Photoinduced Electron Transfer-Promoted Reactions Using Exciplex-Type Organic Photoredox Catalyst Directly Linking Donor and Acceptor Arenes
Abstract
:1. Introduction
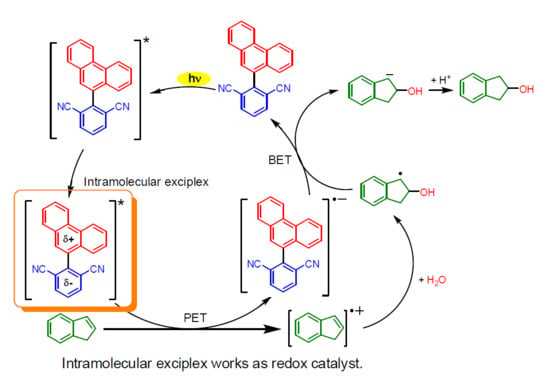
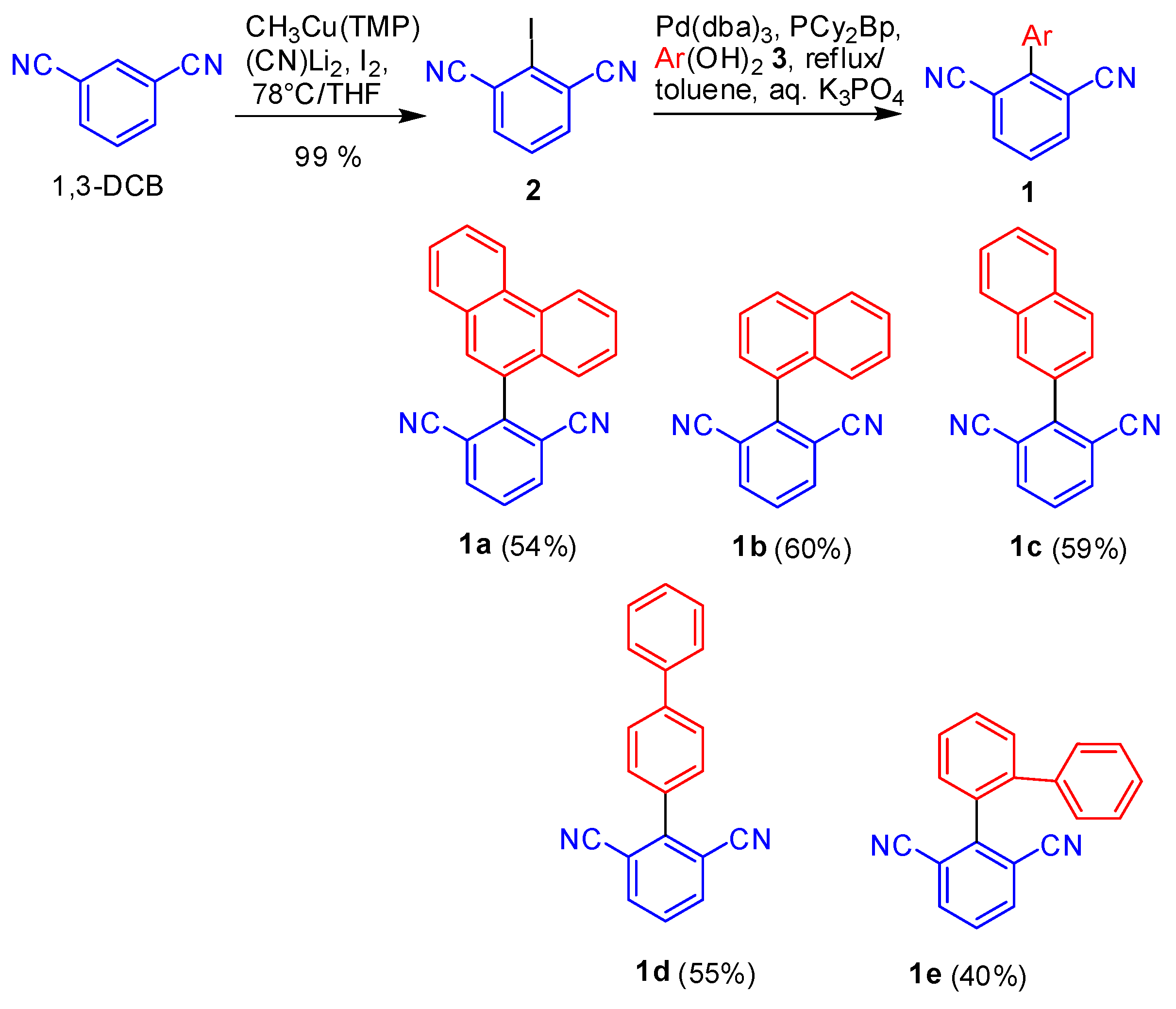
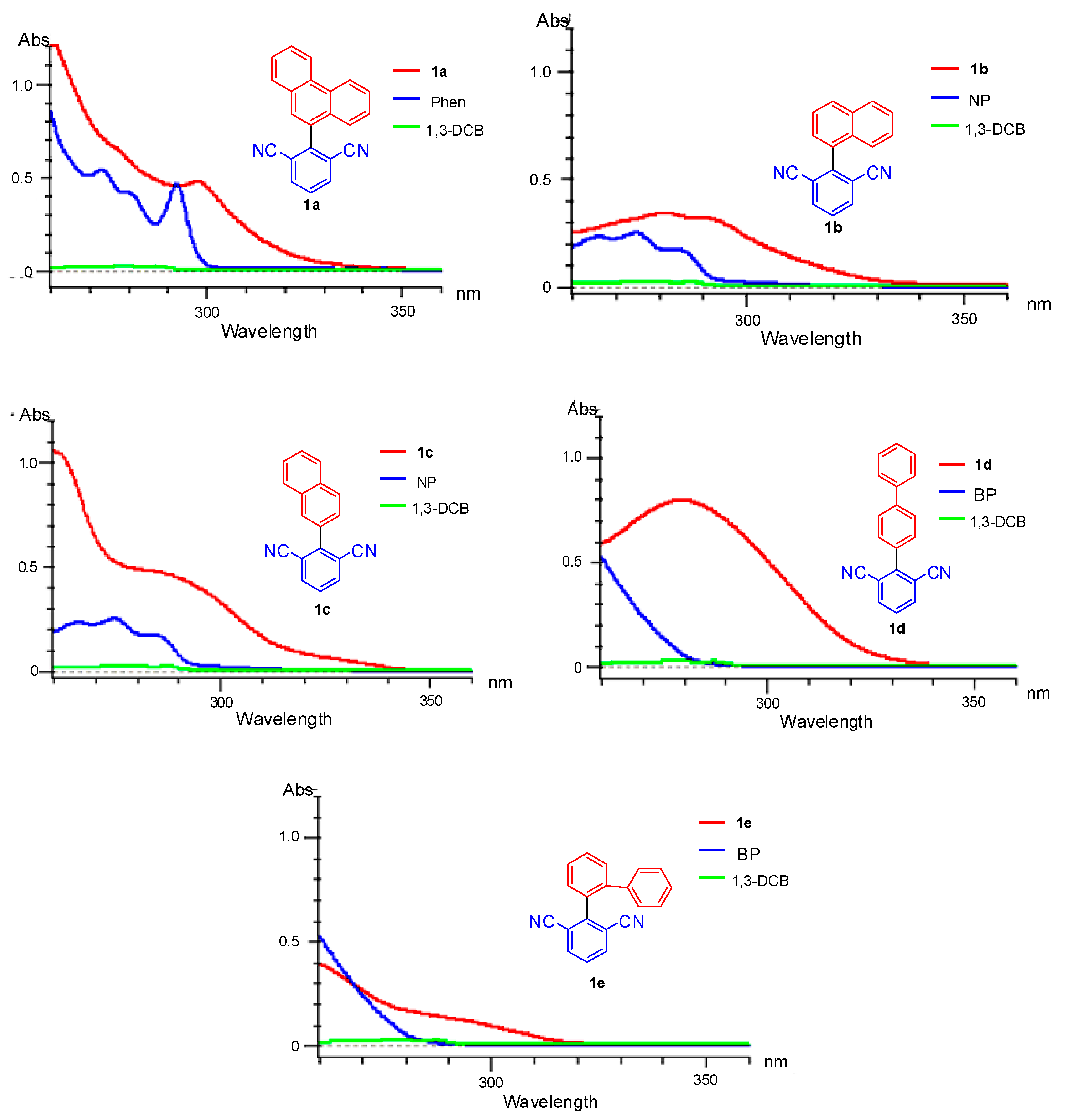
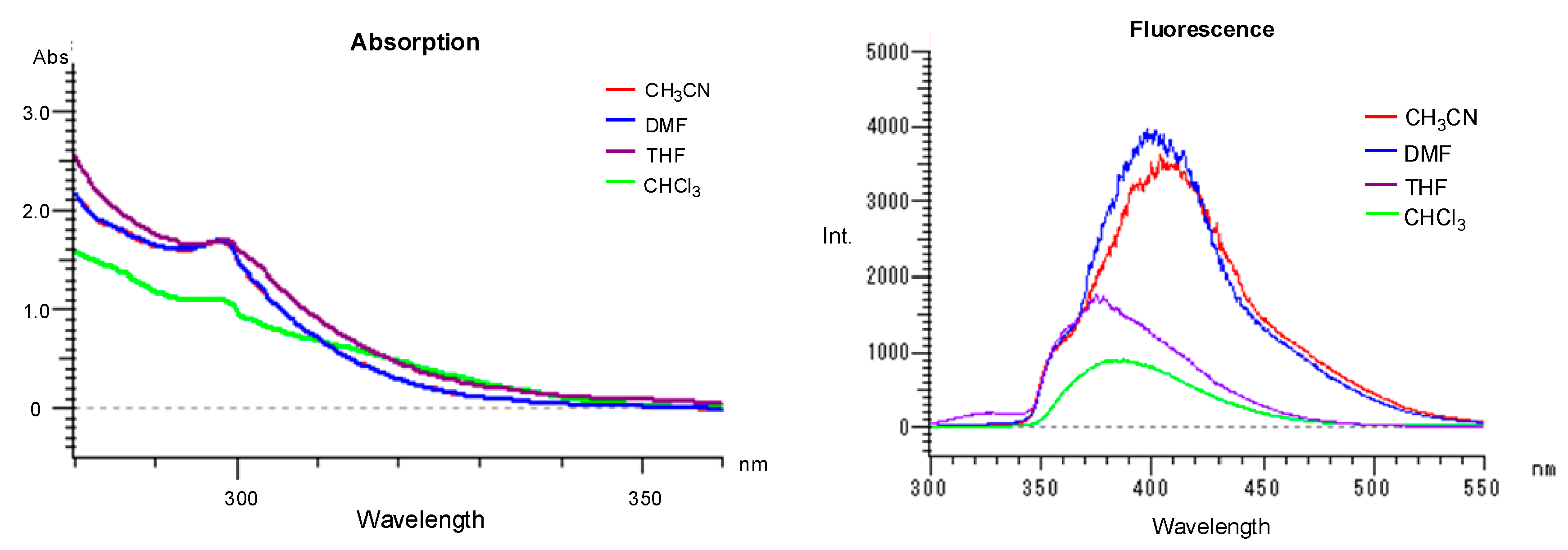
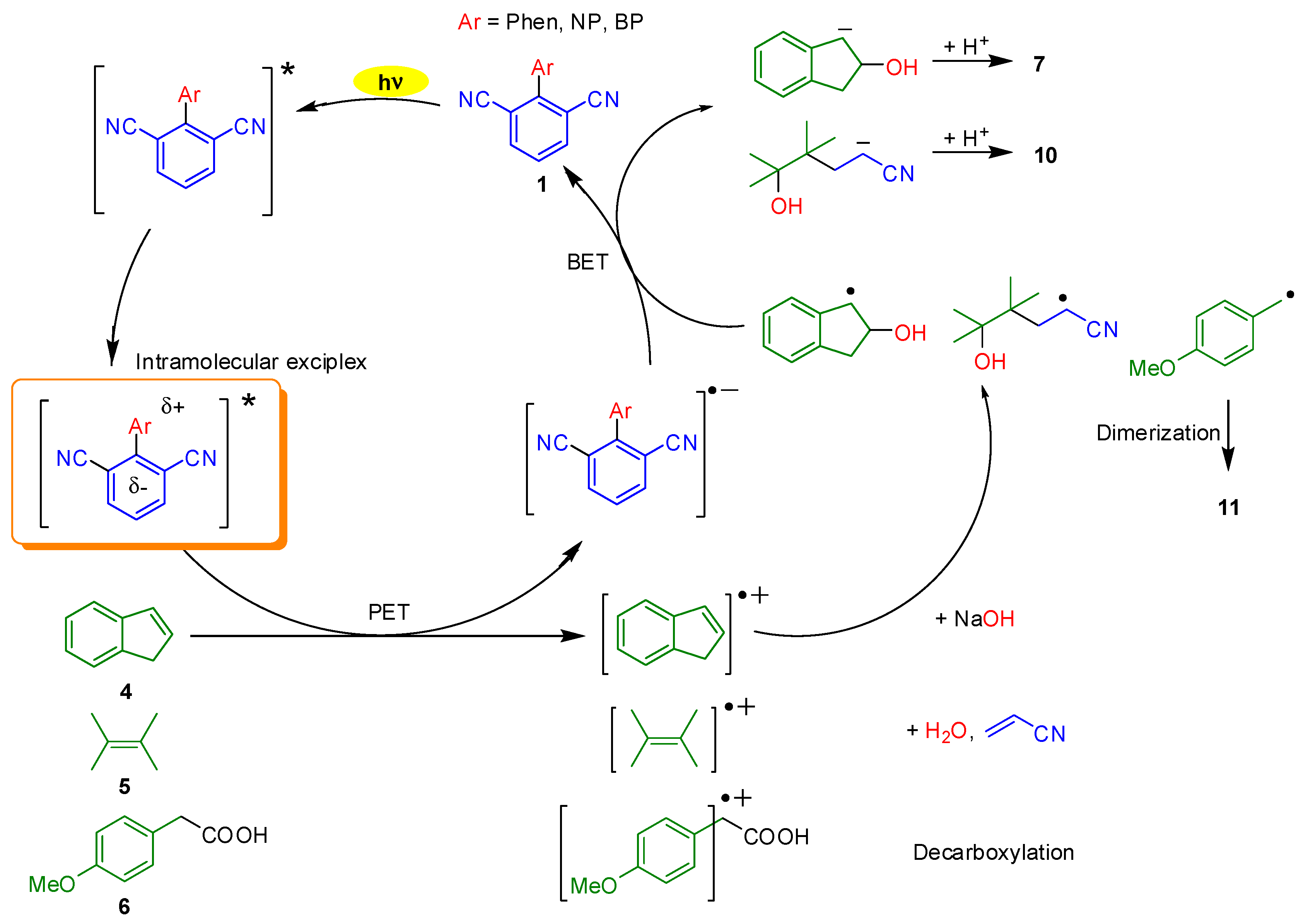
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, E.; Ohta, T.; Tsuji, S.; Mori, K.; Uchida, K.; Miura, T.; Ikoma, T.; Tayama, E.; Iwamoto, H.; Takizawa, S.; et al. Aryl-substituted Dimethylbenzimidazolines as Effective Reductants of Photoinduced Electron Transfer Reactions. Tetrahedron 2015, 71, 5494–5505. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, I.; Marzo, L.; Das, A.; Shaikh, R.; König, B. Visible Light Mediated Photoredox Catalytic Arylation Reactions. Acc. Chem. Res. 2016, 49, 1566–1577. [Google Scholar] [CrossRef] [PubMed]
- Margrey, K.A.; Nicewicz, D.A. A General Approach to Catalytic Alkene Anti-Markovnikov Hydrofunctionalization Reactions via Acridinium Photoredox Catalysis. Acc. Chem. Res. 2016, 49, 1997–2006. [Google Scholar] [CrossRef]
- Skubi, K.L.; Blum, T.R.; Yoon, T.P. Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev. 2016, 116, 10035–10074. [Google Scholar] [CrossRef]
- Dadashi-Silab, S.; Doran, S.; Yagci, Y. Photoinduced Electron Transfer Reactions for Macromolecular Syntheses. Chem. Rev. 2016, 116, 10212–10275. [Google Scholar] [CrossRef]
- Yamawaki, M.; Ukai, A.; Kamiya, Y.; Sugihara, S.; Sakai, M.; Yoshimi, Y. Metal-Free Photoinduced Decarboxylative Radical Polymerization Using Carboxylic Acids as Benign Radical Initiators: Introduction of Complex Molecules into Polymer Chain Ens. ACS Macro Lett. 2017, 6, 381–385. [Google Scholar] [CrossRef]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox Catalysis in Organic Chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Ohkubo, K. Organic Synthetic Transformations Using Organic Dyes as Photoredox Catalysts. Org. Biomol. Chem. 2014, 12, 6059–6071. [Google Scholar] [CrossRef] [Green Version]
- Yoshimi, Y. Photoinduced Electron Transfer-promoted Decarboxylative Radical Reactions of Aliphatic Carboxylic Acids by Organic Photoredox System. J. Photochem. Photobiol. A 2017, 342, 116–130. [Google Scholar] [CrossRef]
- Yoshimi, Y.; Itou, T.; Hatanaka, M. Decarboxylative Reduction of Free Aliphatic Carboxylic Acids by Photogenerated Cation Radical. Chem. Commun. 2007, 48, 5244–5246. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, Y.; Masuda, M.; Mizunashi, T.; Nishikawa, K.; Maeda, K.; Koshida, N.; Itou, T.; Morita, T.; Hatanaka, M. Inter- and Intramolecular Addition Reactions of Electron-Deficient Alkenes with Alkyl Radicals, Generated by SET-Photochemical Decarboxylation of Carboxylic Acids, Serve as a Mild and Efficient Method for the Preparation of γ-Amino Acids and Macrocyclic Lactones. Org. Lett. 2009, 11, 4652–4655. [Google Scholar] [PubMed]
- Yoshimi, Y.; Hayashi, S.; Nishikawa, K.; Haga, Y.; Maeda, K.; Morita, T.; Itou, T.; Okada, Y.; Ichinose, N.; Hatanaka, M. Influence of Solvent, Electron Acceptors and Arenes on Photochemical Decarboxylation of Free Carboxylic Acids via Single Electron Transfer (SET). Molecules 2010, 15, 2623–2630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishikawa, K.; Yoshimi, Y.; Maeda, K.; Morita, T.; Takahashi, I.; Itou, T.; Inagaki, S.; Hatanaka, M. Radical Photocyclization Route for Macrocyclic Lactone Ring Expansion and Conversion to Macrocyclic Lactams and Ketones. J. Org. Chem. 2013, 78, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Osaka, K.; Usami, A.; Iwasaki, T.; Yamawaki, M.; Morita, T.; Yoshimi, Y. Sequential Intermolecular Radical Addition and Reductive Radical Cyclization of Tyrosine and Phenylalanine Derivatives with Alkenes via Photoinduced Decarboxylation: Access to Ring-constrained γ-Amino Acids. J. Org. Chem. 2019, 84, 9480–9488. [Google Scholar] [CrossRef] [PubMed]
- Yoshimi, Y.; Itou, T.; Hatanaka, M. Redox-photosensitized Reaction of Indene Using Photosensitive Surfactant in Emulsion: Dependence on Oil Droplet Size and Surfactant Charge. Tetrahedron Lett. 2006, 47, 3257–3260. [Google Scholar] [CrossRef]
- Iwata, Y.; Tanaka, Y.; Kubosaki, S.; Morita, T.; Yoshimi, Y. A Strategy for Generating Aryl Radicals from Arylborates through Organic Photoredox Catalysis: Photo-Meerwein Type Arylation of Electron-deficient Alkenes. Chem. Commun. 2018, 54, 1257–1260. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kubosaki, S.; Osaka, K.; Yamawaki, M.; Morita, T.; Yoshimi, Y. Two Types of Cross-Coupling Reactions between Electron-Rich and Electron–Deficient Alkenes Assisted by Nucleophilic Addition Using Organic Photoredox Catalyst. J. Org. Chem. 2018, 83, 13625–13635. [Google Scholar] [CrossRef]
- Usui, S.; Hashimoto, Y.; Morey, J.V.; Wheatley, A.E.H.; Uchiyama, M. Direct ortho Cupration: A New Route to Regioselectively Functionalized Aromatics. J. Am. Chem. Soc. 2007, 129, 15102–15103. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Pearson, R.M.; Lim, C.H.; McCarthy, B.G.; Musgrave, C.B.; Miyake, G.M. Organocatalyzed Atom Transfer Radical Polymerization Using N-Aryl Phenoxazines as Photoredox Catalysts. J. Am. Chem. Soc. 2016, 138, 11399–11407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, N.; Kim, T.; Kim, D.; Osuka, A. Porphyrin Arch-Tapes: Synthesis, Contorted Structures, and Full Conjugation. J. Am. Chem. Soc. 2017, 139, 9075–9088. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Liu, Y.; Rao, Y.; Kim, G.; Zhou, M.; Yu, D.; Xu, L.; Yin, B.; Liu, S.; Tanaka, T.; et al. meso-Triaryl-Substituted Smaragdyrins: Facile Aromaticity Switching. J. Am. Chem. Soc. 2018, 140, 16553–16559. [Google Scholar] [CrossRef] [PubMed]
- Roth, H.G.; Romero, N.A.; Nicewicz, D.A. Experimental and Calculated Electrochemical Potentials of Common Organic Molecules for Applications to Single Electron Redox Chemistry. Synlett 2016, 27, 714–723. [Google Scholar]
- Maeda, H.; Mizuno, K. Inter- and Intramolecular Photocycloaddition of Aromatic Compounds. In CRC Handbook of Organic Photochemistry and Photobiology, 3rd ed.; Griesbeck, A., Oelgemöller, M., Ghetti, F., Eds.; CRC Press: Boca Raton, FL, USA, 2012; Volume 1, pp. 489–509. [Google Scholar]
- Maeda, H.; Nakashima, R.; Sugimoto, A.; Mizuno, K. Intramolecular 10,10a-[2+2] Photocycloaddition Reactions of Phenanthrenes with Linked Styrene. J. Photochem. Photobiol. A 2016, 329, 232–237. [Google Scholar] [CrossRef]
- Su, X.; Fox, D.J.; Blackwell, D.T.; Tanaka, K.; Spring, D.R. Copper Catalyzed Oxidation of Organozinc Halides. Chem. Comm. 2006, 37, 3883–3885. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


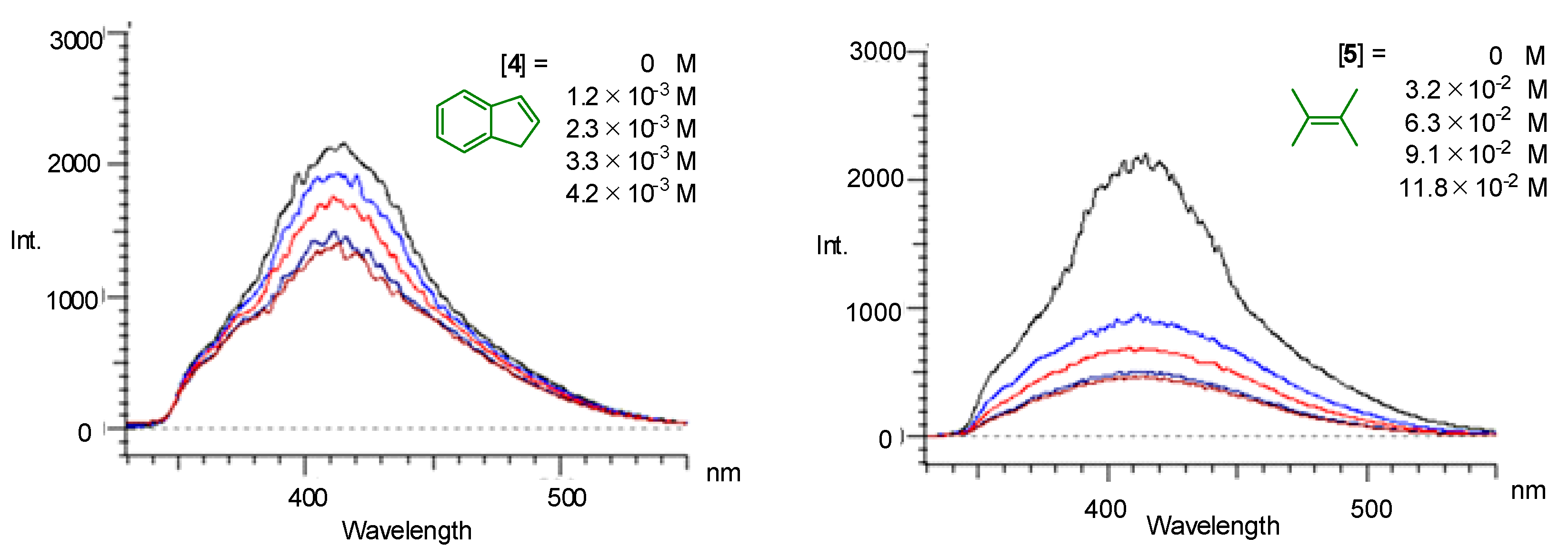
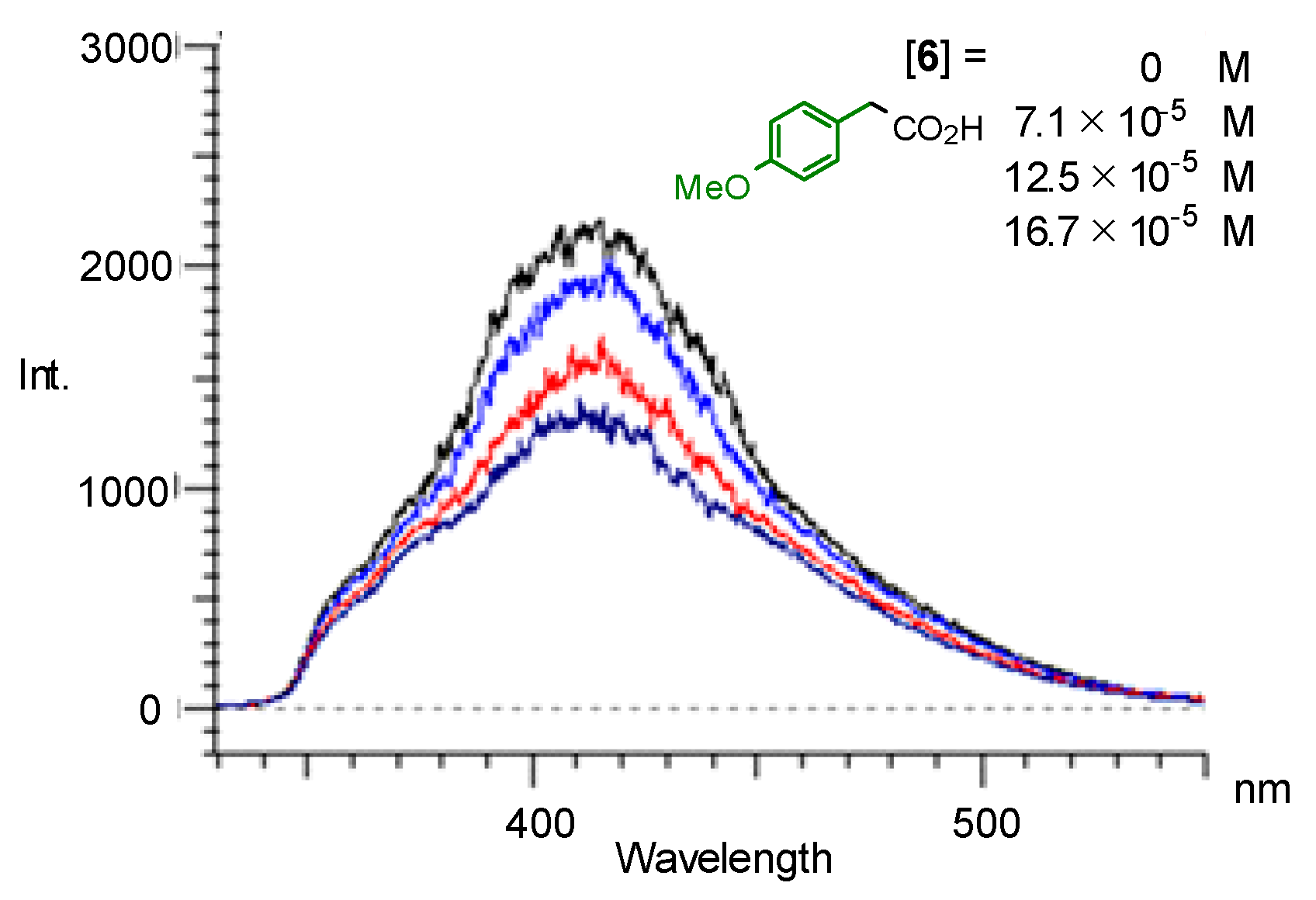
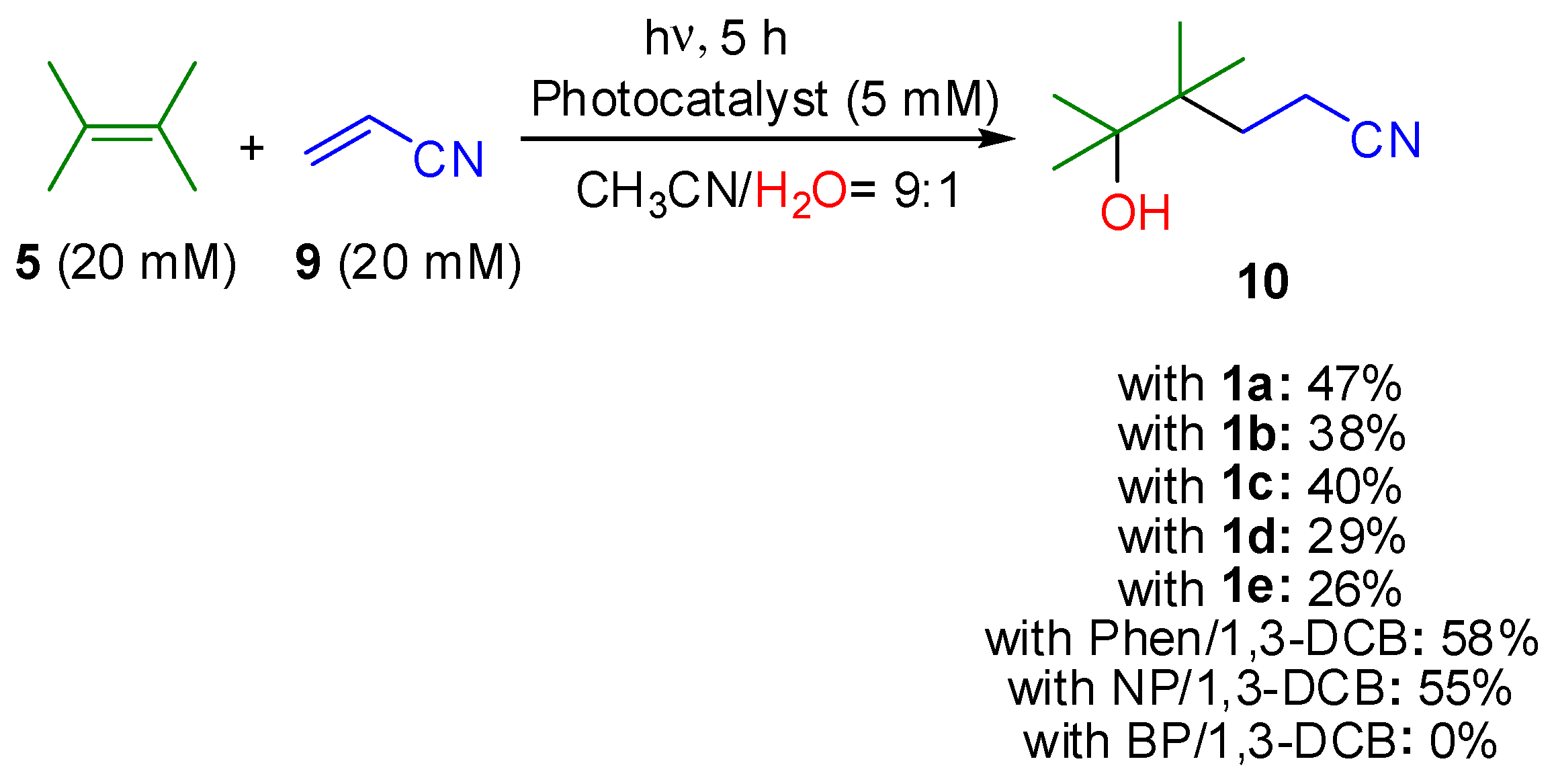
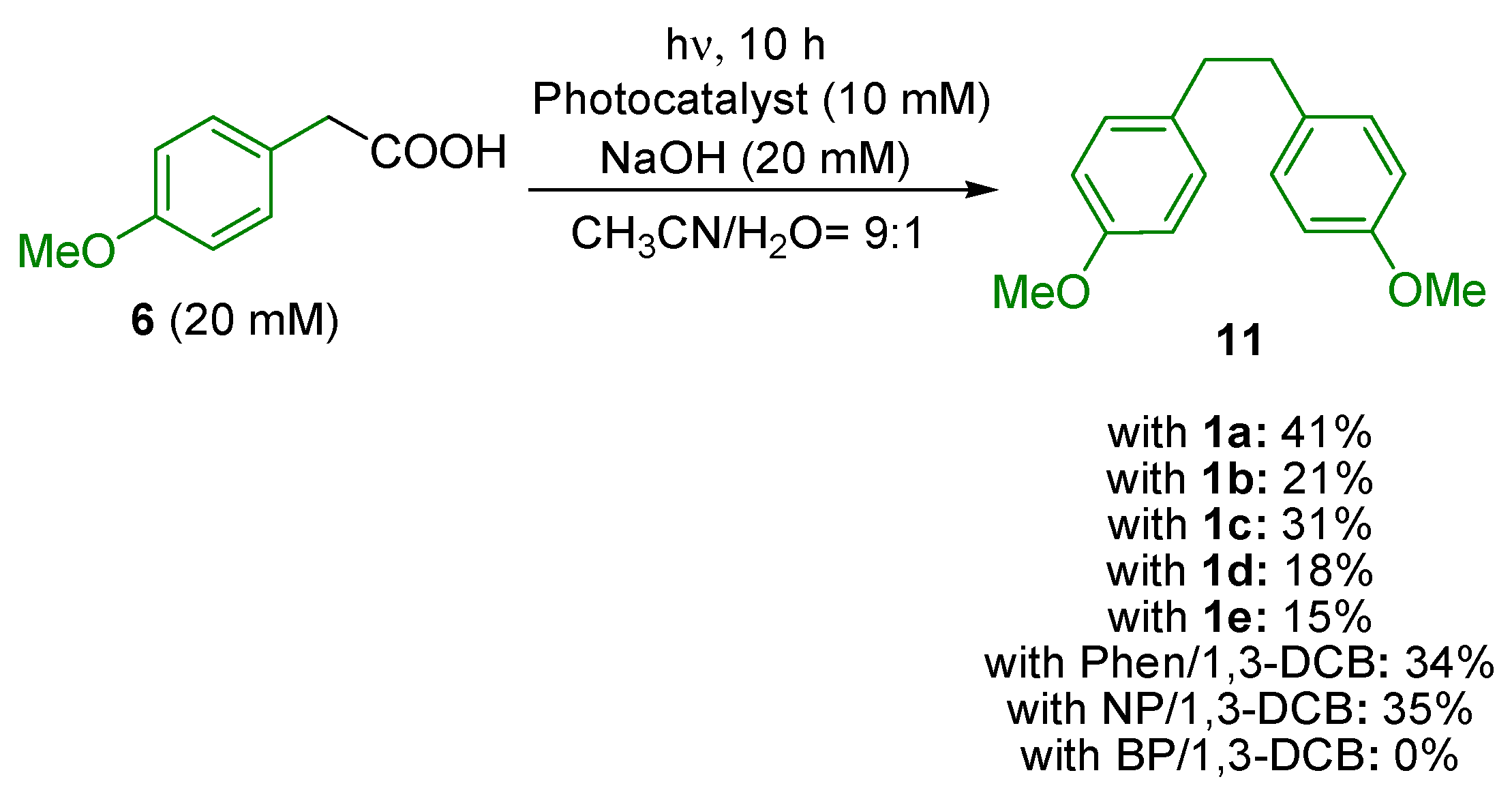
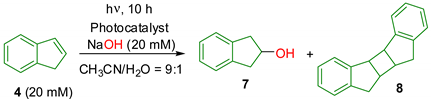
| Entry | Photocatalyst | Yield of 7/% 1 | Yield of 8/% 1 |
|---|---|---|---|
| 1 | 1a (10 mM) | 49 | 0 |
| 2 | none | 0 | 60 |
| 3 | Phen (10 mM), 1,3-DCB (10 mM) | 50 | 6 |
| 4 | Phen (10 mM) | 0 | trace |
| 5 | 1,3-DCB (10 mM) | trace | 19 |
| 6 | 1a (5 mM) | 42 | 3 |
| 7 | 1a (1 mM) | 34 | 14 |
| 8 | 1a (0.5 mM) | 30 | 25 |
| 9 | 1a (0.1 mM) | 10 | 37 |
| 10 | Phen (0.1 mM), 1,3-DCB (0.1 mM) | 0 | 32 |
| 11 | NP (10 mM), 1,3-DCB (10 mM) | 45 | trace |
| 12 | 1b (10 mM) | 45 | trace |
| 13 | 1c (10 mM) | 41 | 0 |
| 14 | BP (10 mM), 1,3-DCB (10 mM) | 0 | 24 |
| 15 | 1d (10 mM) | 36 | 14 |
| 16 | 1e (10 mM) | 27 | 17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamawaki, M.; Asano, A.; Furutani, T.; Izumi, Y.; Tanaka, Y.; Osaka, K.; Morita, T.; Yoshimi, Y. Photoinduced Electron Transfer-Promoted Reactions Using Exciplex-Type Organic Photoredox Catalyst Directly Linking Donor and Acceptor Arenes. Molecules 2019, 24, 4453. https://doi.org/10.3390/molecules24244453
Yamawaki M, Asano A, Furutani T, Izumi Y, Tanaka Y, Osaka K, Morita T, Yoshimi Y. Photoinduced Electron Transfer-Promoted Reactions Using Exciplex-Type Organic Photoredox Catalyst Directly Linking Donor and Acceptor Arenes. Molecules. 2019; 24(24):4453. https://doi.org/10.3390/molecules24244453
Chicago/Turabian StyleYamawaki, Mugen, Akiko Asano, Toshiki Furutani, Yuki Izumi, Yosuke Tanaka, Kazuyuki Osaka, Toshio Morita, and Yasuharu Yoshimi. 2019. "Photoinduced Electron Transfer-Promoted Reactions Using Exciplex-Type Organic Photoredox Catalyst Directly Linking Donor and Acceptor Arenes" Molecules 24, no. 24: 4453. https://doi.org/10.3390/molecules24244453
APA StyleYamawaki, M., Asano, A., Furutani, T., Izumi, Y., Tanaka, Y., Osaka, K., Morita, T., & Yoshimi, Y. (2019). Photoinduced Electron Transfer-Promoted Reactions Using Exciplex-Type Organic Photoredox Catalyst Directly Linking Donor and Acceptor Arenes. Molecules, 24(24), 4453. https://doi.org/10.3390/molecules24244453