results: soil (F = 4080.3, p < 0.05), N (F = 1296.1, p < 0.05), soil × N (F = 39.8, p < 0.05) | ||||||
| benzene acetaldehyde | L | 62.49e ± 1.99 | 47.61c ± 0.46 | 77.56f ± 1.70 | 117.63g ± 1.70 | 147.21h ± 0.91 |
| S | 4.68a ± 0.19 | 37.20b ± 0.98 | 56.42d ± 1.84 | 64.90e ± 1.39 | 63.04e ± 1.71 | |
| Two-way ANOVA results: soil (F = 10,265.3, p < 0.05), N (F = 3750.1, p < 0.05), soil × N (F = 886.7, p < 0.05) | ||||||
| Chemical Variables | Axis 1 | Axis 2 |
|---|---|---|
| (a) | ||
| Eigenvalues | 59,339.4 | 1823.14 |
| Percentage | 94.520 | 2.904 |
| Cumulative percentage | 94.520 | 97.424 |
| (b) | ||
| alpha-pinene | 0.888 | −0.008 |
| germacrene D | 0.187 | 0.692 |
| p-cymene | 0.181 | −0.647 |
| spathulenol | 0.088 | 0.261 |
| benzene acetakdehyde | 0.153 | −0.187 |
| cumene | 0.290 | −0.003 |
| caryophyllene oxide | 0.114 | 0.003 |
| beta-pinene | 0.103 | 0.003 |
| decanal | 0.080 | 0.002 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugier, D.; Olesińska, K.; Sugier, P.; Wójcik, M. Chemical Composition of Essential Oil from Flower Heads of Arnica Chamissonis Less. under a Nitrogen Impact. Molecules 2019, 24, 4454. https://doi.org/10.3390/molecules24244454
Sugier D, Olesińska K, Sugier P, Wójcik M. Chemical Composition of Essential Oil from Flower Heads of Arnica Chamissonis Less. under a Nitrogen Impact. Molecules. 2019; 24(24):4454. https://doi.org/10.3390/molecules24244454
Chicago/Turabian StyleSugier, Danuta, Katarzyna Olesińska, Piotr Sugier, and Małgorzata Wójcik. 2019. "Chemical Composition of Essential Oil from Flower Heads of Arnica Chamissonis Less. under a Nitrogen Impact" Molecules 24, no. 24: 4454. https://doi.org/10.3390/molecules24244454
APA StyleSugier, D., Olesińska, K., Sugier, P., & Wójcik, M. (2019). Chemical Composition of Essential Oil from Flower Heads of Arnica Chamissonis Less. under a Nitrogen Impact. Molecules, 24(24), 4454. https://doi.org/10.3390/molecules24244454







