Co-Adsorption of H2O, OH, and Cl on Aluminum and Intermetallic Surfaces and Its Effects on the Work Function Studied by DFT Calculations
Abstract
1. Introduction
2. Computational Details
2.1. Model Construction
2.2. Description of Energetics
3. Results and Discussion
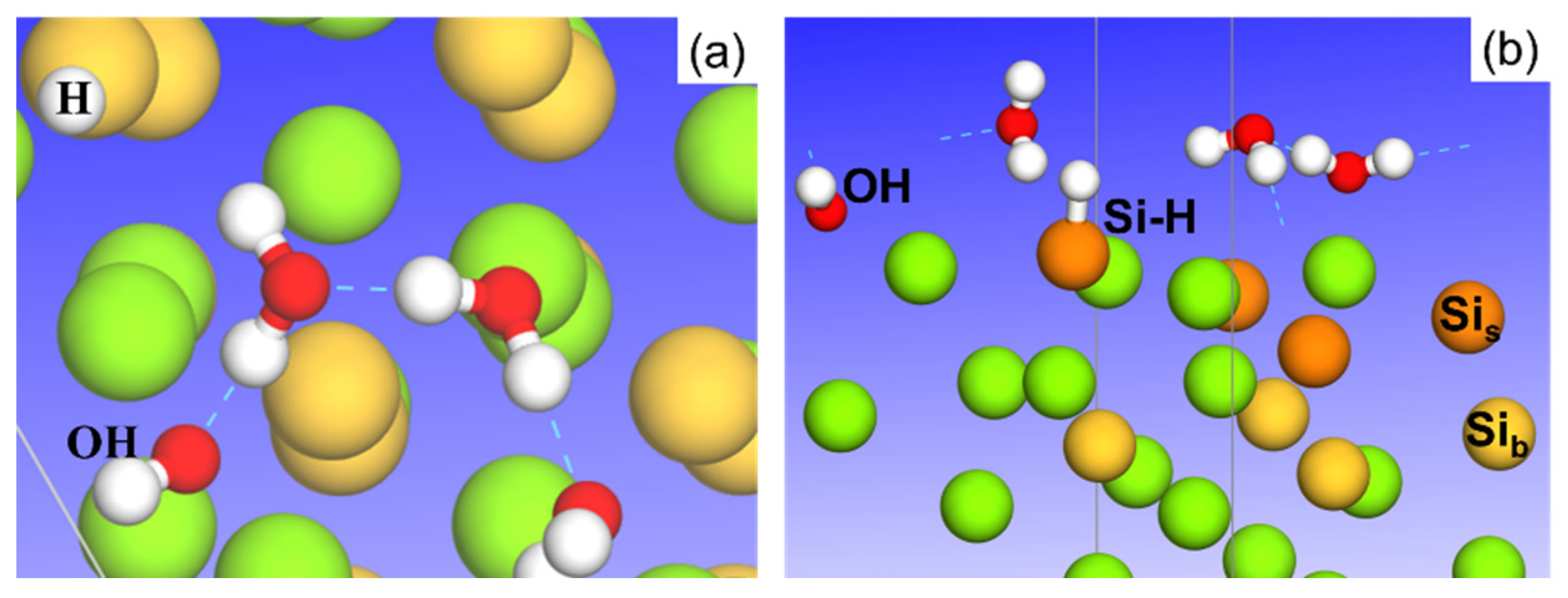
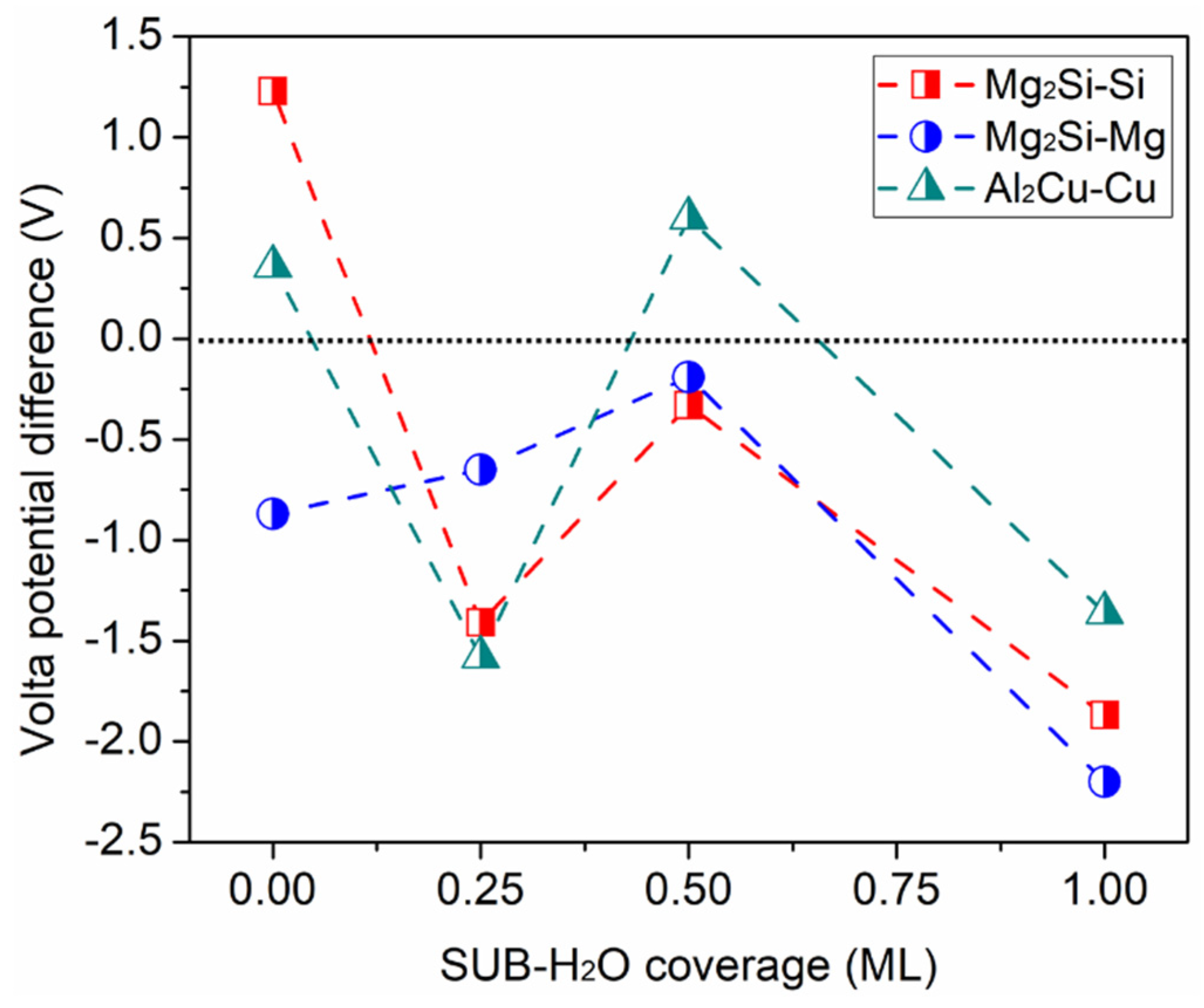
3.1. Adsorption of Pure H2O Ad-Layers
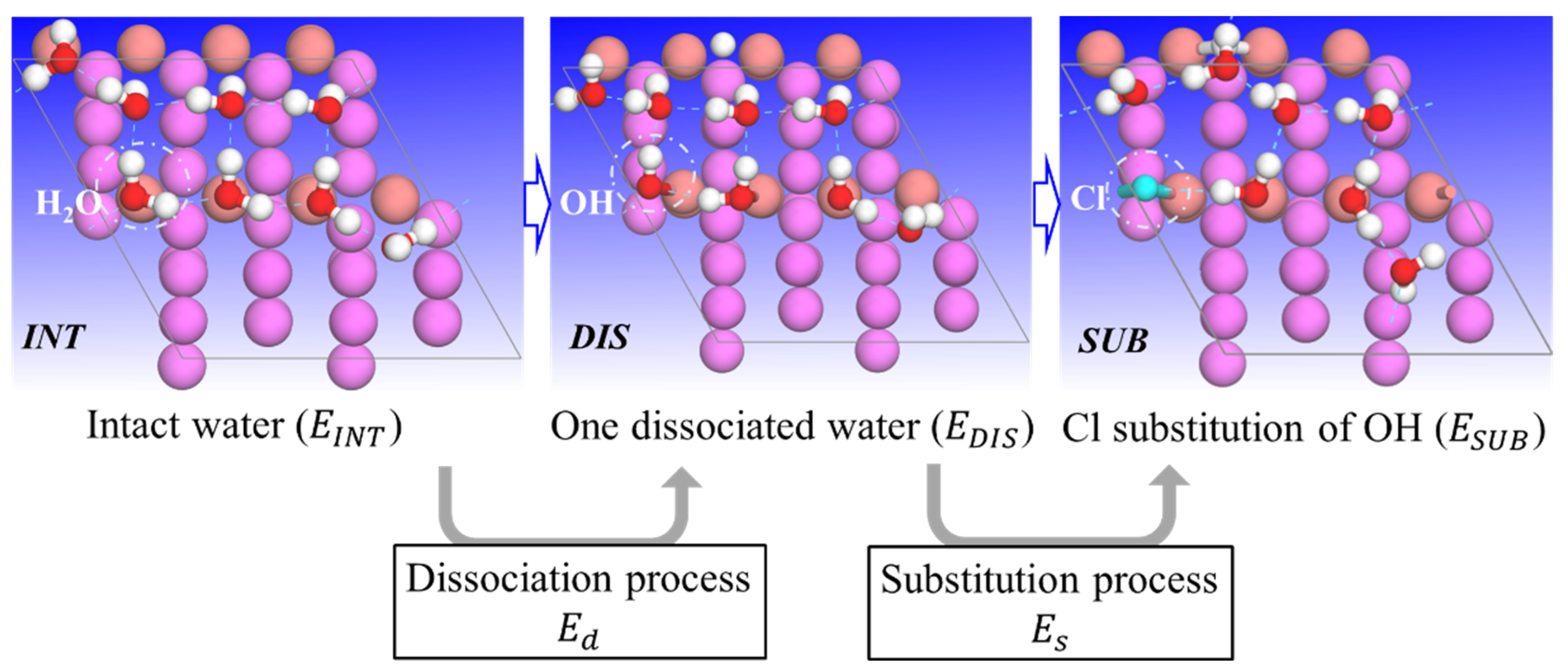
3.2. H2O Dissociation and Cl Substitution of OH
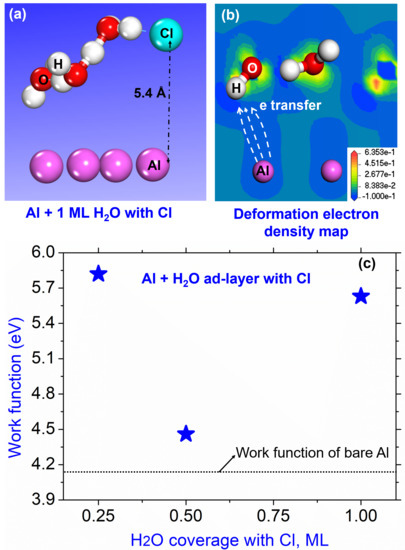
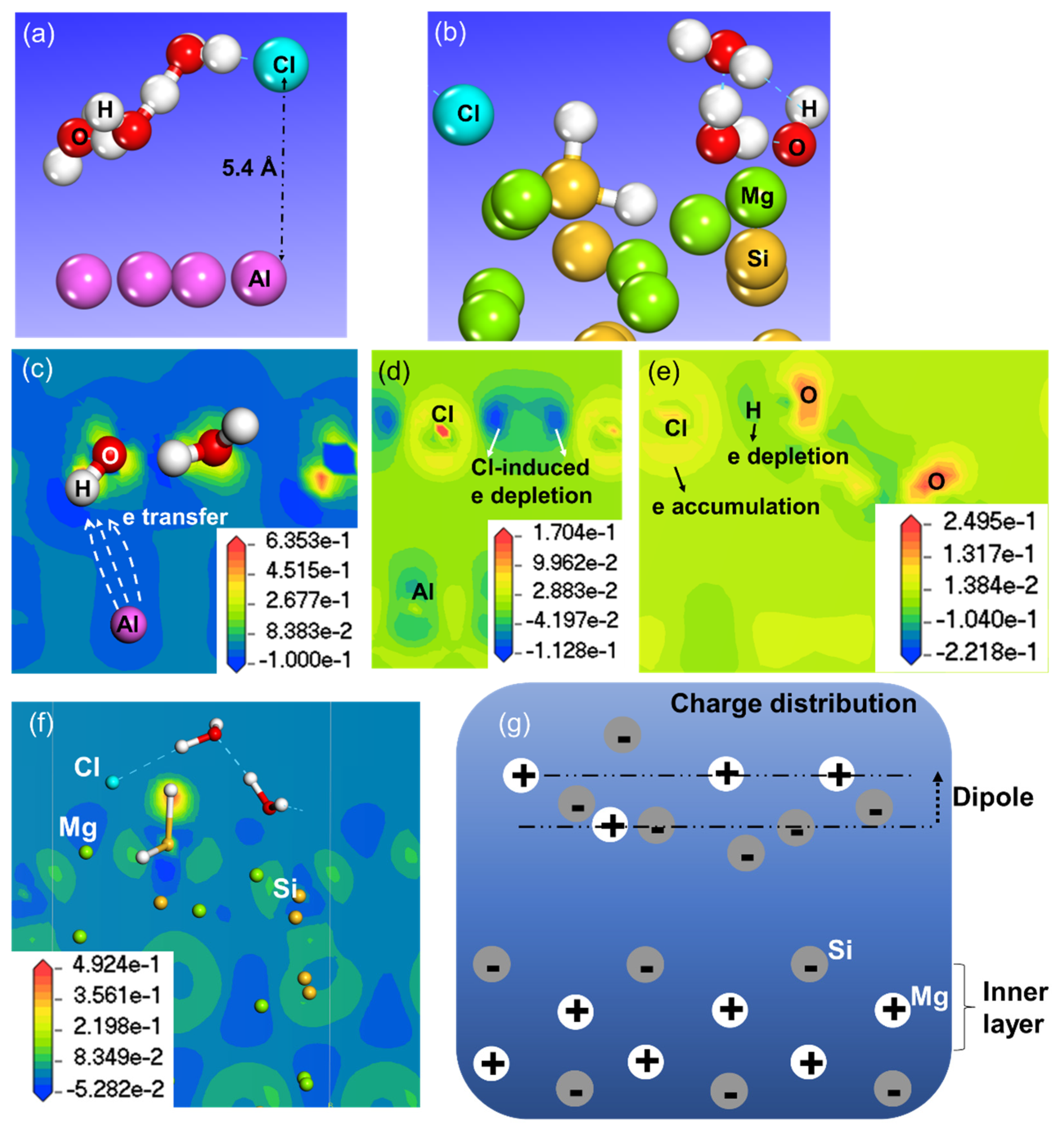
3.3. Effect on Work Function of Cl in the Aqueous Ad-Layer
3.4. Short Summary and Implications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Birbilis, N.; Buchheit, R.G. Electrochemical characteristics of intermetallic phases in aluminum alloys-an experimental survey and discussion. J. Electrochem. Soc. 2005, 152, B140–B151. [Google Scholar] [CrossRef]
- Lebouil, S.; Tardelli, J.; Rocca, E.; Volovitch, P.; Ogle, K. Dealloying of Al2Cu, Al7Cu2Fe, and Al2CuMg intermetallic phases to form nanoparticulate copper films. Mater. Corros. 2014, 65, 416–424. [Google Scholar] [CrossRef]
- Marcus, P.; Maurice, V.; Strehblow, H.H. Localized corrosion (pitting): A model of passivity breakdown including the role of the oxide layer nanostructure. Corros. Sci. 2008, 50, 2698–2704. [Google Scholar] [CrossRef]
- Gründer, Y.; Drünkler, A.; Golks, F.; Wijts, G.; Stettner, J.; Zegenhagen, J.; Magnussen, O.M. Cu (111) in chloride containing acidic electrolytes: Coadsorption of an oxygenated species. J. Electroanal. Chem. 2014, 712, 74–81. [Google Scholar] [CrossRef]
- Liu, M.; Jin, Y.; Zhang, C.H.; Leygraf, C.; Wen, L. Density-functional theory investigation of Al pitting corrosion in electrolyte containing chloride ions. Appl. Surf. Sci. 2015, 357, 2028–2038. [Google Scholar] [CrossRef]
- Bouzoubaa, A.; Diawara, B.; Maurice, M.; Minot, C.; Marcus, P. Ab initio study of the interaction of chlorides with defect-free hydroxylated NiO surfaces. Corros. Sci. 2009, 51, 941–948. [Google Scholar] [CrossRef]
- da Silva, T.H.; Nelson, E.B.; Williamson, I.; Efaw, C.M.; Sapper, E.; Hurley, M.F.; Li, L. First-principles surface interaction studies of aluminum-copper and aluminum-copper-magnesium secondary phases in aluminum alloys. Appl. Surf. Sci. 2018, 439, 910–918. [Google Scholar] [CrossRef]
- Guo, L.Q.; Zhao, X.M.; Bai, Y.; Qiao, L.J. Water adsorption behavior on metal surfaces and its influence on surface potential studied by in situ SPM. Appl. Surf. Sci. 2012, 258, 9087–9091. [Google Scholar] [CrossRef]
- Gossenberger, F.; Roman, T.; Forster-Tonigold, K.; Groß, A. Change of the work function of platinum electrodes induced by halide adsorption. Beilstein J. Nanotech. 2014, 5, 152–161. [Google Scholar] [CrossRef]
- Örnek, C.; Liu, M.; Pan, J.; Jin, Y.; Leygraf, C. Volta potential evolution of intermetallics in aluminum alloy microstructure under thin aqueous adlayers: A combined DFT and experimental study. Top. Catal. 2018, 61, 1169–1182. [Google Scholar] [CrossRef]
- Sarvghad-Moghaddam, M.; Parvizi, R.; Davoodi, A.; Haddad-Sabzevar, M.; Imani, A. Establishing a correlation between interfacial microstructures and corrosion initiation sites in Al/Cu joints by SEM–EDS and AFM–SKPFM. Corros. Sci. 2014, 79, 148–158. [Google Scholar] [CrossRef]
- Schmutz, P.; Frankel, G.S. Characterization of AA2024-T3 by scanning Kelvin probe force microscopy. J. Electrochem. Soc. 1998, 145, 2285–2295. [Google Scholar] [CrossRef]
- Rohwerder, M.; Turcu, F. High-resolution Kelvin probe microscopy in corrosion science: Scanning Kelvin probe force microscopy (SKPFM) versus classical scanning Kelvin probe (SKP). Electrochim. Acta 2007, 53, 290–299. [Google Scholar] [CrossRef]
- Migani, A.; Sousa, C.; Illas, F. Chemisorption of atomic chlorine on metal surfaces and the interpretation of the induced work function changes. Surf. Sci. 2005, 574, 297–305. [Google Scholar] [CrossRef]
- Roman, T.; Gross, A. Periodic density-functional calculations on work-function change induced by adsorption of halogens on Cu (111). Phys. Rev. Lett. 2013, 110, 156804. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, S.Q. Trends and regularities for halogen adsorption on various metal surfaces. J. Electrochem. Soc. 2016, 163, H796–H808. [Google Scholar] [CrossRef]
- Zhou, W.L.; Liu, T.; Li, M.C.; Zhao, T.; Duan, Y.H. Adsorption of bromine on Mg (0001) surface from first-principles calculations. Comput. Mater. Sci. 2016, 111, 47–53. [Google Scholar] [CrossRef]
- Marks, L. Competitive Chloride chemisorption disrupts hydrogen bonding networks: DFT, crystallography, thermodynamics, and morphological consequences. Corros. 2017, 74, 295–311. [Google Scholar] [CrossRef]
- Duan, S.; Xu, X.; Tian, Z.Q.; Luo, Y. Hybrid molecular dynamics and first-principles study on the work function of a Pt (111) electrode immersed in aqueous solution at room temperature. Phys. Rev. B 2012, 86, 045450. [Google Scholar] [CrossRef]
- Meng, S.; Wang, E.G.; Gao, S. Water adsorption on metal surfaces: A general picture from density functional theory studies. Phys. Rev. B 2004, 69, 195404. [Google Scholar] [CrossRef]
- Schnur, S.; Groß, A. Properties of metal–water interfaces studied from first principles. New J. Phys. 2009, 11, 125003. [Google Scholar] [CrossRef]
- Pedroza, L.S.; Poissier, A.; Fernández-Serra, M.V. Local order of liquid water at metallic electrode surfaces. J. Chem. Phys. 2015, 142, 034706. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.L.; Chen, Z.X. Density functional slab model studies of water adsorption on flat and stepped Cu surfaces. Surf. Sci. 2007, 601, 954–964. [Google Scholar] [CrossRef]
- Tzvetkov, G.; Zubavichus, Y.; Koller, G.; Schmidt, T.; Heske, C.; Umbach, E.; Grunze, M.; Ramsey, M.G.; Netzer, F.P. Growth of H2O layers on an ultra-thin Al2O3 film: From monomeric species to ice. Surf. Sci. 2003, 543, 131–140. [Google Scholar] [CrossRef]
- Musumeci, F.; Pollack, G.H. Influence of water on the work function of certain metals. Chem. Phys. Lett. 2012, 536, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, E.; Spitzer, A.; Lüth, H. The adsorption of water on Pt (111) studied by irreflection and UV-photoemission spectroscopy. Surf. Sci. 1984, 147, 179–190. [Google Scholar] [CrossRef]
- McCrum, I.T.; Akhade, S.A.; Janik, M.J. Electrochemical specific adsorption of halides on Cu 111, 100, and 211: A Density Functional Theory study. Electrochim. Acta 2015, 173, 302–309. [Google Scholar] [CrossRef]
- Gossenberger, F.; Roman, T.; Groß, A. Hydrogen and halide co-adsorption on Pt (111) in an electrochemical environment: A computational perspective. Electrochim. Acta 2016, 216, 152–159. [Google Scholar] [CrossRef]
- Bange, K.; Grider, D.; Sass, J.K. Coadsorption of water and ions on Cu (110): Models for the double layer. Surf. Sci. 1983, 126, 437–443. [Google Scholar] [CrossRef]
- Wasileski, S.A.; Janik, M.J. A first-principles study of molecular oxygen dissociation at an electrode surface: A comparison of potential variation and coadsorption effects. Phys. Chem. Chem. Phys. 2008, 10, 3613–3627. [Google Scholar] [CrossRef]
- Dieter, G.E. Mechanical Metallurgy; McGraw-Hill Press: New York, NY, USA, 1986. [Google Scholar]
- Villars, P.; Calvert, L.D. Pearson’s Handbook of Crystallographic Data for Intermetallic Phases; American Society of Metals: Cleveland, OH, USA, 1985. [Google Scholar]
- Jin, Y.; Liu, M.; Zhang, C.H.; Leygraf, C.; Wen, L.; Pan, J. First-principle calculation of Volta potential of intermetallic particles in aluminum alloys and practical implications. J. Electrochem. Soc. 2017, 164, C465–C473. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Pineau, N.; Minot, C.; Maurice, V.; Marcus, P. Density functional theory study of the interaction of Cl− with passivated nickel surfaces. Electrochem. Solid-Sate Lett. 2003, 6, B47–B51. [Google Scholar] [CrossRef]
- Björneholm, O.; Hansen, M.; Hodgson, A.; Liu, L.; Limmer, D.; Michaelides, A.; Pedevilla, P.; Rossmeisl, J.; Shen, H.; Tocci, G.; et al. Water at interfaces. Chem. Rev. 2016, 116, 7698–7726. [Google Scholar]
- Thiel, P.A.; Madey, T.E. The interaction of water with solid surfaces: Fundamental aspects. Surf. Sci. Rep. 1987, 7, 211–385. [Google Scholar] [CrossRef]
- Michaelides, A. Simulating ice nucleation, one molecule at a time, with the ‘DFT microscope’. Faraday Discuss. 2007, 136, 287–297. [Google Scholar] [CrossRef]
- Michaelides, A.; Alavi, A.; King, D.A. Insight into H2O-ice adsorption and dissociation on metal surfaces from first-principles simulations. Phys. Rev. B 2004, 69, 113404. [Google Scholar] [CrossRef]
- Guo, F.Y.; Long, C.G.; Zhang, J.; Zhang, Z.; Liu, C.H.; Yu, K. Adsorption and dissociation of H2O on Al (1 1 1) surface by density functional theory calculation. Appl. Surf. Sci. 2015, 324, 584–589. [Google Scholar] [CrossRef]
- Taheri, P.; Pohl, K.; Grundmeier, G.; Flores, J.R.; Hannour, F.; de Wit, J.H.W.; Mol, J.M.C.; Terryn, H. Effects of surface treatment and carboxylic acid and anhydride molecular dipole moments on the Volta potential values of zinc surfaces. J. Phys. Chem. C 2013, 117, 1712–1721. [Google Scholar] [CrossRef]
- Cai, N.; Zhou, G.; Müller, K.; Starr, D.E. Comparative study of the passivation of Al (111) by molecular oxygen and water vapor. J. Phys. Chem. C 2012, 117, 172–178. [Google Scholar] [CrossRef]
- Digne, M.; Raybaud, P.; Sautet, P.; Guillaume, D.; Toulhoat, H. Atomic scale insights on chlorinated γ-alumina surfaces. J. Am. Chem. Soc. 2008, 130, 11030–11039. [Google Scholar] [CrossRef] [PubMed]
- Huheey, J.E.; Ketter, E.A.; Ketter, R.L.; Medhi, O.K. Inorganic Chemistry: Principles of Structure and Reactivity; Pearson Education India: Delhi, India, 1993. [Google Scholar]
- Bouzoubaa, A.; Costa, D.; Diawara, B.; Audiffren, N.; Marcus, P. Insight of DFT and atomistic thermodynamics on the adsorption and insertion of halides onto the hydroxylated NiO (111) surface. Corros. Sci. 2010, 52, 2643–2652. [Google Scholar] [CrossRef]
- Ren, J.; Meng, S. First-principles study of water on copper and noble metal (110) surfaces. Phys. Rev. B 2008, 77, 054110. [Google Scholar] [CrossRef]
- Jin, X.; Yang, W.; Qian, Z.; Wang, Y.; Bi, S. DFT study on the interaction between monomeric aluminium and chloride ion in aqueous solution. Dalton Trans. 2011, 40, 5052–5058. [Google Scholar] [CrossRef]
- Leung, T.C.; Kao, C.L.; Su, W.S.; Feng, Y.J.; Chan, C.T. Relationship between surface dipole, work function and charge transfer: Some exceptions to an established rule. Phys. Rev. B 2003, 68, 195408. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, K.; Frankel, G.S. Intermetallic phases in aluminum alloys and their roles in localized corrosion. J. Electrochem. Soc. 2018, 165, C807–C820. [Google Scholar] [CrossRef]
- Xue, M.; Xie, J.; Li, W.; Yang, C.; Ai, Y.; Wang, F.; Ou, J.; Yao, J. Dependence of electron work function of Al-Mg alloys on surface structures and relative humidity. Physica B 2011, 406, 4240–4244. [Google Scholar] [CrossRef]
- Crispin, X.; Geskin, V.; Crispin, A.; Cornil, J.; Lazzaroni, R.; Salaneck, W.R.; Bredas, J.L. Characterization of the interface dipole at organic/metal interfaces. J. Am. Chem. Soc. 2002, 124, 8131–8141. [Google Scholar] [CrossRef]
- Migani, A.; Illas, F. A systematic study of the structure and bonding of halogens on low-index transition metal surfaces. J. Phys. Chem. B 2006, 110, 11894–11906. [Google Scholar] [CrossRef]
- Andreatta, F.; Terryn, H.; de Wit, J.H.W. Effect of solution heat treatment on galvanic coupling between intermetallics and matrix in AA7075-T6. Corros. Sci. 2003, 45, 1733–1746. [Google Scholar] [CrossRef]
- Rossmeisl, J.; Nørskov, J.K.; Taylor, C.D.; Janik, M.J.; Neurock, M. Calculated phase diagrams for the electrochemical oxidation and reduction of water over Pt (111). J. Phys. Chem. B 2006, 110, 21833–21839. [Google Scholar] [CrossRef] [PubMed]
- Liu, M. a,b; Busch, M. c; Grönbeck, H. c; Jin, Y. a; Leygraf, C. b; Pan, J. b Aqueous environment around chloride ion studied by first-principle theory, molecular dynamics and x-ray absorption fine structure. (a: University of Science and Technology Beijing, Beijing, China. b: Biotechnology and Health, KTH Royal Institute of Technology, Stockholm, Sweden. c: Chalmers University of Technology, Göteborg). Unpublished work, 2019.
- Wei, X.; Dong, C.; Chen, Z.; Xiao, K.; Li, X. A DFT study of the adsorption of O2 and H2O on Al (111) surfaces. RSC Adv. 2016, 6, 56303–56312. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Jin, Y.; Pan, J.; Leygraf, C. Co-Adsorption of H2O, OH, and Cl on Aluminum and Intermetallic Surfaces and Its Effects on the Work Function Studied by DFT Calculations. Molecules 2019, 24, 4284. https://doi.org/10.3390/molecules24234284
Liu M, Jin Y, Pan J, Leygraf C. Co-Adsorption of H2O, OH, and Cl on Aluminum and Intermetallic Surfaces and Its Effects on the Work Function Studied by DFT Calculations. Molecules. 2019; 24(23):4284. https://doi.org/10.3390/molecules24234284
Chicago/Turabian StyleLiu, Min, Ying Jin, Jinshan Pan, and Christofer Leygraf. 2019. "Co-Adsorption of H2O, OH, and Cl on Aluminum and Intermetallic Surfaces and Its Effects on the Work Function Studied by DFT Calculations" Molecules 24, no. 23: 4284. https://doi.org/10.3390/molecules24234284
APA StyleLiu, M., Jin, Y., Pan, J., & Leygraf, C. (2019). Co-Adsorption of H2O, OH, and Cl on Aluminum and Intermetallic Surfaces and Its Effects on the Work Function Studied by DFT Calculations. Molecules, 24(23), 4284. https://doi.org/10.3390/molecules24234284