Phytofabrication of Nanoparticles as Novel Drugs for Anticancer Applications
Abstract
1. Introduction
2. Advances in Utilizing NPs in Cancer Management and Therapy
2.1. Gold NPs (AuNPs)
2.2. Silver NPs (AgNPs)
2.3. Other Metal NPs
2.4. Plant-Derived Edible NPs
2.5. Plant Lipid-Derived NPs
3. Phytochemicals Conjugated with NPs as Nanomedicine
3.1. Apigenin
3.2. Resveratrol (RES)
3.3. Curcumin (Cur)
3.4. (−)-Epigallocatechin-3-gallate (EGCG)
3.5. 6-Gingerol (6G)
3.6. Quercetin (Qc)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | protein kinase B |
| APLC | apigenin-phospholipid phytosome |
| AuNPs | gold nanoparticles |
| Cur | curcumin |
| EGCG | epigallocatechin-3-gallate |
| EGFR | epidermal growth factor receptor |
| 6G | 6-gingerol |
| IC50 | 50% inhibiting concentration |
| IONP/HAp-NaAlg | pH sensitive sodium alginate, hydroxyapatite bilayer-coated iron oxide nanoparticle composite |
| LPC | cisplatin nanoparticle |
| LPNs | lipid–polymeric NPs |
| m-HAP | magnetic hydroxyapatite |
| MNC | micellar nanocomplex |
| NPs | nanoparticles |
| PI3K | phosphatidylinositol 3-kinase |
| PLA–PEG | polylactic acid and polyethylene glycol |
| PLGA | poly(lactic-co-glycolide acid) |
| Qc | quercetin |
| RES | resveratrol |
| RES–AuNPs | RES-loaded with AuNPs |
| RES–GNPs | gelatin nanoparticles-loaded RES |
| SU | sunitinib |
| Tf–RES–L | transferrin-targeted, resveratrol-loaded liposome |
| VEGF | vascular endothelial growth factor |
| VEGFR | VEGF receptor |
References
- DeVita, V.T., Jr.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Przystupski, D.; Niemczura, M.J.; Górska, A.; Supplitt, S.; Kotowski, K.; Wawryka, P.; Rozborska, P.; Woźniak, K.; Michel, O.; Kiełbik, A.; et al. In search of panacea—review of recent studies concerning nature-derived anticancer agents. Nutrients 2019, 11, 1426. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.K.; Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Dietary polyphenols in prevention and treatment of prostate cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef] [PubMed]
- DiMarco-Crook, C.; Xiao, H. Diet-Based strategies for cancer chemoprevention: The role of combination regimens using dietary bioactive components. Annu. Rev. Food Sci. Technol. 2015, 6, 505–526. [Google Scholar] [CrossRef]
- Baena Ruiz, R.; Salinas Hernández, P. Cancer chemoprevention by dietary phytochemicals: Epidemiological evidence. Maturitas 2016, 94, 13–19. [Google Scholar] [CrossRef]
- Navya, P.N.; Kaphle, A.; Srinivas, S.P.; Bhargava, S.K.; Rotello, V.M.; Daima, H.K. Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 2019, 6, 23. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Rawal, S.; Patel, M.M. Threatening cancer with nanoparticle aided combination oncotherapy. J. Control. Release 2019, 301, 76–109. [Google Scholar] [CrossRef]
- Varma, R.S. Greener approach to nanomaterials and their sustainable applications. Curr. Opin. Chem. Eng. 2012, 1, 123–128. [Google Scholar] [CrossRef]
- Sindhu, K.; Indra, R.; Rajaram, A.; Sreeram, K.J.; Rajaram, R. Investigations on the interaction of gold-curcumin nanoparticles with human peripheral blood lymphocytes. J. Biomed. Nanotechnol 2011, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Mohammadhosseini, M.; Nejati-Koshki, K.; Abadi, A.J.; Moafi, H.F.; Akbarzadeh, A.; Farshbaf, M. Preparation, surface properties, and therapeutic applications of gold nanoparticles in biomedicine. Drug Res. (Stuttg) 2017, 67, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A. Application of gold nanoparticles in biomedical and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 410–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, M.; Webster, T.J. Growth process and anticancer properties of gold nanorods. J. Biomed. Mater. Res. A 2017, 105, 2616–2621. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.J.; Wang, C.; Mathiyalagan, R.; El-Agamy Farh, M.; Yang, D.C. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif. Cells Nanomed. Biotechnol. 2016, 44, 811–816. [Google Scholar] [CrossRef]
- Govindaraju, S.; Rengaraj, A.; Arivazhagan, R.; Huh, Y.S.; Yun, K. Curcumin-conjugated gold clusters for bioimaging and anticancer applications. Bioconjug. Chem. 2018, 29, 363–370. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1163–1170. [Google Scholar] [CrossRef]
- Singh, L.M.; Chakraborty, B.; Pal, R.; Nath, A.; Pal, S.; Shahidur Rahman, D.; Ghosh, S.K.; Sengupta, M. A comparative study on the antioxidant and immunomodulatory properties of curcumin conjugated gold nanospheres and free curcumin. J. Appl. Pharm. Sci. 2017, 7, 56–63. [Google Scholar]
- Singh, D.K.; Jagannathan, R.; Khandelwal, P.; Abraham, P.M.; Poddar, P. In situ synthesis and surface functionalization of gold nanoparticles with curcumin and their antioxidant properties: An experimental and density functional theory investigation. Nanoscale 2013, 5, 1882–1893. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Abdelfatah, E.A.; Khalil, W.A. Antitumor activity of curcumin-green synthesized gold nanoparticles: In vitro study. Bionanoscience 2019. [Google Scholar] [CrossRef]
- Vemuri, S.K.; Banala, R.R.; Mukherjee, S.; Uppula, P.; Gpv, S.; Gurava Reddy, A.V.; Malarvilli, T. Novel biosynthesized gold nanoparticles as anti-cancer agents against breast cancer: Synthesis, biological evaluation, molecular modelling studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Mukherjee, S.; Das, S.; Bhat, F.A.; Raja Singh, P.; Patra, C.R.; Arunakaran, J. Gold nanoparticles–conjugated quercetin induces apoptosis via inhibition of EGFR/PI3K/Akt–mediated pathway in breast cancer cell lines (MCF-7 and MDA-MB-231). Cell Biochem. Funct. 2017, 35, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.; Bhat, F.A.; Raja Singh, P.; Mukherjee, S.; Elumalai, P.; Das, S.; Patra, C.R.; Arunakaran, J. Gold nanoparticle–conjugated quercetin inhibits epithelial–mesenchymal transition, angiogenesis and invasiveness via EGFR / VEGFR–mediated pathway in breast cancer. Cell Prolif. 2016, 49, 678–697. [Google Scholar] [CrossRef] [PubMed]
- Chavva, S.R.; Deshmukh, S.K.; Kanchanapally, R.; Tyagi, N.; Coym, J.W.; Singh, A.P.; Singh, S. Epigallocatechin gallate-gold nanoparticles exhibit superior antitumor activity compared to conventional gold nanoparticles: Potential synergistic interactions. Nanomaterials (Basel) 2019, 9, 396. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ghosh, S.; Das, D.K.; Chakraborty, P.; Choudhury, S.; Gupta, P.; Adhikary, A.; Dey, S.; Chattopadhyay, S. Gold-conjugated green tea nanoparticles for enhanced anti-tumor activities and hepatoprotection-synthesis, characterization and in vitro evaluation. J. Nutr. Biochem. 2015, 26, 1283–1297. [Google Scholar] [CrossRef]
- Favi, P.M.; Valencia, M.M.; Elliott, P.R.; Restrepo, A.; Gao, M.; Huang, H.; Pavon, J.J.; Webster, T.J. Shape and surface chemistry effects on the cytotoxicity and cellular uptake of metallic nanorods and nanospheres. J. Biomed. Mater. Res. A 2015, 103, 3940–3955. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Atapour, A.; Amani, A.M.; Savar Dashtaki, A.; Babapoor, A.; Arjmand, O. Green synthesis of silver nanoparticles toward bio and medical applications: Review study. Artif. Cells Nanomed. Biotechnol. 2018, 46, S855–S872. [Google Scholar] [CrossRef]
- Rafique, M.; Sadaf, I.; Rafique, M.S.; Tahir, M.B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1272–1291. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Jacob, S.J.; Finub, J.S.; Narayanan, A. Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids Surf. B Biointerfaces 2012, 91, 212–214. [Google Scholar] [CrossRef]
- Singh, A.; Dar, M.Y.; Joshi, B.; Sharma, B.; Shrivastava, S.; Shukla, S. Phytofabrication of silver nanoparticles: Novel drug to overcome hepatocellular ailments. Toxicol. Rep. 2018, 5, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.N.; Sharma, T.K.; Bansal, V.; Shukla, R. Phytochemicals as dynamic surface ligands to control nanoparticle-protein interactions. ACS Omega 2018, 3, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Charbgoo, F.; Ahmad, M.B.; Darroudi, M. Cerium oxide nanoparticles: Green synthesis and biological applications. Int. J. Nanomedicine 2017, 12, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhuang, X.; Deng, Z.B.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef]
- Mu, J.; Zhuang, X.; Wang, Q.; Jiang, H.; Deng, Z.B.; Wang, B.; Zhang, L.; Kakar, S.; Jun, Y.; Miller, D.; et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014, 58, 1561–1567. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Xu, C.; Merlin, D. Plant derived edible nanoparticles as a new therapeutic approach against diseases. Tissue Barriers 2016, 4, e1134415. [Google Scholar] [CrossRef]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.G.; Guo, P. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef]
- Akuma, P.; Okagu, O.D.; Udenigwe, C.C. Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Front. Sustain. Food Syst. 2019, 3, 23. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Y.; Mu, J.; Egilmez, N.K.; Zhuang, X.; Deng, Z.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.G. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015, 75, 2520–2529. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.; Merlin, D. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol. Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef]
- Teng, Y.; Mu, J.; Hu, X.; Samykutty, A.; Zhuang, X.; Deng, Z.; Zhang, L.; Cao, P.; Yan, J.; Miller, D.; et al. Grapefruit-derived nanovectors deliver miR-18a for treatment of liver metastasis of colon cancer by induction of M1 macrophages. Oncotarget 2016, 7, 25683–25697. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Han, M.K.; Collins, J.F.; Merlin, D. Oral administration of ginger-derived nanolipids loaded with siRNA as a novel approach for efficient siRNA drug delivery to treat ulcerative colitis. Nanomedicine (Lond) 2017, 12, 1927–1943. [Google Scholar] [CrossRef]
- Seden, K.; Dickinson, L.; Khoo, S.; Back, D. Grapefruit-Drug Interactions. Drugs 2010, 70, 2373–2407. [Google Scholar] [CrossRef]
- Mouly, S.; Lloret-Linares, C.; Sellier, P.O.; Sene, D.; Bergmann, J.F. Is the clinical relevance of drug-food and drug-herb interactions limited to grapefruit juice and Saint-John’s Wort? Pharmacol. Res. 2017, 118, 82–92. [Google Scholar] [CrossRef]
- Madunić, J.; Madunić, I.V.; Gajski, G.; Popić, J.; Garaj-Vrhovac, V. Apigenin: A dietary flavonoid with diverse anticancer properties. Cancer Lett. 2018, 413, 11–22. [Google Scholar] [CrossRef]
- Yan, X.; Qi, M.; Li, P.; Zhan, Y.; Shao, H. Apigenin in cancer therapy: Anti-cancer effects and mechanisms of action. Cell Biosci. 2017, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Jung, N.; Lee, S.; Sohng, J.K.; Jung, H.J. Apigenin inhibits cancer stem cell-like phenotypes in human glioblastoma cells via suppression of c-Met signaling. Phytother. Res. 2016, 30, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Xu, J.; Zhu, G.Y.; Huang, Z.J.; Lu, Y.; Li, X.Q.; Wang, N.; Zhang, F.X. Apigenin suppresses the stem cell-like properties of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Cell Death Discov. 2018, 4, 105. [Google Scholar] [CrossRef] [PubMed]
- Ketkaew, Y.; Osathanon, T.; Pavasant, P.; Sooampon, S. Apigenin inhibited hypoxia induced stem cell marker expression in a head and neck squamous cell carcinoma cell line. Arch. Oral Biol. 2017, 74, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, D.; Huang, Y.; Gao, Y.; Qian, S. Biopharmaceutics classification and intestinal absorption study of apigenin. Int. J. Pharm. 2012, 436, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, J.; Samadder, A.; Paul, A.; Khuda-Bukhsh, A.R. Efficacy of PLGA-loaded apigenin nanoparticles in Benzo[a]pyrene and ultraviolet-B induced skin cancer of mice: Mitochondria mediated apoptotic signalling cascades. Food Chem. Toxicol. 2013, 62, 670–680. [Google Scholar] [CrossRef]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef]
- Wu, W.; Zu, Y.; Wang, L.; Wang, L.; Wang, H.; Li, Y.; Wu, M.; Zhao, X.; Fu, Y. Preparation, characterization and antitumor activity evaluation of apigenin nanoparticles by the liquid antisolvent precipitation technique. Drug Deliv. 2017, 24, 1713–1720. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, K.; Cheng, L.; Yan, B.; Qian, W.; Cao, J.; Li, J.; Wu, E.; Ma, Q.; Yang, W. Resveratrol and cancer treatment: Updates. Ann. N. Y. Acad. Sci. 2017, 1403, 59–69. [Google Scholar] [CrossRef]
- Ray, P.S.; Maulik, G.; Cordis, G.A.; Bertelli, A.A.; Bertelli, A.; Das, D.K. The red wine antioxidant resveratrol protects isolated rat hearts from ischemia reperfusion injury. Free Radic. Biol. Med. 1999, 27, 160–169. [Google Scholar] [CrossRef]
- Wadsworth, T.L.; Koop, D.R. Effects of the wine polyphenolics quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Biochem. Pharmacol. 1999, 57, 941–949. [Google Scholar] [CrossRef]
- Mondal, A.; Bennett, L.L. Resveratrol enhances the efficacy of sorafenib mediated apoptosis in human breast cancer MCF7 cells through ROS, cell cycle inhibition, caspase 3 and PARP cleavage. Biomed. Pharmacother. 2016, 84, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.M.; Yu, W.; Jia, L.; Sanders, B.G.; Kline, K. Vitamin E analog α-TEA, methylseleninic acid, and trans-resveratrol in combination synergistically inhibit human breast cancer cell growth. Nutr. Cancer 2008, 60, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, Š.; Lavrič, Z.; Kristl, J. Stability and solubility of trans-resveratrol are strongly influenced by pH and temperature. Eur. J. Pharm. Biopharm. 2015, 93, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to improve resveratrol systemic and topical bioavailability: An update. Antioxidants (Basel) 2019, 8, 244. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Hoti, S.L.; Prasad, N.R. Resveratrol loaded gelatin nanoparticles synergistically inhibits cell cycle progression and constitutive NF-kappaB activation, and induces apoptosis in non-small cell lung cancer cells. Biomed. Pharmacother. 2015, 70, 274–282. [Google Scholar] [CrossRef]
- Jhaveri, A.; Deshpande, P.; Pattni, B.; Torchilin, V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control. Release 2018, 277, 89–101. [Google Scholar] [CrossRef]
- Nassir, A.M.; Shahzad, N.; Ibrahim, I.A.A.; Ahmad, I.; Md, S.; Ain, M.R. Resveratrol-loaded PLGA nanoparticles mediated programmed cell death in prostate cancer cells. Saudi Pharm. J. 2018, 26, 876–885. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Zeng, J.; Li, Z.; Zuo, H.; Huang, C.; Zhao, X. Nano-gold loaded with resveratrol enhance the anti-hepatoma effect of resveratrol in vitro and in vivo. J. Biomed. Nanotechnol. 2019, 15, 288–300. [Google Scholar] [CrossRef]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [PubMed]
- Joe, B.; Vijaykumar, M.; Lokesh, B.R. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit. Rev. Food Sci. Nutr. 2004, 44, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Harsha, C.; Banik, K.; Gupta, S.C.; Aggarwal, B.B. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin. Sci. (Lond) 2017, 131, 1781–1799. [Google Scholar] [CrossRef]
- Vinod, B.S.; Antony, J.; Nair, H.H.; Puliyappadamba, V.T.; Saikia, M.; Narayanan, S.S.; Bevin, A.; Anto, R.J. Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis. 2013, 4, e505. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Al Zohairy, M.A.; Aly, S.M.; Khan, M.A. Curcumin: A potential candidate in prevention of cancer via modulation of molecular pathways. Biomed Res. Int. 2014, 2014, 761608. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Jankun, J.; Wyganowska-Swiatkowska, M.; Dettlaff, K.; Jelinska, A.; Surdacka, A.; Watróbska-Swietlikowska, D.; Skrzypczak-Jankun, E. Determining whether curcumin degradation/condensation is actually bioactivation (Review). Int. J. Mol. Med. 2016, 37, 1151–1158. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. The pharmacology of curcumin: Is it the degradation products? Trends Mol. Med. 2012, 18, 138–144. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Shi, Z.; Yang, Y.; Xie, X.; Lee, S.M.; Wang, Y.; Leong, K.W.; Chen, M. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 2017, 58, 349–364. [Google Scholar] [CrossRef]
- Singh, N.; Sachdev, A.; Gopinath, P. Polysaccharide functionalized single walled carbon nanotubes as nanocarriers for delivery of curcumin in lung cancer cells. J. Nanosci. Nanotechnol. 2018, 18, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Arya, G.; Das, M.; Sahoo, S.K. Evaluation of curcumin loaded chitosan/PEG blended PLGA nanoparticles for effective treatment of pancreatic cancer. Biomed. Pharmacother. 2018, 102, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Barras, A.; Mezzetti, A.; Richard, A.; Lazzaroni, S.; Roux, S.; Melnyk, P.; Betbeder, D.; Monfilliette-Dupont, N. Formulation and characterization of polyphenol-loaded lipid nanocapsules. Int. J. Pharm. 2009, 379, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Adhami, V.M.; Ahmad, N.; Mukhtar, H. Nanochemoprevention: Sustained release of bioactive food components for cancer prevention. Nutr. Cancer 2010, 62, 883–890. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Bharali, D.J.; Nihal, M.; Adhami, V.M.; Khan, N.; Chamcheu, J.C.; Khan, M.I.; Shabana, S.; Mousa, S.A.; Mukhtar, H. Excellent anti-proliferative and pro-apoptotic effects of (-)-epigallocatechin-3-gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomedicine 2014, 10, 1619–1626. [Google Scholar] [CrossRef]
- Li, K.; Xiao, G.; Richardson, J.J.; Tardy, B.L.; Ejima, H.; Huang, W.; Guo, J.; Liao, X.; Shi, B. Targeted therapy against metastatic melanoma based on self-assembled metal-phenolic nanocomplexes comprised of green tea catechin. Adv. Sci. 2019, 6, 1801688. [Google Scholar] [CrossRef]
- Yongvongsoontorn, N.; Chung, J.E.; Gao, S.J.; Bae, K.H.; Yamashita, A.; Tan, M.H.; Ying, J.Y.; Kurisawa, M. Carrier-enhanced anticancer efficacy of sunitinib-loaded green tea-based micellar nanocomplex beyond tumor-targeted delivery. ACS Nano 2019, 13, 7591–7602. [Google Scholar] [CrossRef]
- De Lima, R.M.T.; Dos Reis, A.C.; de Menezes, A.P.M.; Santos, J.V.O.; Filho, J.W.G.O.; Ferreira, J.R.O.; de Alencar, M.V.O.B.; da Mata, A.M.O.F.; Khan, I.N.; Islam, A.; et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phytother. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef]
- Bhattarai, S.; Tran, V.H.; Duke, C.C. Stability of [6]-gingerol and [6]-shogaol in simulated gastric and intestinal fluids. J. Pharm. Biomed. Anal. 2007, 45, 648–653. [Google Scholar] [CrossRef]
- Manatunga, D.C.; de Silva, R.M.; de Silva, K.M.N.; de Silva, N.; Bhandari, S.; Yap, Y.K.; Costha, N.P. pH responsive controlled release of anti-cancer hydrophobic drugs from sodium alginate and hydroxyapatite bi-coated iron oxide nanoparticles. Eur. J. Pharm. Biopharm. 2017, 117, 29–38. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, Q.; Yang, Q.; Cao, X.; Li, Q.; Shi, F.; Tong, S.S.; Feng, C.; Yu, Q.; Yu, J.; et al. A novel formulation of [6]-gingerol: Proliposomes with enhanced oral bioavailability and antitumor effect. Int. J. Pharm. 2018, 535, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Yang, Q.; Wang, Q.; Sun, C.; Zhu, Y.; Niu, Y.; Yu, J.; Xu, X. Formulation, characterization, and pharmacokinetic studies of 6-gingerol-Loaded nanostructured lipid carriers. AAPS PharmSciTech. 2018, 19, 3661–3669. [Google Scholar] [CrossRef] [PubMed]
- Behroozeh, A.; Mazloumi Tabrizi, M.; Kazemi, S.M.; Choupani, E.; Kabiri, N.; Ilbeigi, D.; Heidari Nasab, A.; Akbarzadeh Khiyavi, A.; Seif Kurdi, A. Evaluation the anti-cancer effect of PEGylated Nano-niosomal Gingerol, on breast cancer cell lines (T47D), in-vitro. Asian Pacific J. Cancer Prev. 2018, 19, 645–648. [Google Scholar]
- Manatunga, D.C.; de Silva, R.M.; de Silva, K.M.N.; Wijeratne, D.T.; Malavige, G.N.; Williams, G. Fabrication of 6-gingerol, doxorubicin and alginate hydroxyapatite into a bio-compatible formulation: Enhanced anti-proliferative effect on breast and liver cancer cells. Chem. Cent. J. 2018, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Michaud-Levesque, J.; Bousquet-Gagnon, N.; Béliveau, R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp. Cell Res. 2012, 318, 925–935. [Google Scholar] [CrossRef]
- Kulisic-Bilusic, T.; Schmöller, I.; Schnäbele, K.; Siracusa, L.; Ruberto, G. The anticarcinogenic potential of essential oil and aqueous infusion from caper (Capparis spinosa L.). Food Chem. 2012, 132, 261–267. [Google Scholar] [CrossRef]
- Moon, J.H.; Eo, S.K.; Lee, J.H.; Park, S.Y. Quercetin-induced autophagy flux enhances TRAIL-mediated tumor cell death. Oncol. Rep. 2015, 34, 375–381. [Google Scholar] [CrossRef]
- Lee, J.; Han, S.I.; Yun, J.H.; Kim, J.H. Quercetin 3-O-glucoside suppresses epidermal growth factor–induced migration by inhibiting EGFR signaling in pancreatic cancer cells. Tumor Biol. 2015, 36, 9385–9393. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Dahmani, F.Z.; Yin, L.; Zhou, J.; Yao, J. Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials 2014, 35, 7654–7665. [Google Scholar] [CrossRef]
- Staedler, D.; Idrizi, E.; Kenzaoui, B.H.; Juillerat-jeanneret, L. Drug combinations with quercetin: Doxorubicin plus quercetin in human breast cancer cells. Cancer Chemother. Pharmacol. 2011, 68, 1161–1172. [Google Scholar] [CrossRef]
- Minaei, A.; Sabzichi, M.; Ramezani, F.; Hamishehkar, H.; Samadi, N. Co-delivery with nano-quercetin enhances doxorubicin-mediated cytotoxicity against MCF-7 cells. Mol. Biol. Rep. 2016, 43, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, S.; Wang, Y.; Yu, Y.; Li, F.; Zhu, H.; Shen, Y.; Huang, S.; Guo, S. Near-infrared triggered co-delivery of doxorubicin and quercetin by using gold nanocages with tetradecanol to maximize anti-tumor effects on MCF-7/ADR cells. J. Colloid Interface Sci. 2018, 509, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Yu, L.; Yue, Q. Co-delivery of vincristine and quercetin by nanocarriers for lymphoma combination chemotherapy. Biomed. Pharmacother. 2017, 91, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Miao, L.; Goodwin, T.J.; Li, J.; Liu, Q.; Huang, L. Quercetin remodels the tumor microenvironment to improve the permeation, retention, and antitumor effects of nanoparticles. ACS Nano 2017, 11, 4916–4925. [Google Scholar] [CrossRef] [PubMed]
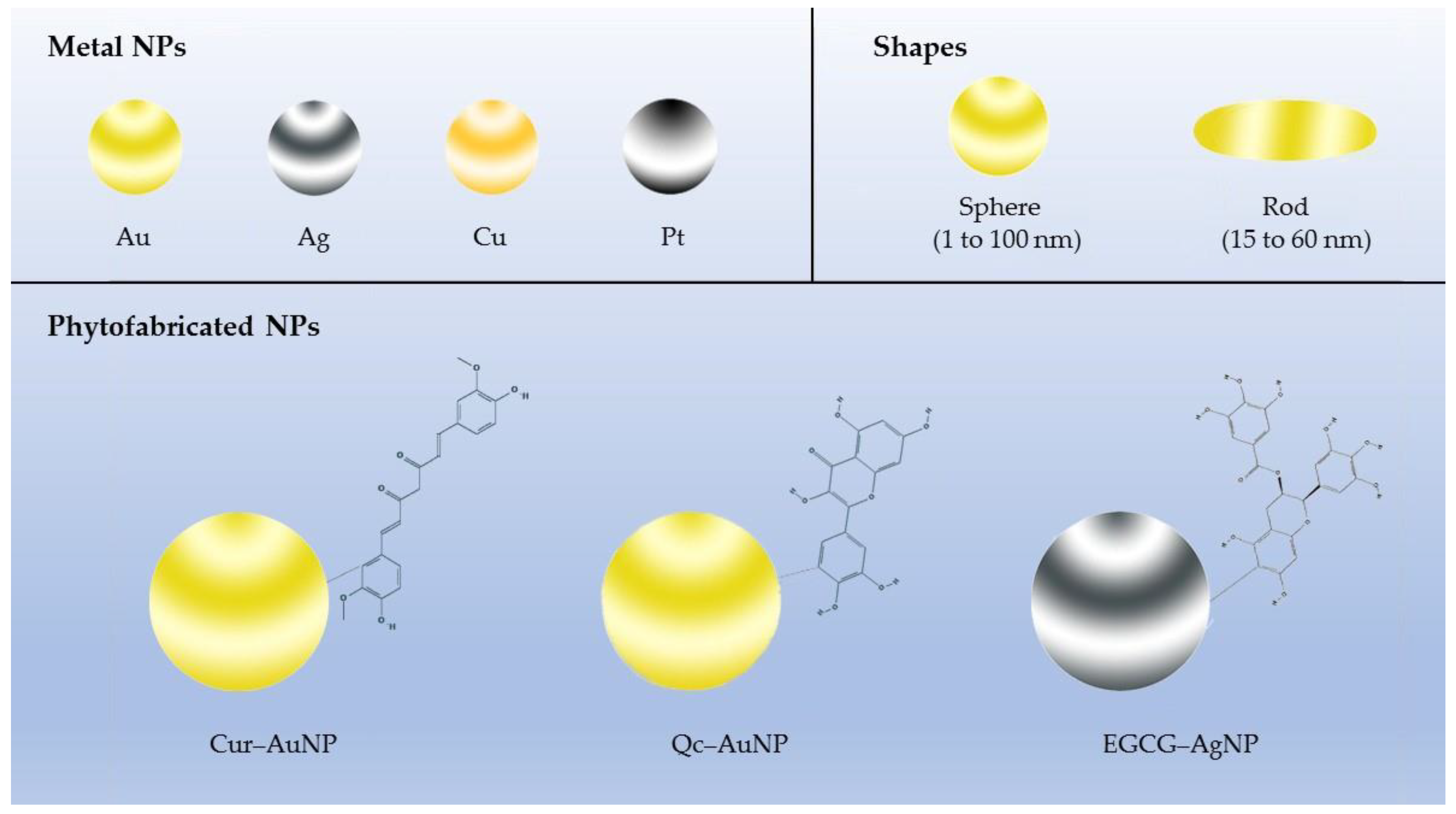


| (Phyto)chemicals | Studying Group | Nanoparticles | Cancer Types | Conditions | Efficacy as Compared with Free Forms | Reference |
|---|---|---|---|---|---|---|
| Apigenin | Das et al. (2013) | PLGA | Skin cancer | In vitro and in vivo | Enhanced anti-carcinogenic effect | [56] |
| Telange et al. (2017) | APLC | Liver cancer | In vitro and in vivo | Improved aqueous solubility, dissolution, in vivo bioavailability, and antioxidant activity | [57] | |
| Wu et al. (2017) | Liposomes | Hepatoma | In vitro and in vivo | Improved solubility and bioavailability | [58] | |
| Resveratrol | Karthikeyan et al. (2015) | Gelatin | Lung cancer | In vitro | Better stability; improved drug- loading capacity; sustained drug-release; improved cytotoxicity | [67] |
| Jhaveri et al. (2018) | Liposomes | Glioblastoma | In vitro and in vivo | Enhanced solubility and stability; sustained drug-release; better tumor selectivity | [68] | |
| Nassir et al. (2018) | PLGA | Prostate cancer | In vitro | Enhanced anti-carcinogenic effect by inducing mitochondrial-dependent apoptosis and cell arrest | [69] | |
| Zhang et al. (2019) | Au | Hepatoma | In vitro and in vivo | Inhibition of tumor growth; induced tumor apoptosis and decreased the expression of VEGF | [70] | |
| Curcumin–Doxorubicin | Zhang et al. (2017) | pH-sensitive nanoparticles | Liver cancer | In vitro and in vivo | Low polydispersity and high encapsulation efficiency; enhanced release in the acidic environment; inhibition of angiogenesis | [80] |
| Curcumin | Singh et al. (2018) | Single walled carbon nanotubes | Lung adenocarcinoma | In vitro | Improved aqueous solubility; a moderate and ideal drug delivery system; enhanced anticancer effect | [81] |
| Arya et al. (2018) | PLGA | Metastatic pancreatic cancer | In vitro | Superior cytotoxicity; enhanced anti-migratory; anti-invasive and apoptosis-inducing ability | [82] | |
| EGCG | Siddiqui et al. (2010) | PLA–PEG | Prostate cancer | In vitro and in vivo | Enhanced bioavailability; superior inhibition of angiogenesis | [84] |
| Siddiqui et al. (2014) | Chitosan | Melanoma | In vitro and in vivo | Excellent anti-proliferation | [85] | |
| Li et al. (2019) | SmIII nanocomplexes | Metastatic melanoma | In vitro and in vivo | Decreased viability; inhibition of wound-induced migration; prevention of metastatic lung melanoma from spreading | [86] | |
| EGCG–Sunitinib | Yongvongsoontorn et al. (2019) | MNC | Renal carcinoma | In vitro and in vivo | Enhanced anticancer effects and less toxicity; inhibition of angiogenesis | [87] |
| 6-Gingerol/Curcumin | Manatunga et al. (2017) | IONP/HAp-NaAlg | Breast cancer | In vitro | Targeted and controlled release over a period of time | [90] |
| 6-Gingerol | Wang et al. (2018) | Nanosized proliposomes | Liver cancer | In vitro and in vivo | Improved water solubility; sustained drug release; enhanced oral bioavailability | [91] |
| Wei et al. (2018) | Lipid nanocapsules | Liver cancer | In vitro | Better stability and slower drug release; targeted delivery | [92] | |
| Behroozeh et al. (2018) | PEGylated nanoniosome | Breast cancer | In vitro and in vivo | Enhanced bioavailability | [93] | |
| Manatunga et al. (2018) | m-HAP | Breast and liver cancers | In vitro | Increased stability; controlled and targeted delivery; minimizing toxicity | [94] | |
| Quercetin–Doxorubicin | Minaei et al. (2016) | Lecithin | Breast cancer | In vitro and in vivo | Elevated efficacy of chemotherapeutics by increasing the permeability of tumor cells to chemical agents | [101] |
| Zhang et al. (2018) | Au nanocages | Breast cancer | In vitro and in vivo | Inhibition of tumor growth | [102] | |
| Quercetin–Vincristine | Zhu et al. (2017) | Lipid-polymeric | Lymphoma | In vitro and in vivo | Improved bioavailability and metabolic stability; remodeled tumor microenvironment and increased the penetration of second-wave nanoparticles into the tumor nests | [103] |
| Quercetin–Cisplatin | Hu et al. (2017) | Lipid calcium phosphate | Bladder carcinoma | In vitro and in vivo | Enhanced permeation and retention effect; selective targeting; greater antitumor efficacy and minimized toxicity | [104] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Q.-Y.; He, K.-M.; Chen, J.-L.; Xu, Y.-M.; Lau, A.T.Y. Phytofabrication of Nanoparticles as Novel Drugs for Anticancer Applications. Molecules 2019, 24, 4246. https://doi.org/10.3390/molecules24234246
Wei Q-Y, He K-M, Chen J-L, Xu Y-M, Lau ATY. Phytofabrication of Nanoparticles as Novel Drugs for Anticancer Applications. Molecules. 2019; 24(23):4246. https://doi.org/10.3390/molecules24234246
Chicago/Turabian StyleWei, Qi-Yao, Kai-Ming He, Jin-Ling Chen, Yan-Ming Xu, and Andy T. Y. Lau. 2019. "Phytofabrication of Nanoparticles as Novel Drugs for Anticancer Applications" Molecules 24, no. 23: 4246. https://doi.org/10.3390/molecules24234246
APA StyleWei, Q.-Y., He, K.-M., Chen, J.-L., Xu, Y.-M., & Lau, A. T. Y. (2019). Phytofabrication of Nanoparticles as Novel Drugs for Anticancer Applications. Molecules, 24(23), 4246. https://doi.org/10.3390/molecules24234246






