Chemometric Characterization of Strawberries and Blueberries according to Their Phenolic Profile: Combined Effect of Cultivar and Cultivation System
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Phenolic Content (TPC), Radical-Scavenging Activity (RSA), and Total Anthocyanin Content (TAC) Results
2.2. Determination of Phenolic Profile using UHPLC-LTQ Orbitrap MS4 Technique
2.3. Differences in Strawberry and Blueberry Phenolic Profilesfrom Fruits and Leaves
2.4. Phenolic Profiles of Plants from Organic and Integrated Production
2.4.1. Blueberry Fruits
2.4.2. Strawberry Fruits
2.4.3. Blueberry Leaves
2.4.4. Strawberry Leaves
2.5. Cis, trans-Abscisic Acid
2.6. Leaf (Source)-Fruit (Sink) Relationship
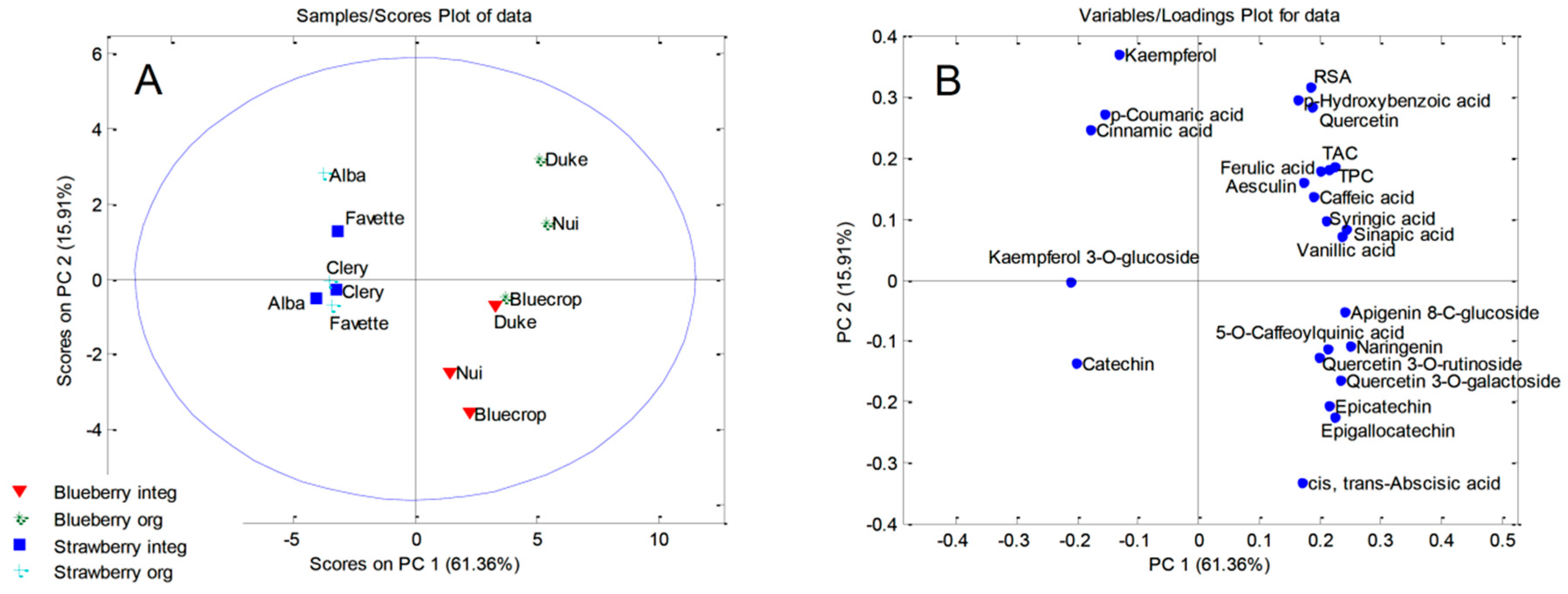
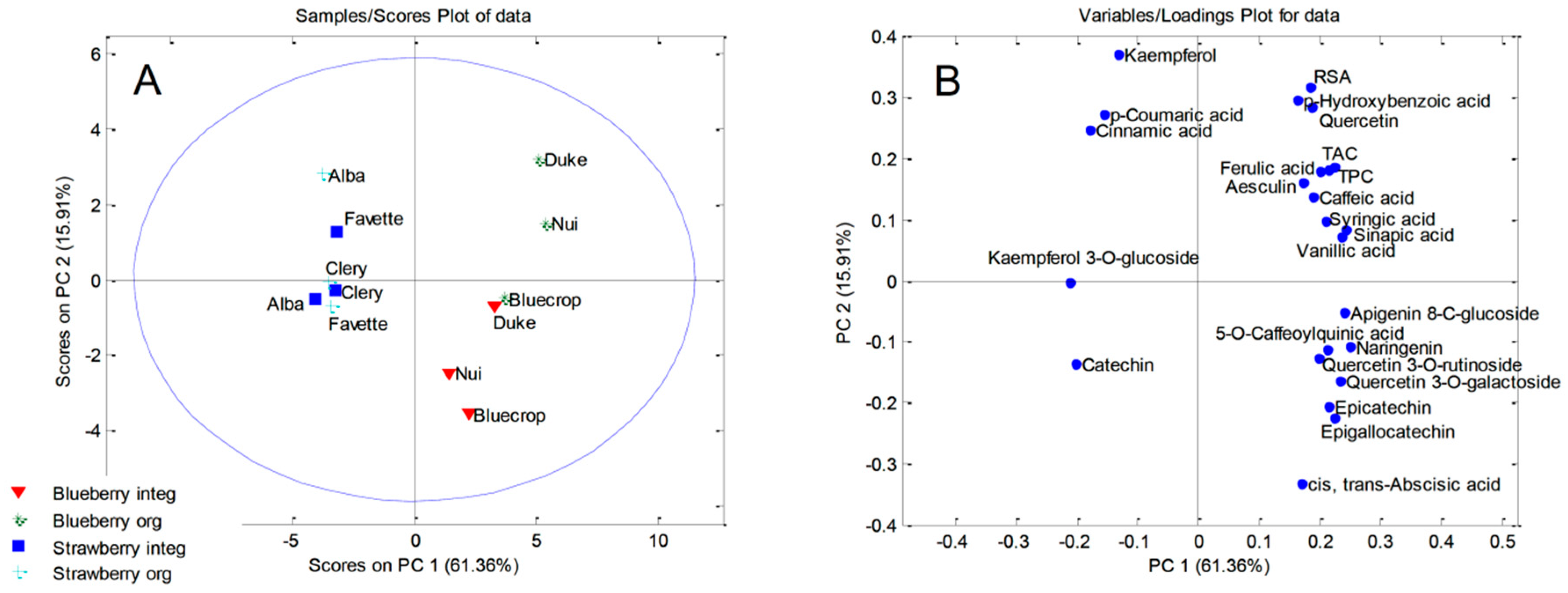
2.7. Principal Component Analysis
3. Materials and Methods
3.1. Plant Material
3.2. Extraction of Phenols from Fruits and Leaves
3.3. Spectrophotometric Determinations
3.4. UHPLC—LTQ Orbitrap MS4
3.5. Statistic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fotirić Akšić, M.; Tosti, T.; Sredojević, M.; Milivojević, J.; Meland, M.; Natić, M. Comparison of sugar profile between leaves and fruits of blueberry and strawberry cultivars grown in organic and integrated production system. Plants 2019, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Vittori, L.D.; Mazzoni, L.; Battino, M.; Mezzetti, B. Pre-harvest factors influencing the quality of berries. Sci. Hortic. Amsterdam 2018, 233, 310–322. [Google Scholar] [CrossRef]
- Milivojević, J.; Radivojevic, D.; Ruml, M.; Dimitrijevic, M.; Dragisic Maksimovic, J. Does microclimate under grey colored hail protection net affect biological and nutritional properties of ′Duke′ highbush blueberry (V. corymbosum L.)? Fruits 2016, 71, 161–170. [Google Scholar] [CrossRef]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Kizos, T.; Veikontis, G.; Marín-Guirao, J.I. Comparison of organic and integrated farming systems: The case of sultana table grapes in Korinthos, Greece. J. Sustain. Agric. 2011, 35, 27–47. [Google Scholar] [CrossRef]
- Stockdale, E.A.; Lampkin, N.H.; Hovi, M.; Keatinge, R.; Lennartsson, E.K.M.; Macdonald, D.W.; Padel, S.; Tattersall, F.H.; Wolfe, M.S.; Watson, C.A. Agronomic and environmental implications of organic farming systems. Adv. Agron. 2001, 70, 261–327. [Google Scholar]
- Ochmian, I.; Kozos, K.; Chelpiñski, P.; Szczepanek, M. Comparison of berry quality in highbush blueberry cultivars grown according to conventional and organic methods. Turk. J. Agric. For. 2015, 39, 174–181. [Google Scholar] [CrossRef]
- Granatstein, D.; Kirby, E.; Willer, H. Global area and trends of organic fruit production. Acta Hortic. 2013, 1001, 383–394. [Google Scholar] [CrossRef]
- The World of Organic Agriculture. Statistics and Emerging Trends. Available online: https://shop.fibl.org/CHen/mwdownloads/download/link/id/1202/?ref=1 (accessed on 29 October 2019).
- You, Q.; Wang, B.; Chen, F.; Huang, Z.; Wang, X.; Luo, P.G. Comparison of anthocyanins and phenolics in organically and conventionally grown blueberries in selected cultivars. Food Chem. 2011, 125, 201–208. [Google Scholar] [CrossRef]
- Olsson, M.E.; Andersson, C.S.; Oredsson, S.; Berglund, R.H.; Gustavsson, K. Antioxidant levels and inhibition of cancer cell proliferation in vitro by extracts from organically and conventionally cultivated strawberries. J. Agric. Food Chem. 2006, 54, 1248–1255. [Google Scholar] [CrossRef]
- Brandt, K.; Mølgaard, J.P. Organic agriculture: Does it enhance or reduce the nutritional value of plant foods. J. Sci. Food Agric. 2001, 81, 924–931. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Milivojević, J.; Rakonjac, V.; Fotirić Akšić, M.; Bogdanović Pristov, J.; Maksimović, V. Classification and fingerprinting of different berries based on biochemical profiling and antioxidant capacity. Pesqui. Agropecu. Bras. 2013, 48, 1285–1294. [Google Scholar] [CrossRef]
- Kalt, W.; Lawand, C.; Ryan, D.; McDonald, J.E.; Donner, H. Oxygen radical absorbing capacity, anthocyanin and phenolic content of highbush blueberries (Vaccinium corymbosum, L.), during ripening and storage. J. Am. Soc. Hortic. Sci. 2003, 128, 917–923. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Calò, R.; Marabini, L. Protective effect of Vaccinium myrtillus extract against UVA- and UVB-induced damage in a human keratinocyte cell line (HaCaT cells). J. Photochem. Photobiol. B. 2014, 132, 27–35. [Google Scholar] [CrossRef]
- Milivojević, J.; Maksimović, V.; Dragišić Maksimović, J.; Radivojević, D.; Poledica, M.; Ercişli, S. A comparison of major taste- and health-related compounds of Vaccinium berries. Turkish, J. Biol. 2012, 36, 738–745. [Google Scholar]
- Rostamian, V.; Shakeri, F.; Estakhr, J. The effect of hydro-alcoholic extract of strawberry leaf on sugar and lipids in serum of diabetic rats. Pharmacology Online 2011, 3, 1171–1175. [Google Scholar]
- Mandave, P.; Rani, S.; Kuvalekar, A.; Ranjekar, P. Antiglycation, antioxidant and antidiabetic activity of mature strawberry (Fragaria x ananassa) fruts. Int. J. Appl. Biol. Pharm. 2013, 4, 168–177. [Google Scholar]
- Routray, W.; Orsat, V. MAE of phenolic compounds from blueberry leaves and comparison with other extraction methods. Ind. Crop. Prod. 2014, 58, 36–45. [Google Scholar] [CrossRef]
- Yuji, K.; Sakaida, H.; Kai, T.; Fukuda, N.; Yukizaki, C.; Sakai, M.; Tsubouchi, H.; Kataoka, H. Effect of dietary blueberry (Vaccinium ashei Reade) leaves on serum and hepatic lipid levels in rats. J. Oleo Sci. 2013, 62, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Vyas, P.; Kalidindi, S.; Chibrikova, L.; Igamberdiev, A.U.; Weber, J.T. Chemical analysis and effect of blueberry and lingonberry fruits and leaves against glutamate-mediated excitotoxicity. J. Agric. Food Chem. 2013, 61, 7769–7776. [Google Scholar] [CrossRef] [PubMed]
- Sakaida, H.; Nagao, K.; Higa, K.; Shirouchi, B.; Inoue, N.; Hidaka, F.; Kai, T.; Yanagita, T. Effect of Vaccinium ashei Reade leaves on angiotensin converting enzyme activity in vitro and on systolic blood pressure of spontaneously hypertensive rats In Vivo. Biosci. Biotechnol. Biochem. 2007, 71, 2335–2337. [Google Scholar] [CrossRef] [PubMed]
- Martineau, L.C.; Couture, A.; Spoor, D.; Benhaddou-Andaloussi, A.; Harris, C.; Meddah, B.; Leduc, C.; Burt, A.; Vuong, T.; Le Mai, P.; et al. Anti-diabetic properties of the Canadian low bush blueberry Vaccinium angustifolium Ait. Phytomedicine 2006, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Naglaa, M.; Saleh, A. Influence of ethanolic extract of strawberry leaves for abrogating bromate hazards in male rats. J. Basic Appl. Zool. 2019, 80, 1–19. [Google Scholar]
- Ibrahim, D.S.; Abd El-Maksoud, M.A.E. Effect of strawberry (Fragaria ananassa) leaf extract on diabetic nephropathy in rats. Int. J. Exp. Pathol. 2015, 96, 87–93. [Google Scholar] [CrossRef]
- Kårlund, A.; Salminen, J.P.; Koskinen, P.; Ahern, J.R.; Karonen, M.; Tiilikkala, K.; Karjalainen, R.O. Polyphenols in strawberry (Fragaria x ananassa) leaves induced by plant activators. J. Agric. Food Chem. 2014, 62, 4592–4600. [Google Scholar] [CrossRef]
- Okan, O.T.; Deniz, I.; Yayli, N.; ¸Sat, I.G.; Öz, M.; Hatipo˘gluSerdar, G. Antioxidant activity, sugar content and phenolic profiling of blueberries cultivars: A comprehensive. Not. Bot. Horti. Agrobo. 2018, 46, 639–652. [Google Scholar] [CrossRef]
- Mezzetti, B.; Balducci, F.; Capocasa, F.; Cappelletti, R.; Di Vittori, L.; Mazzoni, L.; Giampieri, F.; Battino, M. Can we breed a healthier strawberry and claim it? Acta Hortic. 2016, 1117, 7–14. [Google Scholar] [CrossRef]
- Capocasa, F.; Scalzo, J.; Mezzetti, B.; Battino, M. Combining quality and antioxidant attributes in the strawberry: The role of genotype. Food Chem. 2008, 111, 872–878. [Google Scholar] [CrossRef]
- Wang, S.; Chen, C.-T.; Sciarappa, W.; Wang, C.; Camp, M. Fruit quality, antioxidant capacity and flavonoid content of organically and conventionally grown blueberries. J. Agric. Food Chem. 2008, 56, 5788–5794. [Google Scholar] [CrossRef] [PubMed]
- Aaby, K.; Ekeberg, D.; Skrede, G. Characterization of phenolic compounds in strawberry (Fragaria ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J. Agric. Food Chem. 2007, 55, 4395–4406. [Google Scholar] [CrossRef] [PubMed]
- Motilva, M.J.; Serra, A.; Macià, A. Analysis of food polyphenols by ultra high-performance liquid chromatography coupled to mass spectrometry: An overview. J. Chromatogr. A 2013, 1292, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Castrejớn, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum, L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Mikulic Petkovsek, M.; Stampar, F.; Veberic, R. The effect of prohexadione-calcium on the phenolic content in developing fruits and leaves of apple trees. J. Food Agric. Environ. 2009, 7, 369–375. [Google Scholar]
- Kitano, M.; Araki, T. Environmental effects on dynamics of fruit growth and photo assimilate translocation in tomato plants (2)-Analysis of phloem sap and xylem sap fluxes and fruit water balance. Environ. Control Biol. 2001, 39, 43–51. [Google Scholar] [CrossRef]
- Dragišić Maksimović, J.; Poledica, M.; Mutavdžić, D.; Mojović, M.; Radivojević, D.; Milivojević, J. Variation in nutritional quality and chemical composition of fresh strawberry fruit: Combined effect of cultivar and storage. Plant Foods Hum. Nutr. 2015, 70, 77–84. [Google Scholar] [CrossRef]
- Connor, A.M.; Luby, J.J.; Tong, C.B.S.; Finn, C.E.; Hancock, J.F. Variation and heritability estimates for antioxidant activity. Total phenolic content and anthocyanin content in blueberry progenies. J. Am. Soc. Hortic. Sci. 2002, 1, 82–88. [Google Scholar] [CrossRef]
- Jin, P.; Wang, S.Y.; Wang, C.Y.; Zheng, Y. Effect of cultural system and storage temperature on antioxidant capacity and phenolic compounds in strawberries. Food Chem. 2011, 124, 262–270. [Google Scholar] [CrossRef]
- Prodorutti, D.; Pertot, I.; Giongo, L.; Gessler, C. Highbush blueberry: Cultivation, protection, breeding and biotechnology. Eur. J. Plant Sci. Biotechnol. 2007, 1, 44–56. [Google Scholar]
- Häkkinen, S.; Tȍrrȍnen, A.R. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: Influence of cultivar, cultivation site and technique. Food Res. Int. 2000, 33, 517–524. [Google Scholar] [CrossRef]
- Tomić, J.; Milivojević, J.; Pešaković, M. The response to bacterial inoculation is cultivar-related in strawberries. Turk. J. Agric. For. 2015, 39, 332–334. [Google Scholar] [CrossRef]
- Weibel, F.P.; Bickel, R.; Leuthold, S.; Alfoeldi, T.; Niggli, U. Are organically grown apples tastier and healthier? A comparative field study using conventional and alternative methods to measure fruit quality. Acta Hortic. 2000, 517, 417–426. [Google Scholar] [CrossRef]
- Panico, A.M.; Garufi, F.; Nitto, S.; Di Mauro, R.; Longhitano, R.C.; Magrı, G.; Catalfo, A.; Serrentino, M.E.; De Guidi, G. Antioxidant activity and phenolic content of strawberry genotypes from Fragaria x ananassa. Pharm. Biol. 2009, 47, 203–208. [Google Scholar] [CrossRef]
- Crespo, P.; Bordonaba, J.G.; Terry, L.A.; Carlen, C. Characterization of major taste and health-related compounds of four strawberry genotypes grown at different Swiss production sites. Food Chem. 2010, 122, 16–24. [Google Scholar] [CrossRef]
- Stevenson, D.; Scalzo, J. Anthocyanin composition and content of blueberries from around the world. J. Berry Res. 2012, 2, 179–189. [Google Scholar]
- Cezarotto, V.S.; Giacomelli, S.R.; Vendruscolo, M.H.; Vestena, A.S.; Cezarotto, C.S.; Cruz, R.C.; Maurer, L.H.; Ferreira, L.M.; Emanuelli, T.; Cruz, L. Influence of harvest season and cultivar on the variation of phenolic compounds composition and antioxidant properties in Vaccinium ashei Leaves. Molecules 2017, 22, 1603. [Google Scholar] [CrossRef]
- Wu, H.; Chai, Z.; Hutabarat, R.P.; Zeng, Q.; Niu, L.; Li, D.; Yu, H.; Huang, W. Blueberry leaves from 73 different cultivars in southeastern China as nutraceutical supplements rich in antioxidants. Food Res. Int. 2019, 122, 548–560. [Google Scholar] [CrossRef]
- Grace, M.; Xiong, J.; Esposito, D.; Ehlenfeldt, M.; Lila, M. Simultaneous LC-MS quantification of anthocyanins and non-anthocyanin phenolics from blueberries with widely divergent profiles and biological activities. Food Chem. 2019, 227, 336–346. [Google Scholar] [CrossRef]
- Buřičová, L.; Andjelkovic, M.; Čermáková, A.; Réblová, Z.; Jurček, O.; Kolehmainen, E.; Verhé, R.; Kvasnička, F. Antioxidant capacity and antioxidants of strawberry, blackberry, and raspberry leaves. Czech, J. Food Sci. 2011, 29, 181–189. [Google Scholar] [CrossRef]
- Ryan, J.J. Flavonol glycosides of cultivated strawberry. J. Food Sci. 1971, 36, 867–870. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Hayirlioglu-Ayaz, S.; Gruz, J.; Novak, O.; Strnad, M. Separation, Characterization, and Quantitation of Phenolic Acids in a Little-Known Blueberry (Vaccinium arctostaphylos L.) Fruit by HPLC-MS. J. Agric. Food Chem. 2005, 53, 8116–8122. [Google Scholar] [CrossRef] [PubMed]
- La Barbera, G.; Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; Samperi, R.; Chiozzi, R.Z.; Laganà, A. Comprehensive polyphenol profiling of a strawberry extract (Fragaria x ananassa) by ultra-high-performance liquid chromatography coupled with high-resolution mass spectrometry. Anal. Bioanal. Chem. 2017, 409, 2127–2142. [Google Scholar] [CrossRef] [PubMed]
- Ablajan, K.; Abliz, A.; Shang, X.Y.; He, J.M.; Rui- Ping Zhang, R.P.; Shi, J.G. Structural characterization of flavonol 3,7- di-O glycosides and determination of the glycosylation position by using negative ion electrospray ionisation tandem mass spectrometry. J. Mass Spectrom. 2006, 41, 352–360. [Google Scholar] [CrossRef]
- Cuyckens, F.; Claeys, M. Determination of the glycosylation site in flavonoid mono-O-glycosides by collision-induced dissociation of electrospray-generated deprotonated and sodiated molecules. J. Mass Spectrom. 2005, 40, 364–372. [Google Scholar] [CrossRef]
- Mondolot, L.; La Fisca, P.; Buatois, B.; Talansier, E.; De Kochko, A.; Campa, C. Evolution in caffeoylquinic acid content and histolocalization during Coffea canephora leaf development. Ann. Bot. 2006, 98, 33–40. [Google Scholar] [CrossRef]
- Wang, L.; Yue, Z.; Guo, M.; Fang, L.; Bai, L.; Li, X.; Tao, Y.; Wang, S.; Liu, Q.; Zhi, D.; et al. Dietary flavonoid hyperoside induces apoptosis of activated human LX-2 hepatic stellate cell by suppressing canonical NF-κB signaling. Biomed. Res. Int. 2016, 2016, 1068528. [Google Scholar] [CrossRef]
- Zimmer, K.R.; Blum-Silva, C.H.; Souza, A.L.K.; Wulffschuch, M.; Reginatto, H.F.; Pereira, C.M.P.; Macedo, A.J.; Lencina, L.C. The antibiofilm effect of blueberry fruit cultivars against Staphylococcus epidermidis and Pseudomonas aeruginosa. J. Med. Food 2014, 17, 324–331. [Google Scholar] [CrossRef]
- Yang, G.; Yue, J.; Gong, X.; Qian, B.; Wang, H.; Deng, Y.; Zhao, Y. Blueberry leaf extracts incorporated chitosan coatings for preserving postharvest quality of fresh blueberries. Postharvest Biol. Technol. 2014, 92, 46–53. [Google Scholar] [CrossRef]
- Howard, L.R.; Clark, J.R.; Brownmiller, C. Antioxidant capacity and phenolic content in blueberries as affected by genotype and growing season. J. Sci. Food Agric. 2003, 83, 1238–1247. [Google Scholar] [CrossRef]
- Wang, S.Y.; Millner, P. Effect of different cultural systems on antioxidant capacity, phenolic content, and fruit quality of strawberries (Fragaria x aranassaDuch.). J. Agric. Food Chem. 2009, 57, 9651–9657. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef]
- Skupień, K.; Oszmianski, J.; Kostrzewa-Nowak, D.; Tarasiuk, J. In vitro anti-leukemic activity of extracts from berry plant leaves against sensitive and multidrug resistant HL60 cells. Cancer Lett. 2006, 236, 282–291. [Google Scholar] [CrossRef]
- Harris, C.S.; Burt, A.J.; Saleem, A.; Le, P.M.; Martineau, L.C.; Haddad, P.S.; Bennett, S.A.; Arnason, J.T. A single HPLC-PAD-APCI/MS method for the quantitative comparison of phenolic compounds found in leaf, stem, root and and fruit extracts of Vaccinium angustifolium. Phytochem. Anal. 2007, 18, 161–169. [Google Scholar] [CrossRef]
- Oszmianski, J.; Wojdylo, A.; Gorzelany, J.; Kapusta, I. Identification and characterization of low molecular weight polyphenols in berry leaf extracts by HPLC-DAD and LC-ESI/MS. J. Agric. Food Chem. 2011, 59, 12830–12835. [Google Scholar] [CrossRef]
- Li, C.; Feng, J.; Huang, W.-Y.; An, X.-T. Composition of polyphenols and antioxidant activity of rabbiteye blueberry (Vaccinium ashei) in Nanjing. J. Agric. Food Chem. 2013, 61, 523–531. [Google Scholar] [CrossRef]
- Kaur, T.; Bhat, H.A.; Bhat, R.; Kumar, A.; Bindu, K.; Koul, S.; Vyas, D. Physio-chemical and antioxidant profiling of Salvia sclarea L. at different climates in north-western Himalayas. Acta Physiol. Plant. 2015, 37, 132. [Google Scholar] [CrossRef]
- Cheel, J.; Tumova, L.; Areche, C.; Van Antwerpen, P.; Neve, J.; Zouaoui-Boudjeltia, K.; Martin, A.S.; Vokral, I.; Wsol, V.; Neugebauerova, J. Variations in the chemical profile and biological activities of licorice (Glycyrrhiza glabra L.), as influenced by harvest times. Acta Physiol. Plant. 2013, 35, 1337–1349. [Google Scholar] [CrossRef]
- Wasilewska, A.; Vlad, F.; Sirichandra, C.; Redko, Y.; Jammes, F.; Valon, C.; Frei dit Frey, N.; Leung, J. An update on abscisic acid signaling in plants and more. Mol. Plant 2008, 1, 198–217. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Abscisic acid: A seed maturation and anti stress signal. In Plant Physiology; Sinauer Associates, Inc.: Sunderland, MA, USA, 2006; pp. 423–558. [Google Scholar]
- Li, C.; Jia, H.; Chai, Y.; Shen, Y. Abscisic acid perception and signaling transduction in strawberry: A model for non-climacteric fruit ripening. Plant Signal. Behav. 2011, 6, 1950–1953. [Google Scholar] [CrossRef]
- Symons, G.M.; Chua, Y.-J.; Ross, J.J.; Quittenden, L.J.; Davies, N.W.; Reid, J.B. Hormonal changes during non-climacteric ripening in strawberry. J. Exp. Bot. 2012, 63, 4741–4750. [Google Scholar] [CrossRef] [PubMed]
- Karppinen, K.; Tegelberg, P.; Häggman, H.; Jaakola, L. Abscisic acid regulates anthocyanin biosynthesis and gene expression associated with cell wall modification in ripening bilberry (Vaccinium myrtillus L.) fruits. Front. Plant Sci. 2018, 9, 1259. [Google Scholar] [CrossRef] [PubMed]
- Setha, S. Roles of abscisic acid in fruit ripening. Walailak J. Sci. Tech. 2012, 9, 297–308. [Google Scholar]
- Srivastava, L.M. Plant Growth and Development. Hormones and Environment, 1st ed.; Oxford Academic Press: Cambridge, MA, USA, 2002; pp. 1–772. [Google Scholar]
- Harris, J. Abscisic acid: Hidden architect of root system structure. Plants 2015, 4, 548–572. [Google Scholar] [CrossRef] [PubMed]
- Ehlenfeldt, M.K.; Prior, R.L. Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbush blueberry. J. Agric. Food Chem. 2001, 49, 2222–2227. [Google Scholar] [CrossRef]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How can we make plants grow faster? A source–sink perspective on growth rate. J. Exp. Bot. 2016, 67, 31–45. [Google Scholar] [CrossRef]
- Ruiz-Vera, U.; De Souza, A.; Long, S.; Ort, D. The role of sink strength and nitrogen availability in the down-regulation of photosynthetic capacity in field-grown Nicotiana tabacum L. at elevated CO2 concentration. Front. Plant Sci. 2017, 8, 998. [Google Scholar] [CrossRef]
- Kurepa, J.; Shull, T.E.; Smalle, J.A. Quercetin feeding protects plants against oxidative stress. F1000Research 2016, 5, 2430. [Google Scholar] [CrossRef]
- Riaz, U.; Kharal, M.A.; Murtaza, G.; Zaman, Q.; Javaid, S.; Malik, H.A.; Aziz, H.; Abbas, Z. Prospective roles and mechanisms of caffeic acid in counter plant stress: A mini review. Pakistan J. Agri. Res. 2019, 32, 8–19. [Google Scholar] [CrossRef]
- Lan, X.; Wang, W.; Li, Q.; Wang, J. The natural flavonoid pinocembrin: Molecular targets and potential therapeutic applications. Mol. Neurobiol. 2016, 53, 1794–1801. [Google Scholar] [CrossRef]
- Natić, M.; Dabić, D.; Papetti, A.; FotirićAkšić, M.; Ognjanov, V.; Ljubojević, M.; Tešić, Ž. Analysis and characterisation of phytochemicals in mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chem. 2015, 171, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Pantelić, M.; Dabić Zagorac, D.; Ćirić, I.; Pergal, M.; Relić, D.; Todić, S.; Natić, M. Phenolic profiles, antioxidant activity and minerals in leaves of different grapevine varieties grown in Serbia. J. Food Compos. Anal. 2017, 62, 76–83. [Google Scholar] [CrossRef]
- Pantelić, M.; DabićZagorac, D.; Natić, M.; Gašić, U.; Jović, S.; Vujović, D.; Popović-Djordjević, J. Impact of clonal variability on phenolics and radical scavenging activity of grapes and wines: A study on the recently developed Merlot and Cabernet Franc clones (Vitis vinifera L.). PLoS ONE 2016, 11, e0163823. [Google Scholar]
- Vasić, V.; Gašić, U.; Stanković, D.; Lušić, D.; Vukić-Lušić, D.; Milojković-Opsenica, D.; Tešić, Ž. Towards better quality criteria of European honeydew honey: Phenolic profile and antioxidant capacity. Food Chem. 2019, 274, 629–641. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Compound Name | Blueberry Fruits | Strawberry Fruits | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrated | Organic | Integrated | Organic | |||||||||
| Bluecrop | Duke | Nui | Bluecrop | Duke | Nui | Alba | Favette | Clery | Alba | Favette | Clery | |
| Aesculin | 0.00 | 0.00 | 0.00 | 0.06 b,** | 0.03 c | 0.11 a | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 5-O-Caffeoylquinic Acid | 89.04 c | 33.08 f | 48.05 d | 120.75 a | 33.73 e | 119.84 b | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Epigallocatechin | 3.99 a | 2.53 d | 3.00 b | 2.92 c | 2.10 f | 2.31 e | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Catechin | 2.32 e | 1.27i | 1.59 g | 1.43 h | 0.67 j | 1.45 h | 3.19 b | 1.70 f | 2.52 c | 2.33 e | 2.43 d | 3.79 a |
| p-Hydroxybenzoic Acid | 0.54 f | 0.80 c | 0.64 e | 0.74 d | 0.99 a | 1.00 a | 0.32 i | 0.96 b | 0.51 g | 0.73 d | 0.45 h | 0.45 h |
| Caffeic Acid | 0.95 c | 0.61 d | 0.42 f | 2.39 a | 1.26 b | 2.36 a | 0.33 h | 0.45 e | 0.38 g | 0.96 c | 0.22 i | 0.34 h |
| Epicatechin | 0.35 a | 0.30 b | 0.15 e | 0.17 d | 0.14 f | 0.22 c | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Syringic Acid | 0.34 d | 0.90 b | 0.42 c | 0.25 e | 1.27 a | 0.43 c | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Apigenin 8-C-Glucoside | 0.73 d | 0.57 f | 0.75 c | 1.28 b | 0.70 e | 1.38 a | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Quercetin 3-O-Rutinoside | 2.29 d | 0.95 e | 3.31 b | 2.53 c | 0.81 f | 4.65 a | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| p-Coumaric Acid | 0.14 j | 0.13 k | 0.08 l | 0.16 i | 0.26 h | 0.56 g | 0.77 e | 3.64 b | 1.05 d | 5.27 a | 0.59 f | 2.12 c |
| Quercetin 3-O-Galactoside | 30.92 a | 25.39 c | 18.67 f | 30.07 b | 21.15 d | 19.33 e | 0.98 i | 0.38 k | 0.21 l | 1.19 h | 0.46 j | 1.26 g |
| Vanillic Acid | 0.13 f | 0.27 a | 0.11 e | 0.15 d | 0.23 b | 0.21 c | 0.05 h | 0.06 g | 0.04 i | 0.07 g | 0.05 h | 0.02 j |
| Sinapic Acid | 0.11 d | 0.14 b | 0.05 e | 0.13 c | 0.24 a | 0.13 c | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ferulic Acid | 0.28 e | 0.35 d | 0.18 f | 0.47 c | 0.79 b | 1.70 a | 0.01 l | 0.05 i | 0.02 k | 0.17 g | 0.08 h | 0.03 j |
| Kaempferol 3-O-Glucoside | 0.84 f | 0.11 i | 0.16 h | 0.83 f,g | 0.08 j | 0.17 h | 2.17 a | 1.03 e | 0.82 g | 1.54 b | 1.34 c | 1.17 d |
| Quercetin | 3.47 g | 10.12 d | 2.88 h | 19.25 c | 53.69 a | 26.51 b | 3.54 f | 2.46 k | 2.45 k | 3.99 e | 2.57 j | 2.77 i |
| Cinnamic Acid | 0.04 h | 0.03 i | 0.05 g | 0.04 h | 0.03 i | 0.07 f | 0.45 c | 0.80 b | 0.34 d | 1.36 a | 0.26 e | 0.34 d |
| Naringenin | 0.28 a | 0.27 b | 0.24 d | 0.25 c | 0.28 a | 0.25 c | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Kaempferol | 0.00 | 0.00 | 0.00 | 0.73 d | 0.68 f | 0.61 g | 0.80 b | 0.79 b | 0.77 c | 1.74 a | 0.70 e | 0.71 e |
| cis, trans-Abscisic Acid | 1.40 a | 0.70 d | 0.97 c | 1.04 b | 0.25 f | 0.65 e | 0.04 k | 0.13 j | 0.19 h | 0.13 j | 0.24 g | 0.16 i |
| TPC | 2.27 f | 4.08 c | 2.69 e | 4.38 b | 6.26 a | 3.30 d | 1.70 h | 1.58 i | 1.47 j | 2.27 f | 1.18 k | 2.04 g |
| RSA | 18.94 g | 25.36 c | 18.31 h | 23.49 e | 33.83 a | 33.03 b | 16.69 k | 18.15 i | 17.71 j | 24.05 d | 16.32 l | 23.35 f |
| TAC | 0.62 f | 1.80 b | 0.82 e | 1.05 d | 2.86 a | 1.63 c | 0.16 k | 0.23 i | 0.22 i | 0.37 g | 0.19 j | 0.33 h |
| Compound Name | Blueberry Leaves | Strawberry Leaves | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrated | Organic | Integrated | Organic | |||||||||
| Bluecrop | Duke | Nui | Bluecrop | Duke | Nui | Alba | Favette | Clery | Alba | Favette | Clery | |
| Gallocatechin | 21.29 b,** | 15.63 d | 34.60 a | 17.79 c | 14.11 e | 11.17 f | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Aesculin | 3.24 c | 2.57 d | 2.54 d | 3.41 b | 2.40 e | 3.70 a | 0.94 f | 0.64 i | 0.75 h | 0.83 g | 0.17 k | 0.48 j |
| 5-O-Caffeoylquinic Acid | 1826.07 c | 1359.64 f | 1617.33 d | 2108.73 b | 1485.93 e | 2380.63 a | 176.49 g | 176.64 g | 152.88 h | 92.22 i | 5.82 k | 9.11 j |
| Catechin | 7.62 g | 6.00 i | 13.50 d | 7.57 g | 7.08 h | 4.51 j | 0.31 k | 9.47 f | 11.67 e | 14.69 c | 30.36 b | 31.86 a |
| p-Hydroxybenzoic Acid | 4.72 d | 4.25 e | 3.93 f | 5.86 a | 4.80 c | 4.90 b | 1.68 j | 1.96 i | 1.52 l | 2.10 h | 1.60 k | 2.20 g |
| Caffeic Acid | 55.51 b | 26.40 f | 41.76 e | 51.16 c | 42.94 d | 90.68 a | 5.21 l | 13.25 g | 6.76 k | 8.11 j | 12.23 h | 12.02 i |
| Syringic Acid | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.48 b | 0.30 d | 0.30 d | 0.24 e | 0.63 a | 0.33 c |
| Apigenin 8-C-Glucoside | 10.06 c | 7.48 f | 8.03 e | 10.62 b | 9.68 d | 13.17 a | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Quercetin 3-O-Rutinoside | 13.19 d | 6.63 f | 26.37 b | 12.73 e | 14.22 c | 27.01 a | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| p-Coumaric Acid | 2.96 i | 0.16 k | 3.05 h | 3.76 g | 2.40 j | 3.81 g | 5.15 f | 10.36 a | 6.09 e | 6.49 d | 9.47 b | 8.64 c |
| Quercetin 3-O-Galactoside | 124.77 b | 90.72 f | 99.65 e | 125.67 c | 118.57 d | 130.20 a | 4.56 k | 8.25 j | 9.65 h | 9.36 i | 12.53 g | 13.35 g |
| Vanillic Acid | 0.60 h | 0.60 h | 1.32 d | 1.92 b | 1.14 f | 3.51 a | 1.06 g | 1.20 e | 0.61 h | 1.51 c | 0.88 i | 1.31 d |
| Sinapic Acid | 0.48 g | 0.16 i | 0.30 h | 0.32 h | 0.14 j | 0.67 f | 3.23 e | 27.47 a | 15.56 b | 14.47 c | 0.04 k | 5.64 d |
| Ferulic Acid | 0.99 e | 0.48 j | 0.55 i | 0.80 g | 0.46 k | 1.39 c | 1.29 d | 1.54 b | 0.87 f | 0.81 g | 0.73 h | 2.44 a |
| Kaempferol 3-O-Glucoside | 38.62 b | 4.09 f | 11.10 d | 41.07 a | 6.77 e | 12.65 c | 1.20 k | 1.29 j | 1.69 i | 3.28 g | 2.41 h | 0.52 l |
| Quercetin | 151.49 c | 165.65 b | 89.51 e | 83.57 f | 145.90 d | 214.72 a | 36.09 g | 20.82 j | 20.06 k | 21.67 i | 32.35 h | 14.14 l |
| Cinnamic Acid | 0.35 g | 0.26 i | 0.28 h | 0.09 j | 0.27 h,i | 0.56 e,f | 0.57 e | 1.04 b | 0.55 f | 0.66 d | 1.18 a | 0.88 c |
| Naringenin | 0.71 e | 0.52 j | 0.66 g | 0.65 h | 0.57 i | 0.85 b | 0.68 f | 0.83 c | 0.72 e | 0.65 h | 0.91 a | 0.75 d |
| Kaempferol | 14.44 a | 5.22 d | 4.93 e | 9.01 b | 4.00 i | 8.25 c | 4.08 g | 2.83 k | 3.13 j | 4.43 f | 4.04 h | 2.41 l |
| Pinocembrin | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.31 b,c | 0.33 a | 0.32 b | 0.30 c,d | 0.33 a | 0.29 d |
| TPC | 54.56 e | 46.96 g | 55.75 d | 81.06 b | 55.42 d | 77.65 c | 49.89 f | 45.75 h | 44.83 i | 38.22 k | 41.29 j | 82.25 a |
| RSA | 456.50 i | 336.19 l | 423.25 j | 493.62 h | 341.53 k | 679.94 g | 802.29 f | 1880.32 b | 1483.77 c | 1225.43 d | 2237.31 a | 951.24 e |
| No | tR, min | Compound Name | Molecular Formula [M − H]− | Calculated Mass [M − H]− | Exact Mass [M − H]− | Δ ppm | MS 2 Fragments, (% Base Peak) | MS 3 Fragments, (% Base Peak) | MS 4 Fragments, (% Base Peak) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.94 | Gallic Acid Hexoside Isomer 1 | C13H15O10– | 331.06707 | 331.06616 | 2.75 | 294(10), 169(100), 125(5) | 125(100) | 107(100), 81(10) |
| 2 | 4.17 | Dihydroxybenzoic Acid hexoside Isomer 1 | C13H15O9– | 315.07216 | 315.07104 | 3.55 | 153(100), 152(50), 109(15), 108(10) | 109(100) | 123(25), 109(10), 85(10), 81(100) |
| 3 | 4.31 | Gallic Acid Hexoside Isomer 2 | C13H15O10– | 331.06707 | 331.06622 | 2.57 | 169(100), 125(5) | 125(100) | 110(10), 97(30), 81(100), 53(30) |
| 4 | 4.44 | Prodelphinidin Dimer B Type | C30H25O13– | 593.13006 | 593.12878 | 2.16 | 467(15), 425(100), 407(30), 289(20) | 407(100), 381(5), 273(10) | 389(30), 297(30), 285(100), 243(70) |
| 5 | 4.53 | Caffeoyltartaric Acid | C13H11O9– | 311.04031 | 311.03986 | 1.45 | 179(50), 177(10), 149(100) | 131(60), 103(90), 87(100), 59(20) | 59(100) |
| 6 | 4.54 | Chlorogenic Acid Hexoside Isomer 1 | C22H27O14– | 515.14008 | 515.14001 | 0.14 | 353(100), 341(5), 323(10), 191(90), 179(5) | 191(100), 179(10) | 173(65), 127(80), 111(30), 85(100) |
| 7 | 4.59 | Gallocatechin * | C15H13O7− | 305.06668 | 305.06537 | 4.29 | 261(50), 221(70), 219(70), 179(100), 165(35) | 164(100), 151(40), 135(30) | 120(100), 108(20) |
| 8 | 4.64 | Dihydroxybenzoic Acid Hexosyl-Pentoside | C18H23O13– | 447.11441 | 447.11353 | 1.97 | 315(100), 285(10), 153(10) | 153(100), 123(10) | 123(100) |
| 9 | 4.71 | Gallic Acid Hexoside Isomer 3 | C13H15O10– | 331.06707 | 331.06610 | 2.93 | 313(100), 211(10), 169(30), 168(80), 150(10), 125(25) | 193(50), 151(100), 125(80) | 123(100), 107(90), 95(65) |
| 10 | 4.72 | Chlorogenic Acid Hexoside Isomer 2 | C22H27O14– | 515.14008 | 515.13928 | 1.55 | 353(40), 341(100), 335(30), 323(10), 191(15), 179(45) | 179(100), 135(10) | 135(100) |
| 11 | 4.83 | Caffeic Acid Hexoside Isomer 1 | C15H17O9– | 341.08781 | 341.08685 | 2.81 | 191(10), 179(100), 135(10) | 135(100) | 135(100), 107(50) |
| 12 | 4.84 | Dihydroxybenzoic Acid Pentoside | C12H13O8– | 285.06159 | 285.06094 | 2.28 | 153(100), 152(25), 109(5), 108(5) | 109(100) | 81(100) |
| 13 | 4.90 | 3-O-Caffeoylquinic Acid Isomer 1 | C16H17O9– | 353.08781 | 353.08673 | 3.06 | 191(100), 179(30), 135(10) | 173(75), 127(100), 111(40), 93(60), 85(90) | 109(30), 99(40), 85(100) |
| 14 | 5.02 | Hydroxybenzoic Acid Hexoside | C13H15O8– | 299.07724 | 299.07693 | 1.04 | 137(100) | 93(100) | – |
| 15 | 5.09 | 3-O-Caffeoylquinic Acid Isomer 2 | C16H17O9– | 353.08781 | 353.08652 | 3.65 | 191(100), 179(30), 135(10) | 173(75), 127(100), 111(40), 93(60), 85(90) | 109(30), 99(40), 85(100) |
| 16 | 5.10 | Procyanidin Dimer B Type Isomer 1 | C30H25O12– | 577.13515 | 577.13434 | 1.40 | 559(10), 451(30), 425(100), 407(40), 289(20), 287(10) | 407(100), 381(5), 287(5), 273(10) | 389(30), 297(30), 285(100), 281(90) |
| 17 | 5.12 | Aesculin * | C15H15O9– | 339.07216 | 339.07114 | 3.01 | 177(100) | 177(5), 149(10), 133(100), 105(10), 89(5) | 89(100) |
| 18 | 5.28 | Caffeic Acid Hexoside Isomer 2 | C15H17O9– | 341.08781 | 341.08664 | 3.43 | 179(100), 135(10) | 135(100) | 107(100), 79(20) |
| 19 | 5.31 | Coumaric Acid Hexoside Isomer 1 | C15H17O8– | 325.09289 | 325.09171 | 3.63 | 163(100), 119(10) | 119(100) | – |
| 20 | 5.33 | Procyanidin Dimer B Type Isomer 2 | C30H25O12– | 577.13515 | 577.13409 | 1.84 | 559(5), 451(20), 425(100), 407(35), 289(20), 287(10) | 407(100), 381(10), 273(10) | 389(40), 297(40), 285(100), 243(75) |
| 21 | 5.35 | 5-O-Caffeoylquinic Acid * | C16H17O9– | 353.08781 | 353.08616 | 4.67 | 191(100), 179(5) | 173(75), 127(100), 111(40), 93(60), 85(90) | 109(40), 99(50), 85(100) |
| 22 | 5.37 | Epigallocatechin * | C15H13O7– | 305.06668 | 305.06589 | 2.59 | 287(10), 261(40), 247(20), 221(90), 219(80), 179(100) | 164(100), 151(40), 135(30) | 120(100), 108(20) |
| 23 | 5.44 | Dihydroxybenzoic Acid Hexoside Isomer 2 | C13H15O9– | 315.07216 | 315.07123 | 2.95 | 153(100), 135(10), 109(10) | 135(100), 109(50) | 91(100) |
| 24 | 5.45 | Quercetin 3-O-Hexoside-7-O-hexuronide | C27H27O18– | 639.12029 | 639.11963 | 1.03 | 463(100), 301(20) | 343(5), 301(100) | 179(70), 151(100), 107(10) |
| 25 | 5.47 | Catechin * | C15H13O6– | 289.07176 | 289.07068 | 3.74 | 271(5), 245(100), 205(40), 179(15), 125(5) | 227(30), 203(100), 187(25), 175(10), 161(20) | 188(70), 185(20), 175(100), 161(40), 157(10) |
| 26 | 5.48 | p-Hydroxybenzoic Acid * | C7H5O3– | 137.02442 | 137.02420 | 1.61 | 109(10), 93(100) | 93(10) | – |
| 27 | 5.57 | Coumaric Acid Hexoside Isomer 2 | C15H17O8– | 325.09289 | 325.09128 | 4.95 | 289(20), 265(20), 235(10), 187(40), 163(80), 145(100) | 117(100) | – |
| 28 | 5.60 | 4-O-Caffeoylquinic Acid | C16H17O9– | 353.08781 | 353.08688 | 2.63 | 191(60), 179(75), 173(100), 135(15) | 115(20), 111(50), 93(100), 71(20) | – |
| 29 | 5.61 | Procyanidin Dimer B Type Isomer 3 | C30H25O12– | 577.13515 | 577.13312 | 3.52 | 559(10), 451(20), 425(100), 407(40), 289(20), 287(10) | 407(100), 381(5), 273(10) | 389(30), 297(30), 285(100), 243(75) |
| 30 | 5.80 | Methyl 3-caffeoylquinate | C17H19O9– | 367.10346 | 367.10251 | 2.59 | 193(20), 179(5), 161(100), 135(10) | 133(100) | 77(100) |
| 31 | 5.84 | Caffeic Acid * | C9H7O4– | 179.03498 | 179.03444 | 3.02 | 135(100) | 135(60), 117(15), 107(100), 91(55), 79(15) | – |
| 32 | 5.92 | Epicatechin * | C15H13O6– | 289.07176 | 289.07104 | 2.49 | 271(5), 245(100), 205(40), 179(15), 125(5) | 227(35), 203(100), 187(30), 175(15), 161(25) | 188(60), 185(20), 175(100), 161(35), 157(15) |
| 33 | 5.92 | 5-Caffeoylquinic acid Isomer | C16H17O9– | 353.08781 | 353.08624 | 4.45 | 191(100), 179(5) | 173(75), 127(100), 111(40), 93(60), 85(90) | 109(40), 99(50), 85(100) |
| 34 | 6.00 | Syringic Acid * | C9H9O5− | 197.04555 | 197.04477 | 3.96 | 183(100), 153(40), 138(10) | 167(100), 138(10), 123(5) | – |
| 35 | 6.01 | Caffeoylshikimic Acid | C16H15O8– | 335.07724 | 335.07587 | 4.09 | 179(100), 135(25) | 135(100) | 107(100) |
| 36 | 6.06 | Myricetin 3-O-rutinoside | C27H29O17– | 625.14102 | 625.14014 | 1.41 | 607(10), 359(5), 329(5), 317(65), 316(100), 287(10) | 287(30), 271(100), 179(30), 151(10) | 271(10), 243(100), 227(40), 215(15) |
| 37 | 6.14 | Quercetin 3-O-Hexosyl-hexuronide | C27H27O18– | 639.12029 | 639.11865 | 2.57 | 337(10), 301(100) | 273(20), 257(20), 179(100), 151(75) | 151(100) |
| 38 | 6.18 | Myricetin 3-O-hexoside | C21H19O13– | 479.08311 | 479.08176 | 2.82 | 317(100), 316(80) | 273(60), 179(100), 151(40) | 151(100) |
| 39 | 6.22 | Methyl 4-caffeoylquinate | C17H19O9– | 367.10346 | 367.10211 | 3.68 | 193(5), 179(5), 161(100), 135(30) | 133(100) | 105(100) |
| 40 | 6.26 | Ellagic Acid Pentoside | C19H13O12– | 433.04125 | 433.04047 | 1.80 | 301(100), 300(80) | 301(95), 284(25), 257(100), 229(70), 222(15) | 229(70), 213(30), 201(15), 185(100) |
| 41 | 6.29 | Methyl 3-p-coumaroylquinate | C17H19O8– | 351.10854 | 351.10767 | 2.48 | 163(5), 145(100), 119(10), 117(10) | 117(100) | – |
| 42 | 6.40 | Ellagic Acid Rhamnoside | C20H15O12– | 447.05690 | 447.05576 | 2.55 | 301(50), 300(100) | 300(100), 284(15), 271(20), 257(30), 244(30) | 216(100), 200(40), 188(15), 172(20) |
| 43 | 6.44 | Apigenin 8-C-glucoside * | C21H19O10− | 431.09837 | 431.09720 | 2.71 | 341(20), 311(100) | 283(100) | 283(50), 239(100), 224(40), 197(50), 183(60) |
| 44 | 6.45 | Methyl 5-caffeoylquinate isomer 1 | C17H19O9– | 367.10346 | 367.10190 | 4.25 | 191(20), 179(100), 161(10), 135(50) | 135(100) | 135(60), 107(100), 91(25), 79(20) |
| 45 | 6.46 | Coumaric Acid Hexoside Isomer 3 | C15H17O8– | 325.09289 | 325.09180 | 3.35 | 289(10), 265(10), 163(100), 161(50), 119(60), 101(20) | 91(100) | – |
| 46 | 6.47 | Quercetin 3-O-rutinoside * | C27H29O16– | 609.14611 | 609.14496 | 1.89 | 343(5), 301(100), 300(30), 271(10), 255(5) | 273(25), 257(20), 179(100), 151(75) | 151(100) |
| 47 | 6.50 | Myricetin 3-O-pentoside | C20H17O12– | 449.07255 | 449.07169 | 1.92 | 387(5), 317(35), 316(100) | 287(30), 271(100), 179(30), 151(10) | 271(10), 243(100), 227(40), 215(15) |
| 48 | 6.65 | p-Coumaric acid * | C9H7O3– | 163.04007 | 163.03932 | 4.60 | 119(100) | 119(60), 101(20), 93(25), 91(100), 72(10) | – |
| 49 | 6.67 | Quercetin 3-O-galactoside | C21H19O12– | 463.08820 | 463.08719 | 2.18 | 301(100), 300(30) | 273(25), 257(20), 179(100), 151(75) | 151(100) |
| 50 | 6.70 | Methyl 5-caffeoylquinate Isomer 2 | C17H19O9– | 367.10346 | 367.10269 | 2.10 | 191(20), 179(100), 161(10), 135(50) | 135(100) | 109(100), 107(70) |
| 51 | 6.75 | Ellagic Acid | C14H5O8– | 300.99899 | 300.99805 | 3.12 | 284(40), 271(60), 257(100), 229(85), 185(40) | 229(100), 213(20), 185(85) | 201(100), 185(95), 157(30), 145(20) |
| 52 | 6.82 | Kaempferol 7-O-rutinoside | C27H29O15– | 593.15119 | 593.14972 | 2.48 | 285(100) | 267(40), 257(100), 241(30), 229(40), 213(30) | 255(10), 239(30), 229(100), 163(40) |
| 53 | 6.85 | Quercetin 3-O-rhamnosyl-hexuronide | C27H27O17– | 623.12537 | 623.12341 | 3.15 | 605(15), 491(10), 475(5), 315(40), 301(60), 300(100) | 271(100), 255(60), 179(10), 151(10) | 243(100), 227(80), 215(20), 199(20) |
| 54 | 6.87 | Vanillic Acid * | C8H7O4– | 167.03498 | 167.03419 | 4.73 | 153(10), 152(80), 124(10), 123(100), 108(20) | 108(100) | 79(100) |
| 55 | 6.89 | Isorhamnetin 3-O-rutinoside | C28H31O16– | 623.16176 | 623.16010 | 2.66 | 315(100), 300(20), 271(10), 255(5) | 300(100), 287(5), 272(5) | 271(100), 255(50), 151(5) |
| 56 | 6.97 | Quercetin 3-O-pentoside | C20H17O11– | 433.07763 | 433.07669 | 2.17 | 343(5), 301(80), 300(100) | 271(100), 255(60), 179(10), 151(10) | 243(100), 227(80), 215(20), 199(20) |
| 57 | 6.99 | Sinapic Acid * | C11H11O5− | 223.06120 | 223.06058 | 2.78 | 208(100), 179(30), 164(20) | 193(10), 164(100), 149(15), 135(5) | 149(100), 135(35) |
| 58 | 7.02 | Ferulic Acid * | C10H9O4– | 193.05063 | 193.04990 | 3.78 | 178(70), 149(100), 134(40) | 134(100) | – |
| 59 | 7.05 | Methyl 5-p-coumaroylquinate Isomer 1 | C17H19O8– | 351.10854 | 351.10773 | 2.31 | 163(100), 145(5), 119(15) | 119(100) | – |
| 60 | 7.08 | Kaempferol 3-O-glucoside * | C21H19O11– | 447.09329 | 447.09244 | 1.90 | 327(20), 285(80), 284(100), 255(10) | 255(100), 227(10) | 227(100), 211(60) |
| 61 | 7.12 | Syringetin 3-O-hexoside | C23H23O13– | 507.11441 | 507.11292 | 2.94 | 479(10), 387(20), 345(80), 344(100), 299(15) | 330(90), 316(100), 301(90), 287(10), 273(70) | 301(100), 300(20), 287(10), 273(60) |
| 62 | 7.16 | Isorhamnetin 3-O-hexoside | C22H21O12– | 477.10385 | 477.10321 | 1.34 | 357(20), 315(50), 314(100), 300(5), 299(5), 285(10) | 300(30), 285(100), 271(75), 257(10), 243(25) | 270(100) |
| 63 | 7.20 | Quercetin 3-O-acetyl-hexoside Isomer 1 | C23H21O13– | 505.09876 | 505.09756 | 2.38 | 463(20), 343(20), 301(100), 300(60), 299(50) | 273(20), 257(20), 179(100), 151(75) | 151(100) |
| 64 | 7.24 | Isorhamnetin 3-O-hexuronide | C22H19O13– | 491.08311 | 491.08221 | 1.83 | 473(10), 315(70), 301(100), 300(60) | 283(15), 272(20), 256(10), 179(100), 151(75) | 151(100) |
| 65 | 7.29 | Dicaffeoylquinic Acid Isomer 1 | C25H23O12– | 515.11950 | 515.11789 | 3.13 | 353(100) | 191(100), 179(40), 135(10) | 173(100), 127(50), 111(40), 85(70) |
| 66 | 7.31 | Methyl 5-p-coumaroylquinate Isomer 2 | C17H19O8– | 351.10854 | 351.10773 | 2.31 | 163(100), 145(5), 119(15) | 119(100) | – |
| 67 | 7.38 | Quercetin 3-O-acetyl-hexoside Isomer 2 | C23H21O13– | 505.09876 | 505.09744 | 2.61 | 463(30), 445(30), 343(5), 301(100), 300(90), 299(10) | 272(40), 256(25), 179(100), 151(75) | 151(100) |
| 68 | 7.44 | Quercetin 3-O-methyl-malonyl-hexoside | C25H23O15– | 563.10424 | 563.10309 | 2.04 | 531(100), 463(80) | 463(100) | 343(5), 301(100), 300(30) |
| 69 | 7.45 | Quercetin 7-O-hexuronide | C21H17O13– | 477.06692 | 477.06580 | 2.35 | 301(100) | 273(20), 257(20), 179(100), 151(75) | 151(100) |
| 70 | 7.46 | Isorhamnetin 3-O-pentoside | C21H19O11– | 447.09329 | 447.09265 | 1.43 | 357(10), 315(30), 314(100), 285(5), 271(5) | 300(10), 285(100), 271(70), 257(10), 243(20) | 270(100) |
| 71 | 7.49 | Quercetin 3-O-malonyl-hexoside | C24H21O15– | 549.08859 | 549.08752 | 1.95 | 505(100) | 463(30), 301(100), 300(50) | 273(15), 257(15), 179(100), 151(85) |
| 72 | 7.54 | Dicaffeoylquinic Acid Isomer 2 | C25H23O12– | 515.11950 | 515.11865 | 1.65 | 353(100) | 191(100), 179(40), 173(20), 135(10) | 173(60), 127(100), 111(40), 85(80) |
| 73 | 7.60 | Kaempferol 7-O-hexuronide | C21H17O12– | 461.07200 | 461.07141 | 1.28 | 285(100) | 267(40), 257(100), 241(30), 229(50), 213(25) | 255(10), 239(30), 229(100), 163(60) |
| 74 | 7.63 | Isorhamnetin 3-O-malonyl-rutinoside | C31H33O19– | 709.16160 | 709.16040 | 1.69 | 666(30), 665(100) | 623(15), 315(100), 300(20), 271(15), 255(10) | 300(100), 287(10), 272(10), 256(5) |
| 75 | 7.64 | Myricetin | C15H9O8− | 317.03029 | 317.02927 | 3.22 | 287(30), 271(15), 193(10), 179(100),1151(45) | 151(100) | 107(100), 83(15) |
| 76 | 7.70 | Methyl 3,4-dicaffeoylquinate | C26H25O12– | 529.13515 | 529.13397 | 2.23 | 367(100), 161(10) | 335(5), 193(10), 179(5), 161(100), 135(20) | 133(100) |
| 77 | 7.73 | Kaempferol 3-O-hexuronide methyl Ether | C22H19O12− | 475.08820 | 475.08701 | 2.50 | 327(10), 301(10), 285(70), 284(100), 255(35), 227(5) | 255(100), 227(10) | 227(100), 211(60) |
| 78 | 8.00 | Kaempferol 3-O-malonyl-hexoside | C24H21O14– | 533.09368 | 533.09229 | 2.61 | 489(100) | 285(100) | 267(40), 257(100), 241(30), 229(50) |
| 79 | 8.02 | Methyl Caffeate | C10H9O4– | 193.05063 | 193.05019 | 2.28 | 178(30), 161(100), 134(70), 111(10) | 133(100) | – |
| 80 | 8.04 | Methyl 3,5-dicaffeoylquinate | C26H25O12– | 529.13515 | 529.13391 | 2.34 | 367(100), 349(10), 179(10), 161(10) | 335(5), 193(10), 191(25), 179(100), 161(80) | 135(100) |
| 81 | 8.16 | Feruloyl-coumaroylquinic acid isomer 1 | C26H25O11– | 513.14024 | 513.13953 | 1.38 | 367(100), 351(5), 161(10) | 335(5), 193(10), 179(5), 161(100), 135(30) | 133(100) |
| 82 | 8.29 | Methyl 4,5-dicaffeoylquinate | C26H25O12– | 529.13515 | 529.13385 | 2.46 | 367(100), 349(10), 179(15), 161(10) | 335(10), 193(10), 191(20), 179(100), 161(80) | 135(100) |
| 83 | 8.30 | Kaempferol3-O-p-coumaroyl-hexoside | C30H25O13– | 593.13006 | 593.12769 | 4.00 | 447(15), 307(10), 285(100) | 257(100), 241(50), 229(35), 213(40), 151(90) | 256(10), 239(25), 229(100), 213(20) |
| 84 | 8.49 | cis, trans-Abscisic acid * | C15H19O4− | 263.12888 | 263.12839 | 1.86 | 219(15), 153(100), 151(5) | 138(100), 109(10), 97(15) | 122(100) |
| 85 | 8.53 | Feruloyl-coumaroylquinic Acid Isomer 2 | C26H25O11– | 513.14024 | 513.13904 | 2.34 | 367(70), 349(100), 179(5), 161(10) | 305(20), 179(10), 161(100), 133(15) | 133(100) |
| 86 | 8.62 | Quercetin * | C15H9O7− | 301.03538 | 301.03442 | 3.19 | 271(50), 255(20), 179(100), 151(80), 107(5) | 151(100) | 107(100), 83(10) |
| 87 | 8.78 | Feruloyl-coumaroylquinic Acid Isomer 3 | C26H25O11– | 513.14024 | 513.14008 | 0.31 | 367(70), 349(100), 337(10), 179(5), 163(15), 161(10) | 305(10), 193(20), 173(15), 161(100), 133(10) | 133(100) |
| 88 | 9.03 | Cinnamic Acid * | C9H7O2− | 147.04515 | 147.04463 | 3.54 | 104(10), 103(100), 87(10) | 119(100) | – |
| 89 | 9.32 | Naringenin * | C15H11O5− | 271.06120 | 271.06039 | 2.99 | 177(10), 151(100) | 107(100) | 65(100) |
| 90 | 9.51 | Kaempferol * | C15H9O6− | 285.04046 | 285.03909 | 4.81 | 255(100), 227(10) | 211(100), 195(5), 167(15) | 211(40), 137(100) |
| 91 | 9.57 | Syringetin | C17H13O8– | 345.06159 | 345.06036 | 3.56 | 330(100), 315(10), 300(5) | 315(100) | 287(100), 271(40), 259(25), 243(15) |
| 92 | 9.69 | Isorhamnetin | C16H11O7– | 315.05103 | 315.04985 | 3.75 | 301(20), 300(100) | 283(40), 271(80), 255(30), 227(30), 151(100) | 107(100), 83(15) |
| 93 | 11.46 | Pinocembrin * | C15H11O4− | 255.06628 | 255.06580 | 1.88 | 213(100), 187(15), 151(30), 145(10), 107(5) | 185(100), 169(20), 145(20) | 185(10), 157(15), 143(100), 141(50) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotirić Akšić, M.; Dabić Zagorac, D.; Sredojević, M.; Milivojević, J.; Gašić, U.; Meland, M.; Natić, M. Chemometric Characterization of Strawberries and Blueberries according to Their Phenolic Profile: Combined Effect of Cultivar and Cultivation System. Molecules 2019, 24, 4310. https://doi.org/10.3390/molecules24234310
Fotirić Akšić M, Dabić Zagorac D, Sredojević M, Milivojević J, Gašić U, Meland M, Natić M. Chemometric Characterization of Strawberries and Blueberries according to Their Phenolic Profile: Combined Effect of Cultivar and Cultivation System. Molecules. 2019; 24(23):4310. https://doi.org/10.3390/molecules24234310
Chicago/Turabian StyleFotirić Akšić, Milica, Dragana Dabić Zagorac, Milica Sredojević, Jasminka Milivojević, Uroš Gašić, Mekjell Meland, and Maja Natić. 2019. "Chemometric Characterization of Strawberries and Blueberries according to Their Phenolic Profile: Combined Effect of Cultivar and Cultivation System" Molecules 24, no. 23: 4310. https://doi.org/10.3390/molecules24234310
APA StyleFotirić Akšić, M., Dabić Zagorac, D., Sredojević, M., Milivojević, J., Gašić, U., Meland, M., & Natić, M. (2019). Chemometric Characterization of Strawberries and Blueberries according to Their Phenolic Profile: Combined Effect of Cultivar and Cultivation System. Molecules, 24(23), 4310. https://doi.org/10.3390/molecules24234310








