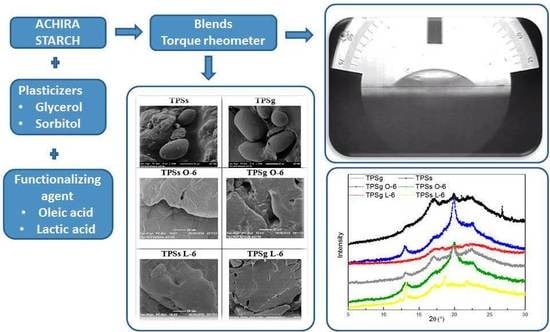
Rheological, Thermal, Superficial, and Morphological Properties of Thermoplastic Achira Starch Modified with Lactic Acid and Oleic Acid
Abstract
:1. Introduction
2. Results
2.1. Rheometric Analysis
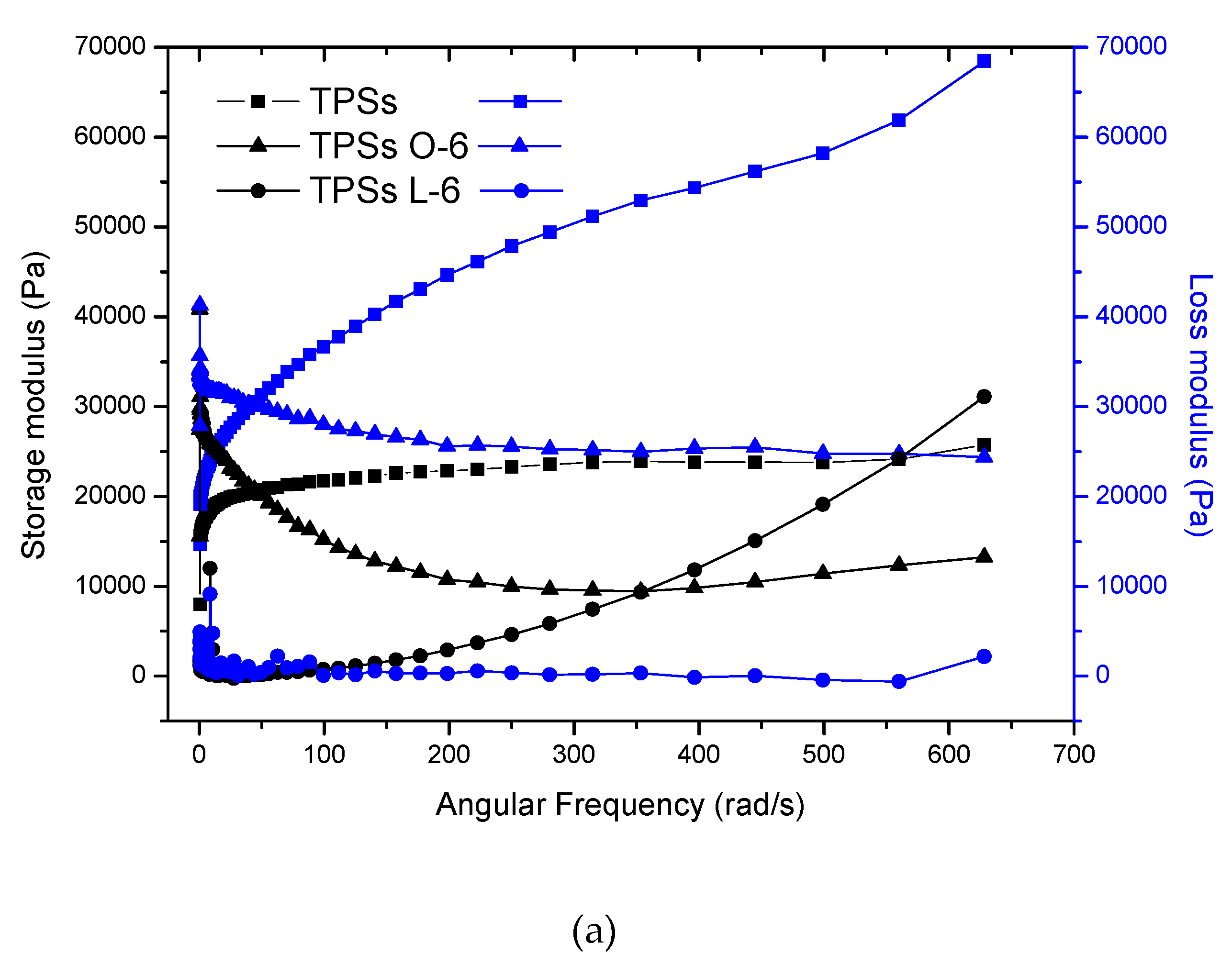
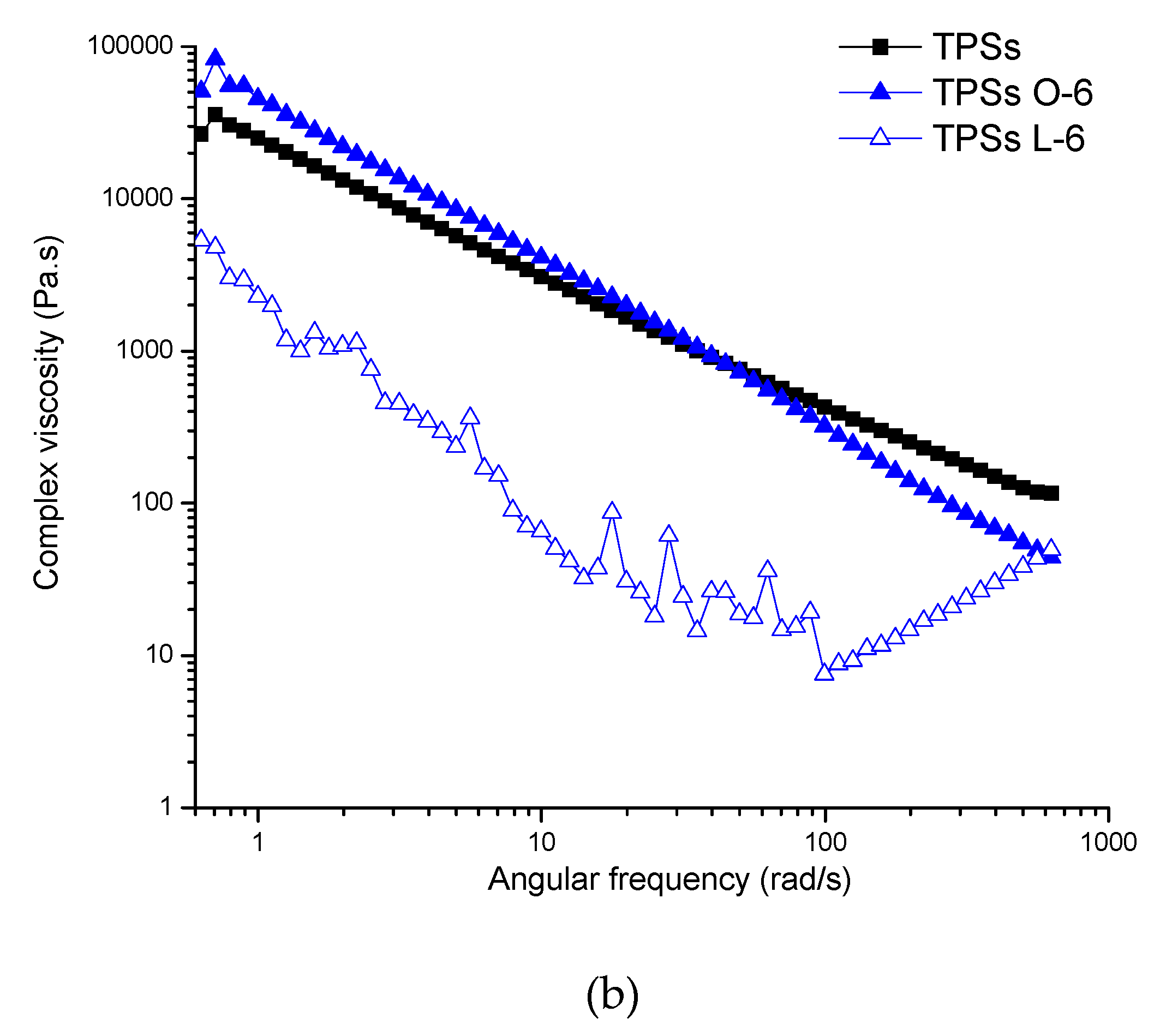
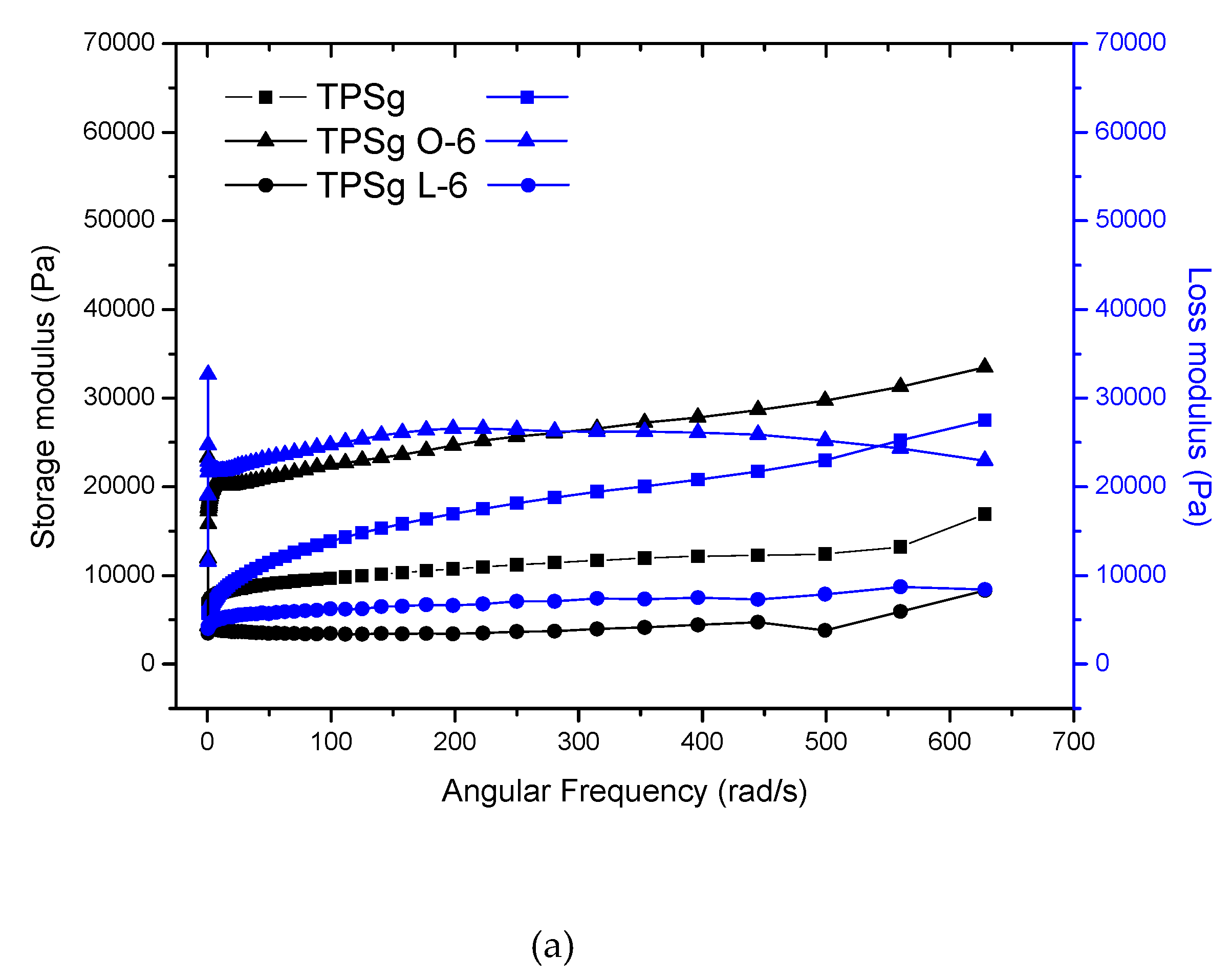
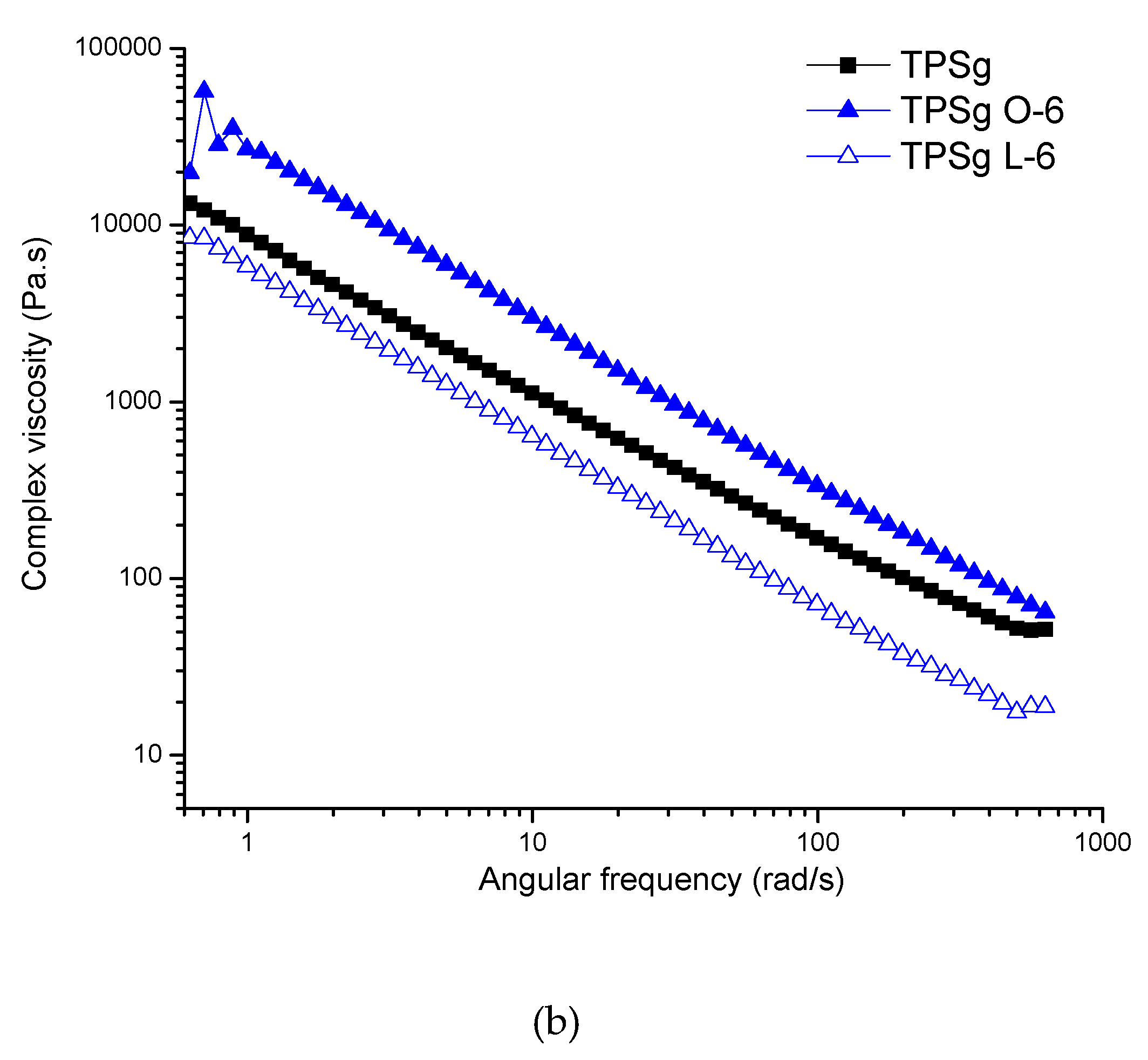
2.2. Rheological Analysis
2.3. IR Analysis
2.4. SEM Analysis
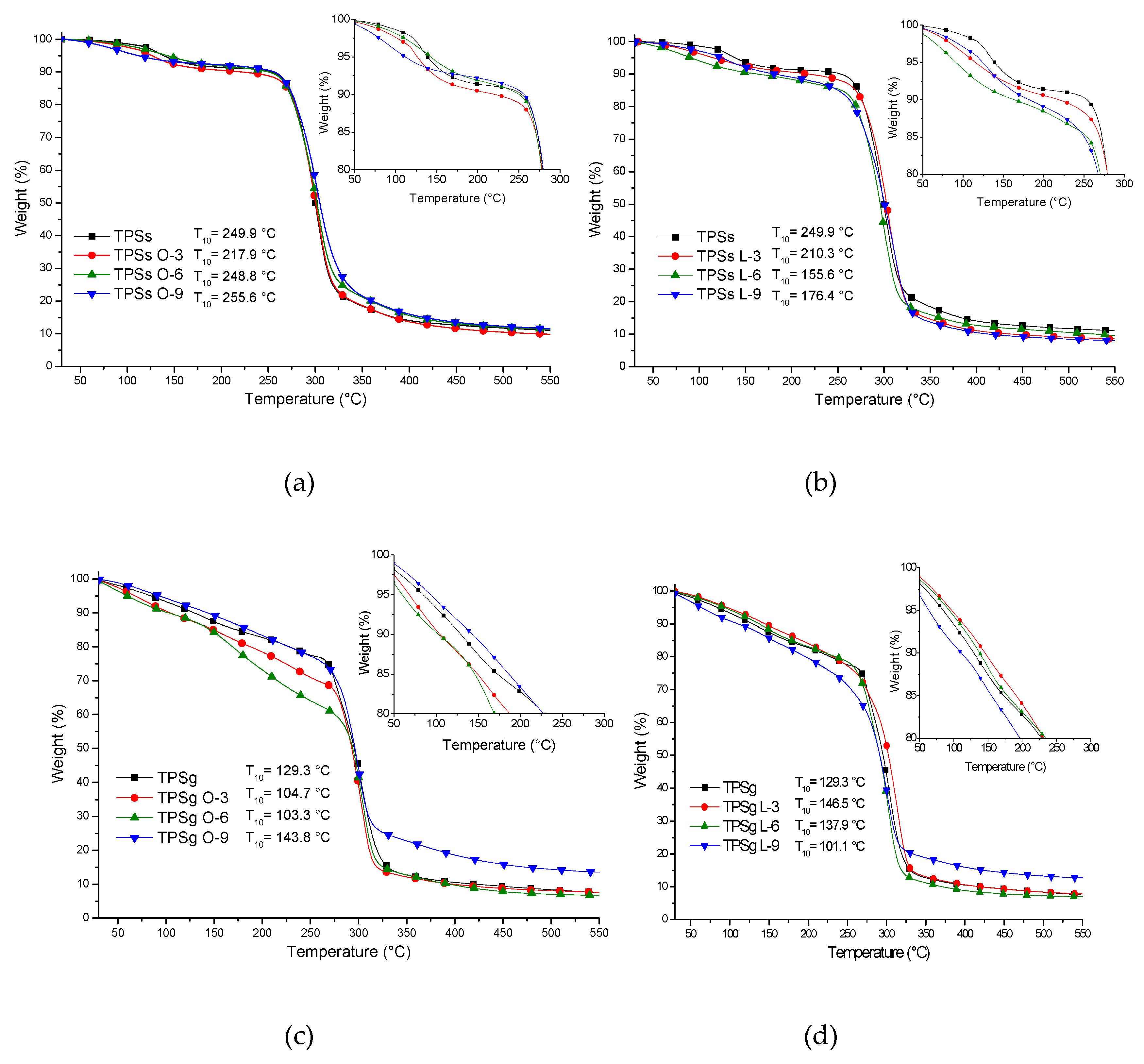
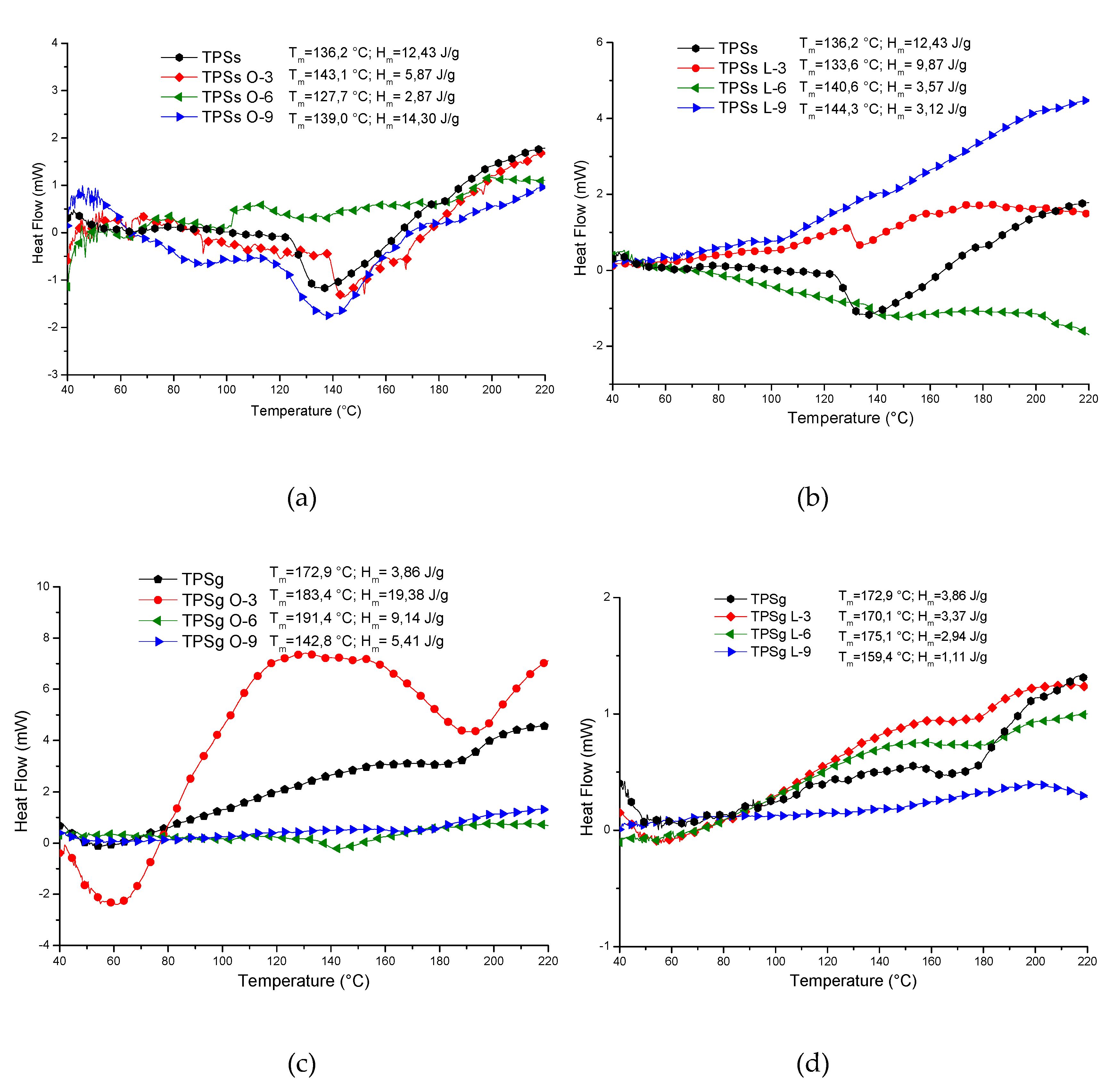
2.5. Thermal Analysis
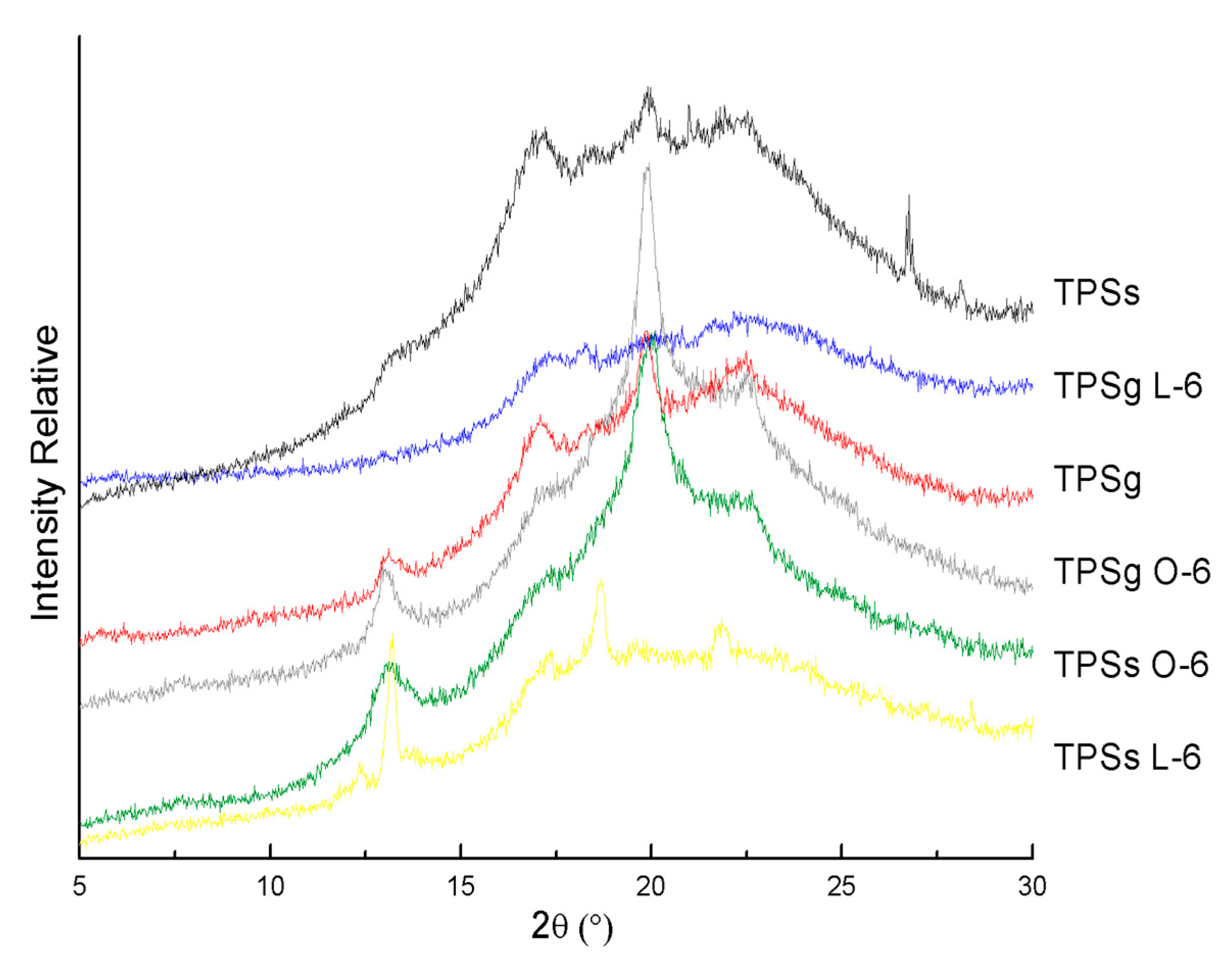
2.6. X-Ray Diffraction (XRD) Analysis
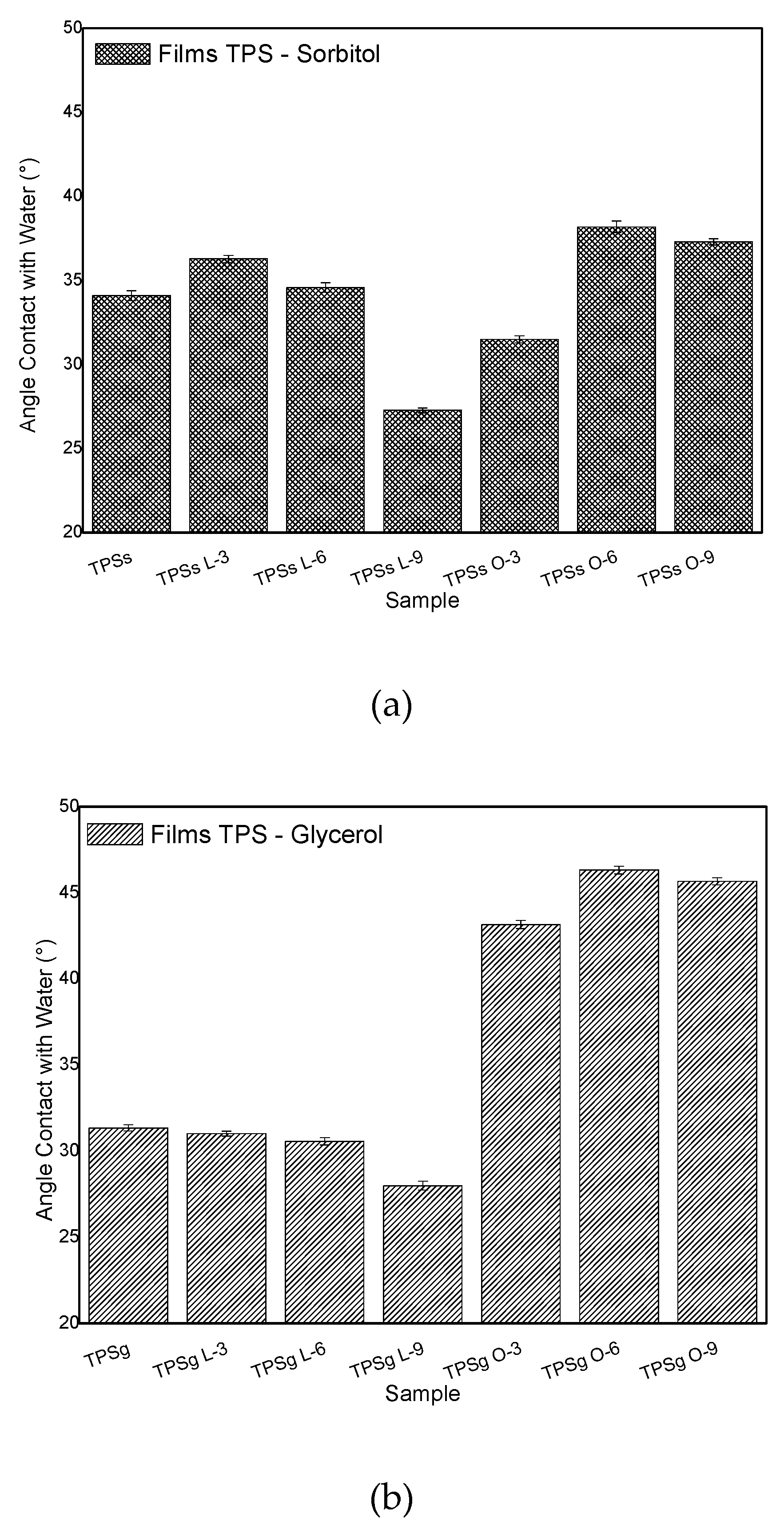
2.7. Contact Angle Analysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of Polymeric Materials
3.3. Characterization
3.3.1. Rheometric Analysis
3.3.2. Rheological Analysis
3.3.3. IR Analysis
3.3.4. Scanning Electron Microscopy Analysis
3.3.5. Thermal Analysis
3.3.6. XRD Analysis
3.3.7. Contact Angle Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, G.-Q.; Patel, M.K. Plastics derived from biological sources: Present and future: A technical and environmental review. Chem. Rev. 2011, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Halden, R.U. Plastics and health risks. Ann. Rev. Public Health 2010, 31, 179–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siracusa, V.; Rocculi, P.; Romani, S.; Dalla Rosa, M. Biodegradable polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of bioplastics: Opportunities and challenges. Curr. Opin. Green Sustain. Chem. 2018, 13, 68–75. [Google Scholar] [CrossRef] [Green Version]
- European Bioplastics. Market Drivers and Development; Institute for Bioplastics and Biocomposites and Nova-Institute: Berlin, Marientrate, Germany, 2017. [Google Scholar]
- Aeschelmann, F.; Carus, M. Biobased building blocks and polymers in the world: Capacities, production, and applications–status quo and trends towards 2020. Ind. Biotechnol. 2015, 11, 154–159. [Google Scholar] [CrossRef]
- Hildebrandt, J.; Bezama, A.; Thrän, D. Cascade use indicators for selected biopolymers: Are we aiming for the right solutions in the design for recycling of bio-based polymers? Waste Manag. Res. 2017, 35, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Fatyeyeva, K.; Chappey, C.; Marais, S. Biopolymer/clay nanocomposites as the high barrier packaging material: Recent advances. In Food Packaging; Elsevier: Rouen, Normadie, France, 2017; pp. 425–463. [Google Scholar] [CrossRef]
- de Léis, C.M.; Nogueira, A.R.; Kulay, L.; Tadini, C.C. Environmental and energy analysis of biopolymer film based on cassava starch in Brazil. J. Clean. Prod. 2017, 143, 76–89. [Google Scholar] [CrossRef]
- Mohan, C.C.; Harini, K.; Karthikeyan, S.; Sudharsan, K.; Sukumar, M. Effect of film constituents and different processing conditions on the properties of starch based thermoplastic films. Int. J. Biol. Macromol. 2018, 120, 2007–2016. [Google Scholar] [CrossRef]
- Masina, N.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A review of the chemical modification techniques of starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef]
- Tabasum, S.; Younas, M.; Zaeem, M.A.; Majeed, I.; Majeed, M.; Noreen, A.; Iqbal, M.N.; Zia, K.M. A review on blending of corn starch with natural and synthetic polymers, and inorganic nanoparticles with mathematical modeling. Int. J. Biol. Macromol. 2019, 122, 969–996. [Google Scholar] [CrossRef]
- Zhu, F. Composition, structure, physicochemical properties, and modifications of cassava starch. Carbohydr. Polym. 2015, 122, 456–480. [Google Scholar] [CrossRef] [PubMed]
- Hulleman, S.; Kalisvaart, M.; Janssen, F.; Feil, H.; Vliegenthart, J. Origins of B-type crystallinity in glycerol-plasticised, compression-moulded potato starches. Carbohydr. Polym. 1999, 39, 351–360. [Google Scholar] [CrossRef]
- Jane, J.-l.; Spence, K.E. Biodegradable thermoplastic composition of aldehyde starch and protein. U.S. Patent No. 5,397,834, 03 September 1993. [Google Scholar]
- García-Tejeda, Y.V.; López-González, C.; Pérez-Orozco, J.P.; Rendón-Villalobos, R.; Jiménez-Pérez, A.; Flores-Huicochea, E.; Solorza-Feria, J.; Bastida, C.A. Physicochemical and mechanical properties of extruded laminates from native and oxidized banana starch during storage. Lwt-Food Sci. Technol. 2013, 54, 447–455. [Google Scholar]
- Dias, A.B.; Müller, C.M.; Larotonda, F.D.; Laurindo, J.B. Biodegradable films based on rice starch and rice flour. J. Cereal Sci. 2010, 51, 213–219. [Google Scholar] [CrossRef]
- Bello-Pérez, L.A.; Agama-Acevedo, E.; Zamudio-Flores, P.B.; Mendez-Montealvo, G.; Rodriguez-Ambriz, S.L. Effect of low and high acetylation degree in the morphological, physicochemical and structural characteristics of barley starch. LWT-Food Sci. Technol. 2010, 43, 1434–1440. [Google Scholar]
- Condés, M.C.; Añón, M.C.; Dufresne, A.; Mauri, A.N. Composite and nanocomposite films based on amaranth biopolymers. Food Hydrocoll. 2018, 74, 159–167. [Google Scholar] [CrossRef]
- Dick, M.; Henrique Pagno, C.; Haas Costa, T.M.; Gomaa, A.; Subirade, M.; de Oliveira Rios, A.; Hickmann Flôres, S. Edible films based on chia flour: Development and characterization. J. Appl. Polym. Sci. 2016, 133, 42455. [Google Scholar] [CrossRef]
- Andrade-Mahecha, M.M.; Tapia-Blácido, D.R.; Menegalli, F.C. Development and optimization of biodegradable films based on achira flour. Carbohydr. Polym. 2012, 88, 449–458. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Ferreira, D.C.; Louzada, L.B.; dos Santos, F.; Corrêa, A.C.; Moreira, F.K.V.; Mattoso, L.H. Starch-Based Edible Films and Coatings: An Eco-friendly Alternative for Food Packaging. In Starches for Food Application; Elsevier: Sao Carlos, Santa Catarina, Brasil, 2019; pp. 359–420. [Google Scholar] [CrossRef]
- Leonel, M.; Sarmento, S.; Cereda, M.; Guerreiro, L. Extração e caracterização do amido de biri (Canna edulis). Braz. J. Food Technol. 2002, 5, 27–32. [Google Scholar]
- Peroni, F.; Rocha, T.; Franco, C. Some structural and physicochemical characteristics of tuber and root starches. Food Sci. Technol. Int. 2006, 12, 505–513. [Google Scholar] [CrossRef]
- Andrade-Mahecha, M.M.; Tapia-Blácido, D.R.; Menegalli, F.C. Physical–chemical, thermal, and functional properties of achira (Canna indica L.) flour and starch from different geographical origin. Starch-Stärke 2012, 64, 348–358. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Alias, A.K.; Ariffin, F.; Mahmud, S. Physico-mechanical and microstructural properties of semolina flour films as influenced by different sorbitol/glycerol concentrations. Int. J. Food Prop. 2018, 21, 983–995. [Google Scholar] [CrossRef]
- Zain, A.M.; Kahar, A.; Noriman, N. Chemical-mechanical hydrolysis technique of modified thermoplastic starch for better mechanical performance. Procedia Chem. 2016, 19, 638–645. [Google Scholar] [CrossRef]
- Shah, N.N.; Singhal, R.S. A two-tier modified starch-oxidation followed by n-octenyl succinylation as gum Arabic substitute: Process details and characterization. J. Food Eng. 2018, 226, 96–104. [Google Scholar] [CrossRef]
- Sun, S.; Lin, X.; Zhao, B.; Wang, B.; Guo, Z. Structural properties of lotus seed starch prepared by octenyl succinic anhydride esterification assisted by high hydrostatic pressure treatment. LWT-Food Sci. Technol. 2019, 108698. [Google Scholar] [CrossRef]
- Bakouri, H.; Guemra, K. Etherification and cross-linking effect on physicochemical properties of Zea mays starch executed at different sequences in 1-butyl-3-methylimidazolium chloride [BMIM] Cl ionic liquid media. Int. J. Boil. Macromol. 2018. [Google Scholar] [CrossRef]
- Saliu, O.; Olatunji, G.; Olosho, A.; Adeniyi, A.; Azeh, Y.; Samo, F.; Adebayo, D.; Ajetomobi, O. Barrier property enhancement of starch citrate bioplastic film by an ammonium-thiourea complex modification. J. Saudi Chem. Soc. 2019, 23, 141–149. [Google Scholar] [CrossRef]
- López, O.V.; Zaritzky, N.E.; Grossmann, M.V.; García, M.A. Acetylated and native corn starch blend films produced by blown extrusion. J. Food Eng. 2013, 116, 286–297. [Google Scholar] [CrossRef]
- Bergel, B.F.; Osorio, S.D.; da Luz, L.M.; Santana, R.M.C. Effects of hydrophobized starches on thermoplastic starch foams made from potato starch. Carbohydr. Polym. 2018, 200, 106–114. [Google Scholar] [CrossRef]
- Murillo, E.A.; Cardona, A.; López, B.L. Rheological behavior in the molten state and solution of hyperbranched polyester of fourth and fifth generation. J. Appl. Polym. Sci. 2011, 119, 929–935. [Google Scholar] [CrossRef]
- Guzmán, M.; Murillo, E.A. Structural, thermal, rheological, morphological and mechanical properties of thermoplastic starch obtained by using hyperbranched polyester polyol as plasticizing agent. Dyna 2018, 85, 178–186. [Google Scholar] [CrossRef]
- Zhang, S.; He, Y.; Lin, Z.; Li, J.; Jiang, G. Effects of tartaric acid contents on phase homogeneity, morphology and properties of poly (butyleneadipate-co-terephthalate)/thermoplastic starch bio-composities. Polym. Test. 2019, 76, 385–395. [Google Scholar] [CrossRef]
- Aguirre-Loredo, R.Y.; Velazquez, G.; Gutierrez, M.C.; Castro-Rosas, J.; Rangel-Vargas, E.; Gómez-Aldapa, C.A. Effect of airflow presence during the manufacturing of biodegradable films from polymers with different structural conformation. Food Packag. Shelf Life 2018, 17, 162–170. [Google Scholar] [CrossRef]
- Gómez-Aldapa, C.A.; Velazquez, G.; Gutierrez, M.C.; Rangel-Vargas, E.; Castro-Rosas, J.; Aguirre-Loredo, R.Y. Effect of polyvinyl alcohol on the physicochemical properties of biodegradable starch films. Mater. Chem. Phys. 2020, 239, 122027. [Google Scholar] [CrossRef]
- Georges, A.; Lacoste, C.; Damien, E. Effect of formulation and process on the extrudability of starch-based foam cushions. Ind. Crop. Prod. 2018, 115, 306–314. [Google Scholar] [CrossRef]
- Jantanasakulwong, K.; Leksawasdi, N.; Seesuriyachan, P.; Wongsuriyasak, S.; Techapun, C.; Ougizawa, T. Reactive blending of thermoplastic starch and polyethylene-graft-maleic anhydride with chitosan as compatibilizer. Carbohydr. Polym. 2016, 153, 89–95. [Google Scholar] [CrossRef]
- Aranda-García, F.; González-Núñez, R.; Jasso-Gastinel, C.; Mendizábal, E. Water absorption and thermomechanical characterization of extruded starch/poly (lactic acid)/agave bagasse fiber bioplastic composites. Int. J. Polym. Sci. 2015, 2015, 343294. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, S.; Hanna, M.A. Properties of extruded starch-based plastic foam. Ind. Crop. Prod. 1995, 4, 71–77. [Google Scholar] [CrossRef]
- Chen, L.; Reddy, N.; Wu, X.; Yang, Y. Thermoplastic films from wheat proteins. Ind. Crop. Prod. 2012, 35, 70–76. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Effects of controlled storage on thermal, mechanical and barrier properties of plasticized films from different starch sources. J. Food Eng. 2006, 75, 453–460. [Google Scholar] [CrossRef]
- Hung, P.V.; Morita, N. Physicochemical properties and enzymatic digestibility of starch from edible canna (Canna edulis) grown in Vietnam. Carbohydr. Polym. 2005, 61, 314–321. [Google Scholar] [CrossRef]
- Puncha-arnon, S.; Puttanlek, C.; Rungsardthong, V.; Pathipanawat, W.; Uttapap, D. Changes in physicochemical properties and morphology of canna starches during rhizomal development. Carbohydr. Polym. 2007, 70, 206–217. [Google Scholar] [CrossRef]
- Watcharatewinkul, Y.; Puttanlek, C.; Rungsardthong, V.; Uttapap, D. Pasting properties of a heat-moisture treated canna starch in relation to its structural characteristics. Carbohydr. Polym. 2009, 75, 505–511. [Google Scholar] [CrossRef]
- Thitipraphunkul, K.; Uttapap, D.; Piyachomkwan, K.; Takeda, Y. A comparative study of edible canna (Canna edulis) starch from different cultivars. Part I. Chemical composition and physicochemical properties. Carbohydr. Polym. 2003, 53, 317–324. [Google Scholar] [CrossRef]
- Gunaratne, A.; Hoover, R. Effect of heat–moisture treatment on the structure and physicochemical properties of tuber and root starches. Carbohydr. Polym. 2002, 49, 425–437. [Google Scholar] [CrossRef]
- Eliasson, A.-C. Carbohydrates in Food; CRC press: Quebec, QC, Canada, 2006. [Google Scholar]
- Maniglia, B.C.; Tessaro, L.; Ramos, A.P.; Tapia-Blácido, D.R. Which plasticizer is suitable for films based on babassu starch isolated by different methods? Food Hydrocoll. 2019, 89, 143–152. [Google Scholar] [CrossRef]
- Wiącek, A.E. Effect of surface modification on starch biopolymer wettability. Food Hydrocoll. 2015, 48, 228–237. [Google Scholar] [CrossRef]
- Chen, J.; Chen, F.; Meng, Y.; Wang, S.; Long, Z. Oxidized microcrystalline cellulose improve thermoplastic starch-based composite films: Thermal, mechanical and water-solubility properties. Polymer 2019, 168, 228–235. [Google Scholar] [CrossRef]
- Chevalier, E.; Assezat, G.; Prochazka, F.; Oulahal, N. Development and characterization of a novel edible extruded sheet based on different casein sources and influence of the glycerol concentration. Food Hydrocoll. 2018, 75, 182–191. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Whey protein edible films modified with almond and walnut oils. Food Hydrocoll. 2016, 52, 78–86. [Google Scholar] [CrossRef]
- Nayak, S. Biodegradable PBAT/starch nanocomposites. Polym. Plast. Technol. Eng. 2010, 49, 1406–1418. [Google Scholar] [CrossRef]
- Müller, C.M.; Yamashita, F.; Laurindo, J.B. Evaluation of the effects of glycerol and sorbitol concentration and water activity on the water barrier properties of cassava starch films through a solubility approach. Carbohydr. Polym. 2008, 72, 82–87. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds area available from the authors. |

| Sample | Torque max (Nm) | Torque min (Nm) | T min (°C) | Energy (kJ) |
|---|---|---|---|---|
| TPSs | 12.8 | 10.3 | 109.2 | 57.4 |
| TPSs O-3 | 9.9 | 9.4 | 99.6 | 53.7 |
| TPSs O-6 | 8.3 | 8.0 | 95.1 | 43.0 |
| TPSs O-9 | 20.0 | 13.7 | 105.8 | 80.4 |
| TPSs L-3 | 6.2 | 3.2 | 111.7 | 4.7 |
| TPSs L-6 | 9.1 | 5.9 | 89.0 | 35.1 |
| TPSs L-9 | 11.5 | 7.3 | 100.2 | 24.3 |
| TPSg | 47.0 | 31.2 | 93.6 | 166.3 |
| TPSg O-3 | 9.1 | 8.8 | 111.0 | 48.2 |
| TPSg O-6 | 11.4 | 9.5 | 113.5 | 44.3 |
| TPSg O-9 | 32.5 | 30.1 | 98.5 | 156.2 |
| TPSg L-3 | 7.1 | 0.4 | 98.6 | 4.7 |
| TPSg L-6 | 10.8 | 2.3 | 110.0 | 18.3 |
| TPSg L-9 | 38.5 | 13.5 | 84.6 | 119.4 |
| Samples | Starch (wt.%) | Plasticizing (wt.%) | Acid Agent (wt.%) |
|---|---|---|---|
| TPSs | 70.0 | 30.0 | 0 |
| TPSs O-3 | 67.9 | 29.1 | 3 |
| TPSs O-6 | 65.8 | 28.2 | 6 |
| TPSs O-9 | 63.7 | 27.3 | 9 |
| TPSs L-3 | 67.9 | 29.1 | 3 |
| TPSs L-6 | 65.8 | 28.2 | 6 |
| TPSs L-9 | 63.7 | 27.3 | 9 |
| TPSg | 70.0 | 30.0 | 0 |
| TPSg O-3 | 67.9 | 29.1 | 3 |
| TPSg O-6 | 65.8 | 28.2 | 6 |
| TPSg O-9 | 63.7 | 27.3 | 9 |
| TPSg L-3 | 67.9 | 29.1 | 3 |
| TPSg L-6 | 65.8 | 28.2 | 6 |
| TPSg L-9 | 63.7 | 27.3 | 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caicedo, C.; Aguirre Loredo, R.Y.; Fonseca García, A.; Ossa, O.H.; Vázquez Arce, A.; Calambás Pulgarin, H.L.; Ávila Torres, Y. Rheological, Thermal, Superficial, and Morphological Properties of Thermoplastic Achira Starch Modified with Lactic Acid and Oleic Acid. Molecules 2019, 24, 4433. https://doi.org/10.3390/molecules24244433
Caicedo C, Aguirre Loredo RY, Fonseca García A, Ossa OH, Vázquez Arce A, Calambás Pulgarin HL, Ávila Torres Y. Rheological, Thermal, Superficial, and Morphological Properties of Thermoplastic Achira Starch Modified with Lactic Acid and Oleic Acid. Molecules. 2019; 24(24):4433. https://doi.org/10.3390/molecules24244433
Chicago/Turabian StyleCaicedo, Carolina, Rocío Yaneli Aguirre Loredo, Abril Fonseca García, Omar Hernán Ossa, Aldo Vázquez Arce, Heidy Lorena Calambás Pulgarin, and Yenny Ávila Torres. 2019. "Rheological, Thermal, Superficial, and Morphological Properties of Thermoplastic Achira Starch Modified with Lactic Acid and Oleic Acid" Molecules 24, no. 24: 4433. https://doi.org/10.3390/molecules24244433
APA StyleCaicedo, C., Aguirre Loredo, R. Y., Fonseca García, A., Ossa, O. H., Vázquez Arce, A., Calambás Pulgarin, H. L., & Ávila Torres, Y. (2019). Rheological, Thermal, Superficial, and Morphological Properties of Thermoplastic Achira Starch Modified with Lactic Acid and Oleic Acid. Molecules, 24(24), 4433. https://doi.org/10.3390/molecules24244433