Study on the Separation of H2 from CO2 Using a ZIF-8 Membrane by Molecular Simulation and Maxwell-Stefan Model
Abstract
:1. Introduction
2. Materials and Methods
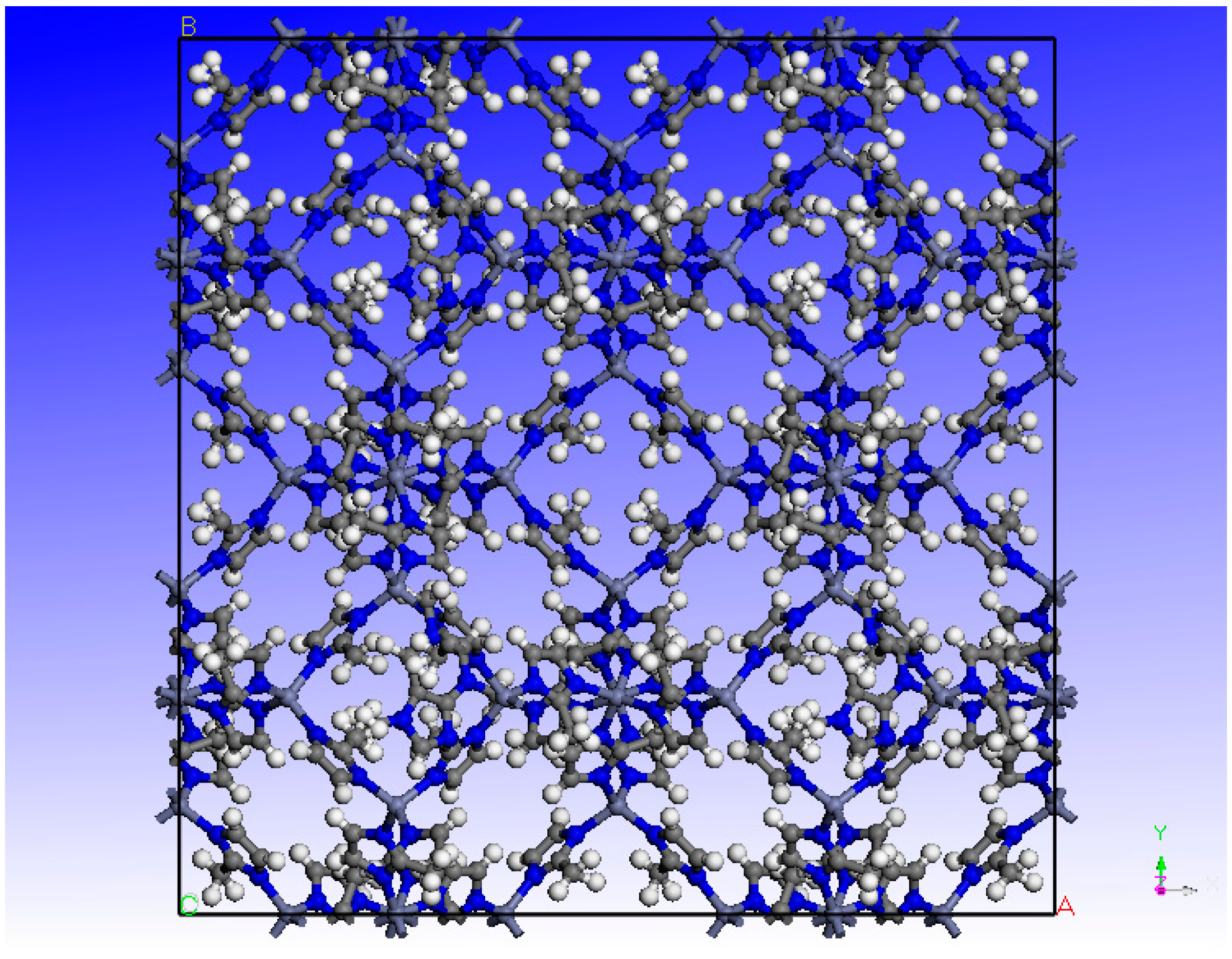
2.1. Molecular Simulation Details
2.2. Maxwell–Stefan Model
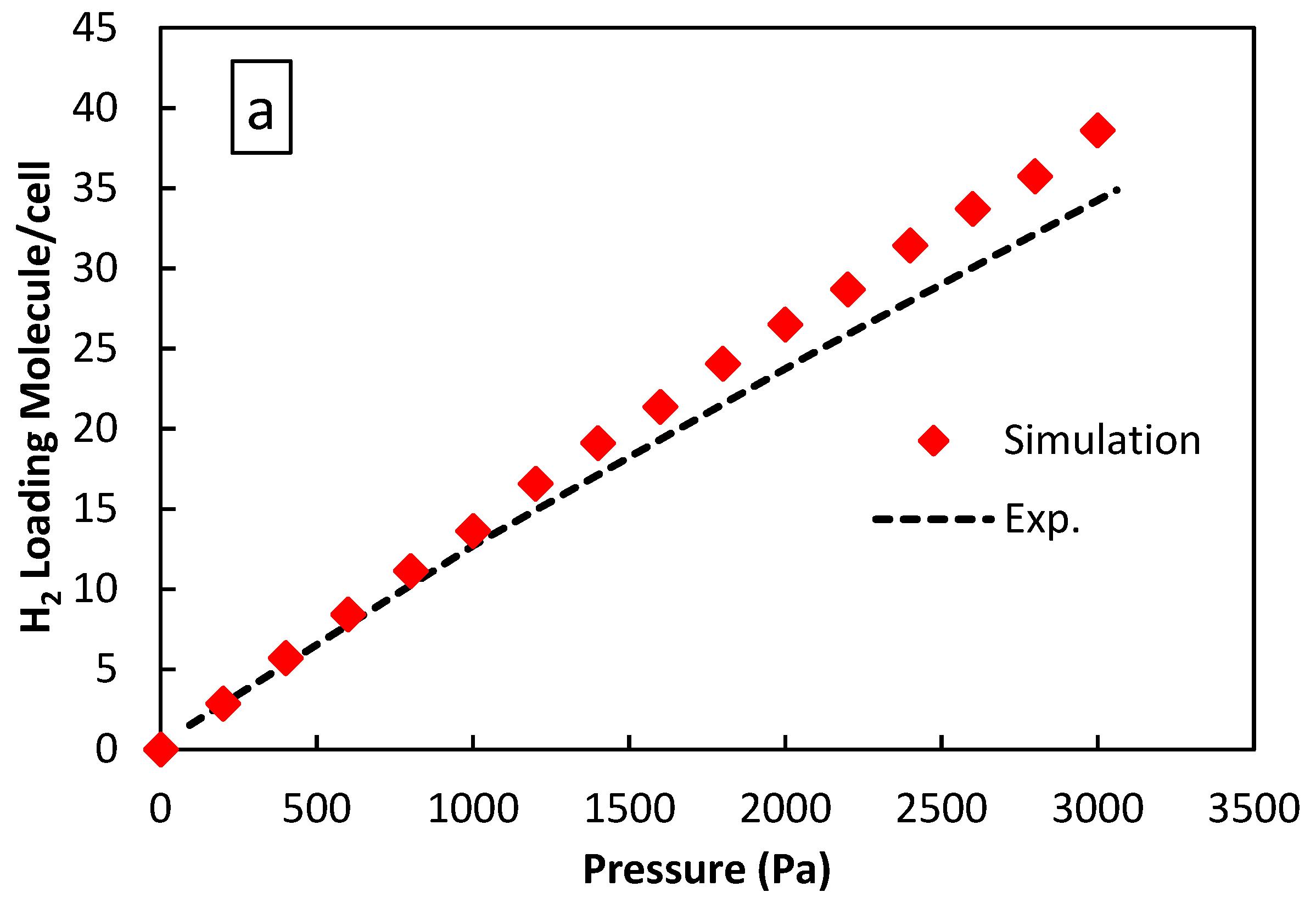
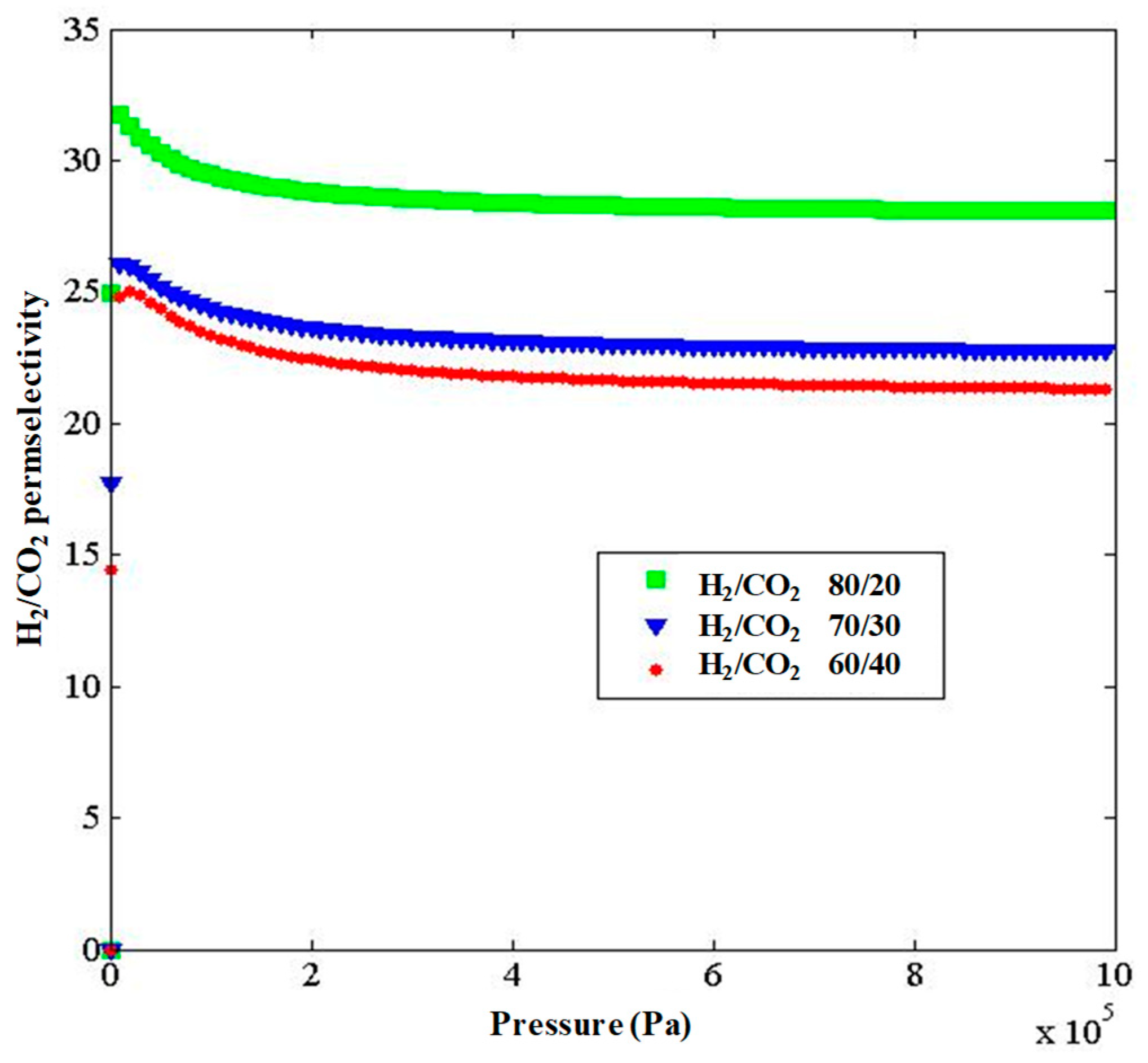
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Handayani, K.; Krozer, Y.; Filatova, T. From fossil fuels to renewables: An analysis of long-term scenarios considering technological learning. Energy Policy 2019, 127, 134–146. [Google Scholar] [CrossRef]
- Dunn, S. Hydrogen futures: Toward a sustainable energy system. Int. J. Hydrog. Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- US Department of Energy. Hydrogen Production Pathways. 2017. Available online: http://energy.gov/eere/fuelcells/hydrogen-production-pathways (accessed on 31 October 2019).
- Biegler, T. The hydrogen economy. Skeptic 2005, 25, 1–29. [Google Scholar]
- Armaroli, N.; Balzani, V. The Hydrogen Issue. ChemSusChem 2011, 4, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Iulianelli, A.; Longo, T.; Liguori, S.; Basile, A. Production of hydrogen via glycerol steam reforming in a Pd-Ag membrane reactor over Co-Al2O3 catalyst. Asia-Pac. J. Chem. Eng. 2010, 5, 138–145. [Google Scholar] [CrossRef]
- Iulianelli, A.; Liguori, S.; Huang, Y.; Basile, A. Model biogas steam reforming in a thin Pd-supported membrane reactor to generate clean hydrogen for fuel cells. J. Power Sources 2015, 273, 25–32. [Google Scholar] [CrossRef]
- Chou, C.-T.; Chen, F.-H.; Huang, Y.-J.; Yang, H.-S. Carbon dioxide capture and hydrogen purification from synthesis gas by pressure swing adsorption. Chem. Eng. Trans. 2013, 32, 1855–1860. [Google Scholar]
- Bredesen, R.; Kumakiri, I.; Peters, T. CO2 capture with membrane systems. In Membrane Operations; Chapter 9; Drioli, E., Giorno, L., Eds.; Wiley-VCH: Weinheim, Germany, 2009; pp. 195–220. [Google Scholar]
- Verweij, H.; Lin, Y.; Dong, J. Microporous silica and zeolite membranes for hydrogen purification. MRS Bull. 2006, 31, 756–764. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Kasik, A.; Li, Z.; Lin, Y.S. Gas separation properties of Metal Organic Framework (MOF-5) membranes. Ind. Eng. Chem. Res. 2013, 52, 1102–1108. [Google Scholar] [CrossRef]
- Bux, H.; Chmelik, C.; van Baten, J.M.; Krishna, R.; Caro, J. Novel MOF-membrane for molecular sieving predicted by IR-diffusion studies and molecular modeling. Adv. Mater. 2010, 22, 4741–4743. [Google Scholar] [CrossRef]
- Fu, J.; Das, S.; Xing, G.; Ben, T.; Valtchev, V.; Qiu, S. Fabrication of COF-MOF composite membranes and their highly selective separation of H2/CO2. J. Am. Chem. Soc. 2016, 138, 7673–7680. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, T.; Lestari, G.; Lai, Z. Effective separation of propylene/propane binary mixtures by ZIF-8 membranes. J. Membr. Sci. 2012, 390, 93–98. [Google Scholar] [CrossRef]
- Lai, L.S.; Yeong, Y.F.; Lau, K.K.; Shariff, A.M. CO2 and CH4 permeation through zeolitic imidazolate framework (ZIF)-8 membrane synthesized via in situ layer-by-layer growth: An experimental and modeling study. RSC Adv. 2015, 5, 79098–79106. [Google Scholar] [CrossRef]
- Verploegh, R.J.; Nair, S.; Sholl, D.S. Temperature and loading-dependent diffusion of light hydrocarbons in ZIF-8 as predicted through fully flexible molecular simulations. J. Am. Chem. Soc. 2015, 137, 15760–15771. [Google Scholar] [CrossRef]
- Chokbunpiam, T.; Chanajaree, R.; Saengsawang, O.; Reimann, S.; Chmelik, C.; Fritzsche, S.; Caro, J.; Remsungnen, T.; Hannongbua, S. The importance of lattice flexibility for the migration of ethane in ZIF-8: Molecular dynamics simulations. Microp. Mesop. Mater. 2013, 174, 126–134. [Google Scholar] [CrossRef]
- Krishna, R.; Wesselingh, J. The Maxwell-Stefan approach to mass transfer. Chem. Eng. Sci. 1997, 52, 861–911. [Google Scholar] [CrossRef]
- Kapteijn, F.; Moulijn, J.; Krishna, R. The generalized Maxwell–Stefan model for diffusion in zeolites: Sorbate molecules with different saturation loadings. Chem. Eng. Sci. 2000, 55, 2923–2930. [Google Scholar] [CrossRef]
- Krishna, R.; van den Broeke, L.J.P. The Maxwell-Stefan description of mass transport across zeolite membranes. Chem. Eng. J. 1995, 57, 155–162. [Google Scholar] [CrossRef]
- Kapteijn, F.; Bakker, W.J.W.; Zheng, G.; Poppe, J.; Moulijn, J.A. Permeation and separation of light hydrocarbons through a silicalite-1 membrane. Application of the generalized Maxwell-Stefan equations. Chem. Eng. J. 1995, 57, 145–153. [Google Scholar]
- Krishna, R. Multicomponent surface diffusion of adsorbed species: A description based on the generalized Maxwell-Stefan equations. Chem. Eng. Sci. 1990, 45, 1779–1791. [Google Scholar] [CrossRef]
- Bakker, W.J.W.; Zheng, G.; Kapteijn, F.; Kakkee, M.; Moulijn, J.A. Single and Multicomponent Transport through Metal-Supported MFI Zeolite Membranes; Weijnen, M.P.C., Drinkenburg, A.A.H., Eds.; Precision Process Technology: Kluwer, The Netherlands, 1993; pp. 425–436. [Google Scholar]
- Krishna, R. Problems and pitfalls in the use of the Fick formulation for intraparticle diffusion. Chem. Eng. Sci. 1993, 48, 845–861. [Google Scholar] [CrossRef]
- Rahmati, M. A molecular simulation of natural gas dehydration by 3A zeolite nanostructure. Iran. J. Oil Gas Sci. Technol. 2017, 6, 68–78. [Google Scholar]
- Cheng, P.; Hu, Y.H. H2O-functionalized zeolitic Zn (2-methylimidazole) 2 framework (ZIF-8) for H2 storage. J. Phys. Chem. C 2014, 118, 21866–21872. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Y.; Xia, Q.; Li, Z.; Xi, H. Experimental and molecular simulation studies of CO2 adsorption on zeolitic imidazolate frameworks: ZIF-8 and amine-modified ZIF-8. Adsorption 2013, 19, 25–37. [Google Scholar] [CrossRef]
- Hertäg, L.; Bux, H.; Caro, J.; Chmelik, C.; Remsungnen, T.; Knauth, M.; Fritzsche, S. Diffusion of CH4 and H2 in ZIF-8. J. Membr. Sci. 2011, 377, 36–41. [Google Scholar] [CrossRef]
- Pusch, A.-K.; Splith, T.; Moschkowitz, L.; Karmakar, S.; Biniwale, R.; Sant, M.; Suffritti, G.B.; Demontis, P.; Cravillon, J.; Pantatosaki, E. NMR studies of carbon dioxide and methane self-diffusion in ZIF-8 at elevated gas pressures. Adsorption 2012, 18, 359–366. [Google Scholar] [CrossRef]
- Demontis, P.; Gulín-González, J.; Jobic, H.; Suffritti, G.B. Diffusion of water in zeolites Na A and NaCa A: A molecular dynamics simulation study. J. Phys. Chem. C 2010, 114, 18612–18621. [Google Scholar] [CrossRef]
- Du, X. Molecular-Dynamics Simulation of self-diffusion of molecular hydrogen in x-type zeolite. J. Phys. Chem. 2013, 2013, 545367. [Google Scholar] [CrossRef]
- Khanmohammadi, H.; Bayati, B.; Rahbar-Shahrouzi, J.; Babaluo, A.-A.; Ghorbani, A. Molecular simulation of the ion exchange behavior of Cu2+, Cd2+ and Pb2+ ions on different zeolites exchanged with sodium. J. Environ. Chem. Eng. 2019, 7, 103040. [Google Scholar] [CrossRef]
- Zito, P.F.; Caravella, A.; Brunetti, A.; Drioli, E.; Barbieri, G. Knudsen and surface diffusion competing for gas permeation inside silicalite membranes. J. Membr. Sci. 2017, 523, 456–469. [Google Scholar] [CrossRef]
- Bakker, W.J.W.; van den Broeke, L.J.P.; Kapteijn, F.; Moulijn, J.A. Temperature dependence of one-component permeation through a silicalite-1 membrane. AIChE J. 1997, 43, 2203–2214. [Google Scholar] [CrossRef]
- Zito, P.F.; Caravella, A.; Brunetti, A.; Drioli, E.; Barbieri, G. Discrimination among gas translation, surface and Knudsen diffusion in permeation through zeolite membranes. J. Membr. Sci. 2018, 564, 166–173. [Google Scholar] [CrossRef]
- Van den Bergh, J.; Tihaya, A.; Kapteijn, F. High temperature permeation and separation characteristics of an all-silica DDR zeolite membrane. Microp. Mesop. Mater. 2010, 132, 137–147. [Google Scholar] [CrossRef]
- Caravella, A.; Zito, P.F.; Brunetti, A.; Drioli, E.; Barbieri, G. A novel modelling approach to surface and Knudsen multicomponent diffusion through NaY zeolite membranes. Microp. Mesop. Mater. 2016, 235, 87–99. [Google Scholar] [CrossRef]
- Zito, P.F.; Brunetti, A.; Caravella, A.; Drioli, E.; Barbieri, G. Mutual influence in permeation of CO2-containing mixtures through a SAPO-34 membrane. J. Membr. Sci. 2019, in press. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Component | Langmuir Model | R2 | Diffusion Coefficient (m2/s) | |
|---|---|---|---|---|
| This Work | Literature | |||
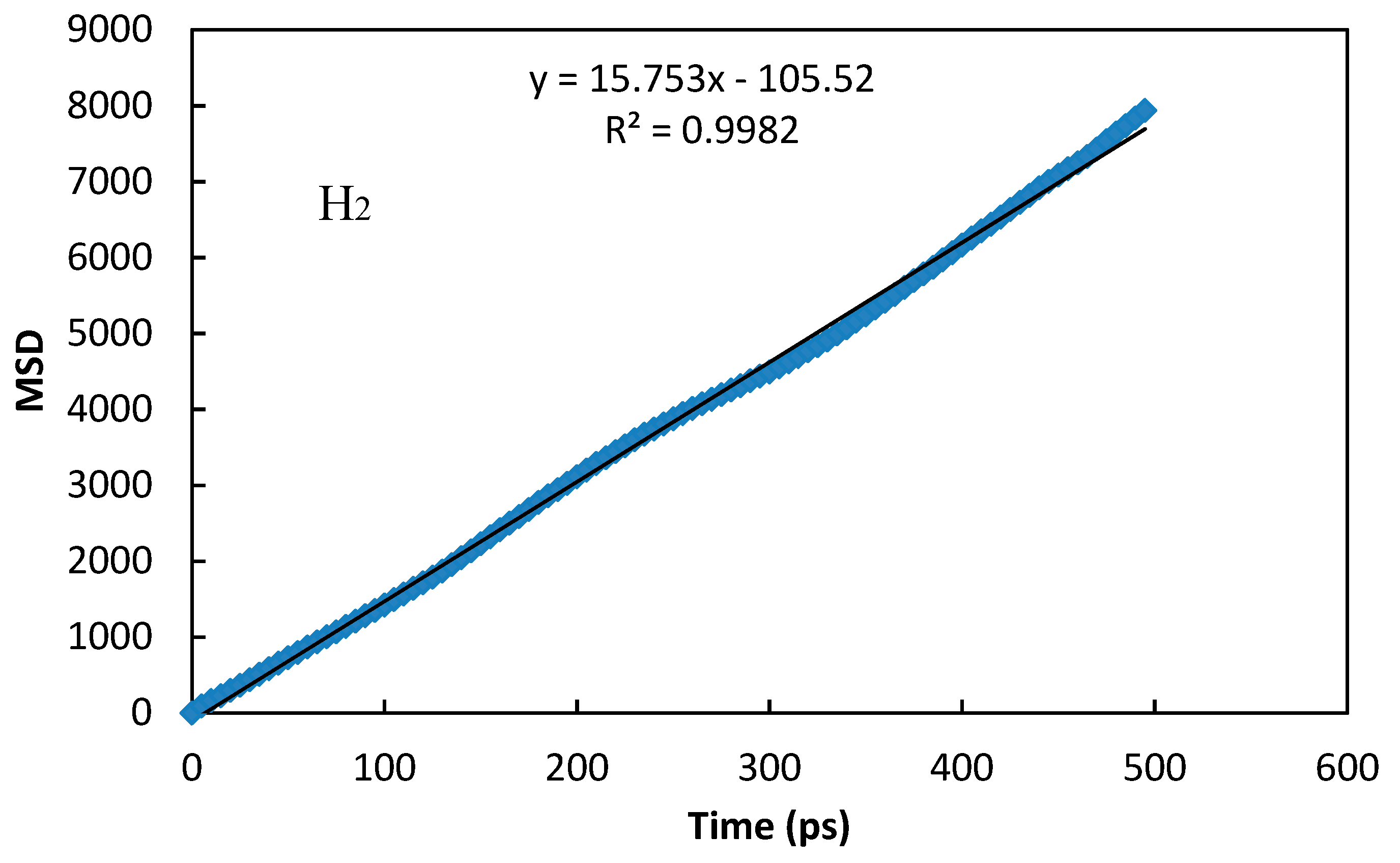
| H2 | Θs = 356; b = 4.02 × 10−5 | 0.999 | 2.62 × 10−8 | 2.5 × 10−8 [28] |
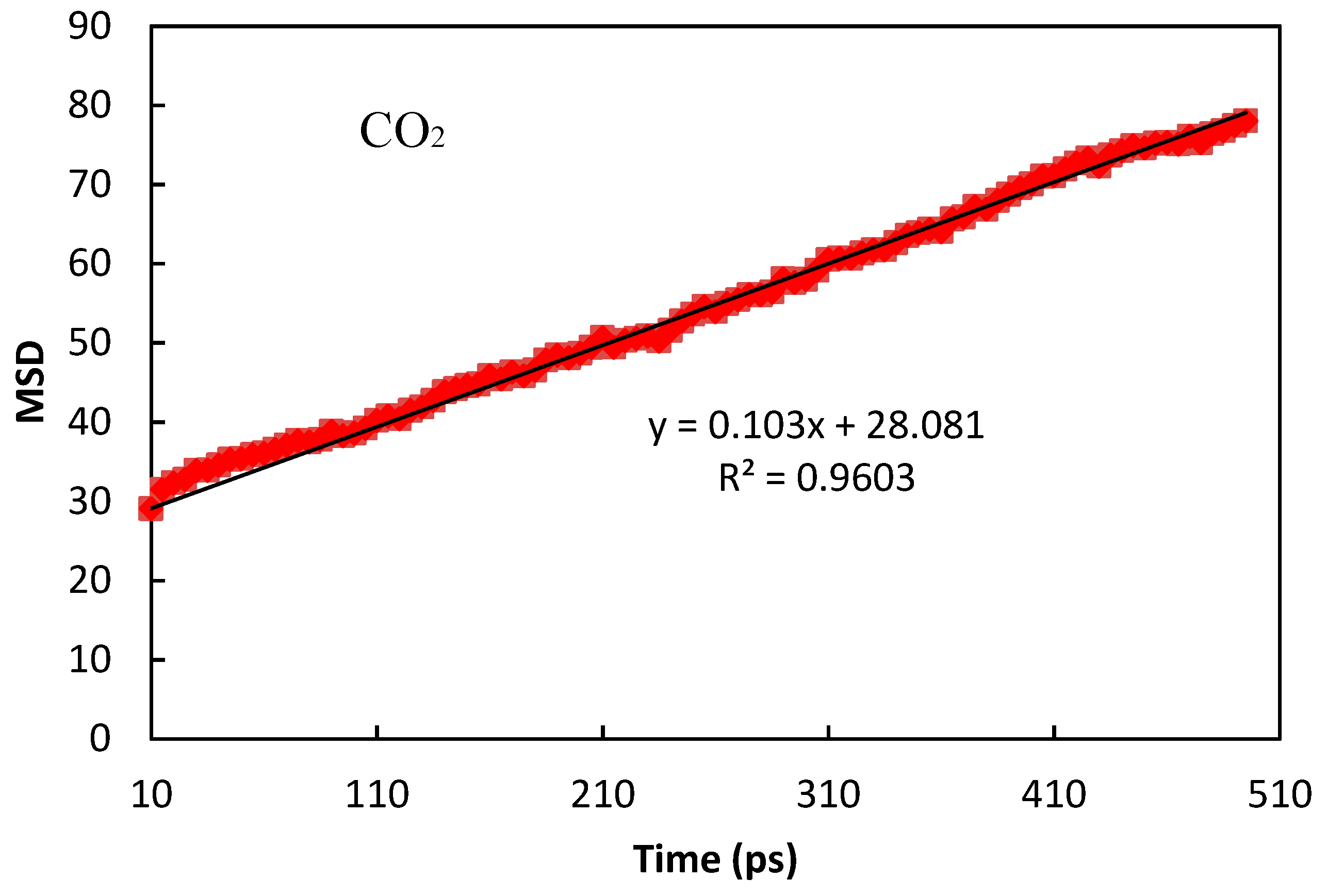
| CO2 | Θs = 127; b = 1.31 × 10−3 | 0.998 | 1.71 × 10−10 | 2.2 × 10−10 [29] |
| DH2 [m2/s] | DCO2 [m2/s] | Membrane | Ref. |
|---|---|---|---|
| 2.62 × 10−8 | 1.71 × 10−10 | ZIF-8 | This work |
| 1.73 × 10−8 | 5.22 × 10−10 | Silicalite | [33] |
| 5.06 × 10−8 | 1.45 × 10−10 | Silicalite | [34] |
| 1.33 × 10−9 | 8.82 × 10−11 | DDR zeolite | [35] |
| 5.01 × 10−9 | 1.58 × 10−11 | DDR zeolite | [36] |
| 5.79 × 10−10 | 4.12 × 10−11 | NaY zeolite | [37] |
| 1.27 × 10−9 | 1.23 × 10−10 | SAPO-34 | [38] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayati, B.; Ghorbani, A.; Ghasemzadeh, K.; Iulianelli, A.; Basile, A. Study on the Separation of H2 from CO2 Using a ZIF-8 Membrane by Molecular Simulation and Maxwell-Stefan Model. Molecules 2019, 24, 4350. https://doi.org/10.3390/molecules24234350
Bayati B, Ghorbani A, Ghasemzadeh K, Iulianelli A, Basile A. Study on the Separation of H2 from CO2 Using a ZIF-8 Membrane by Molecular Simulation and Maxwell-Stefan Model. Molecules. 2019; 24(23):4350. https://doi.org/10.3390/molecules24234350
Chicago/Turabian StyleBayati, Behrouz, Asma Ghorbani, Kamran Ghasemzadeh, Adolfo Iulianelli, and Angelo Basile. 2019. "Study on the Separation of H2 from CO2 Using a ZIF-8 Membrane by Molecular Simulation and Maxwell-Stefan Model" Molecules 24, no. 23: 4350. https://doi.org/10.3390/molecules24234350
APA StyleBayati, B., Ghorbani, A., Ghasemzadeh, K., Iulianelli, A., & Basile, A. (2019). Study on the Separation of H2 from CO2 Using a ZIF-8 Membrane by Molecular Simulation and Maxwell-Stefan Model. Molecules, 24(23), 4350. https://doi.org/10.3390/molecules24234350








