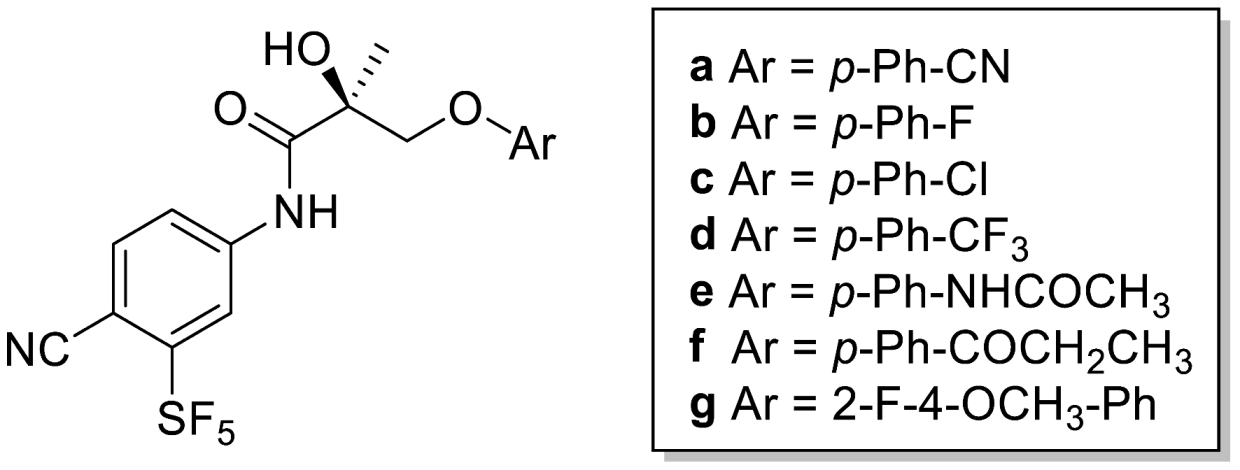
Abstract
The pentafluorosulfane (SF5) group, as a more electronegative bioisostere than the trifluoromethyl (CF3) group, has been gaining greater attention and increasingly reported usage in medicinal chemistry. Ostarine is the selective androgen receptor modulators (SARMs) containing a CF3 group in clinical trial III. In this study, 21 ostarine derivatives for replacing the CF3 group with SF5 substituents were synthesized. Some SF5-derivatives showed androgen receptor (AR) agonistic activities in vitro. The results pointed to the potential of using this scaffold to develop new AR agonists.
1. Introduction
Fluorine has been used widely in drug design and development, and 20–25% of pharmaceuticals contain a fluorine atom at present [1]. It is known that the existence of fluorine can influence electrostatic interactions and hydrogen bonding of a ligand [2]. Besides, the introduction of fluorine into a compound can change its lipophilicity, pKa, and metabolic stability [1]. Direct fluorination and addition of fluorinated functional groups are common methods to incorporate fluorine. The most common example of functional groups is trifluoromethyl (CF3).
The pentafluorosulfanyl (SF5) group, not only a bioisostere of the CF3 group, has been gaining greater attention and increasingly reported usage in medicinal chemistry according to the literature [3,4,5]. Some of the special properties of SF5 have been described, such as chemical and hydrolytically stability [5], steric demand and symmetry (volume of SF5 is slightly less than t-butyl and greater than CF3), electron-withdrawing effect [6,7], electronegativity (SF5 = 3.65, CF3 = 3.36), and nontoxicity of its degradation products [3]. However, the synthetic accessibility of the SF5 group has limited its use. Access to SF5-substituted benzenes is primarily accomplished through two methods, namely, treatment of thiophenols with fluorine gas and chlorofluorination of dibenzyl disulfides to give chlorotetrafluorosulfanyl benzenes (ArSF4Cl), which are, in turn, fluorinated to SF5 analogs [7,8]. The shortcomings of these methods may include low yield and reaction safety. SF5-bearing building blocks are attractive due to their reported bioactivities [9].
Androgen exerts its action via the androgen receptor (AR). Upon activation, AR translocates to the nucleus to mediate both androgenic and anabolic effects. Selective androgen receptor modulators (SARMs) bind AR and display tissue-selective activation of androgenic signaling [10,11,12]. Several nonsteroidal SARMs, such as ostarine [13,14], GSK2881078 [15], and LGD4033 [16], were developed for clinical trials (Figure 1). Among them, ostarine was shown to improve muscle functions in patients with cancer cachexia [17].
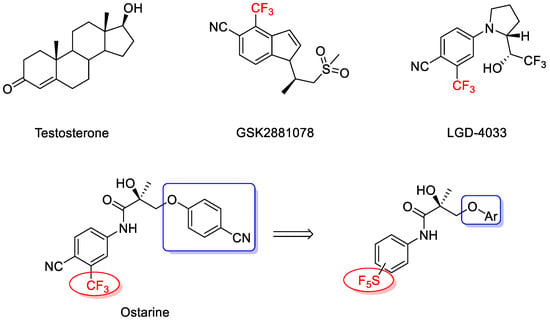
Figure 1.
Structures of testosterone and selected SARMs (selective androgen receptor modulators).
It appears that the strong electron-withdrawing effect of trifluoromethyl in SARMs plays a key role in AR binding [18,19,20]. Since none of the reported SARMs bears SF5 moiety, we decided to study the possibility and effect of replacing the CF3 group with SF5 substituents.
2. Results and Discussion
2.1. Chemistry
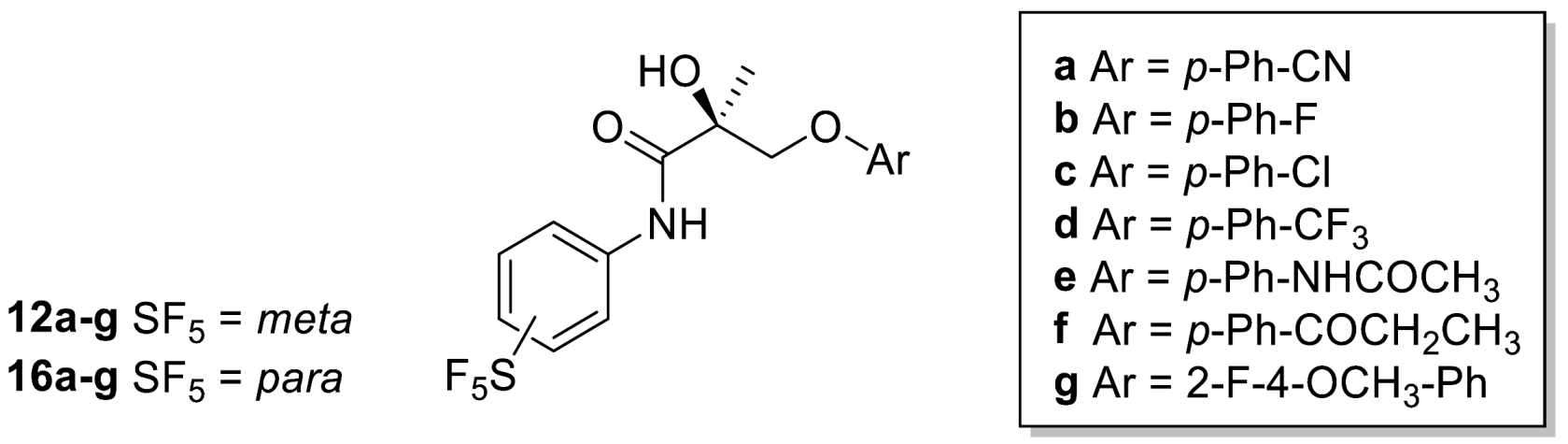
Meta-SF5 derivatives 12a–g and 13a–g were prepared, as illustrated in Scheme 1. 3-(pentafluorosulfanyl)aniline (1) was brominated with 1,3-Dibromo-5,5-dimethylhydantoin in DMAc (N,N-dimethylacetamide) in order to form compound 4-Bromo-3-(pentafluorosulfanyl)aniline (2). The second step consisted of the aromatic nucleophilic displacement of bromide from compound 2 using copper cyanide, leading to the formation of 4-amino-2-(pentafluorosulfanyl)benzonitrile (3).
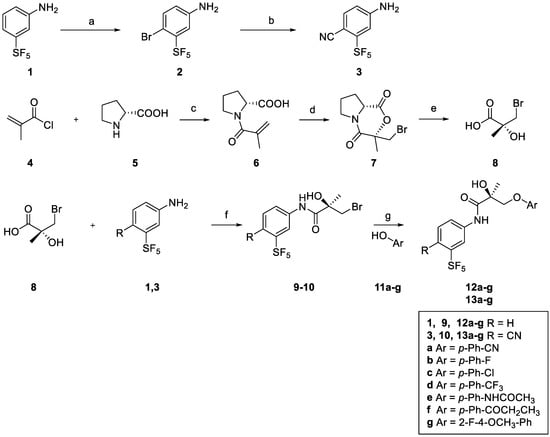
Scheme 1.
Synthesis of (S)-meta-SF5 derivatives 12a–g, 13a–g. Reagents and conditions: (a) DBDMH (1,3-Dibromo-5,5-dimethylhydantoin), DMAc, 10 °C 30 min; (b) CuCN, NMP (1-Methyl-2-pyrrolidinone), 180 °C, 2 h; (c) 2N NaOH, Acetone, 0 °C to rt, 3 h; (d) NBS (N-Bromosuccinimide), DMF, rt, 16 h; (e) 20% HBr(aq), reflux, 16 h; (f) 8, SOCl2, DMAc, −10 °C, 3 h followed by 21, DMAc, rt, 16 h; (g) K2CO3, 2-Propanol, reflux, 4 h.
(R)-N-methacryloylproline 6, prepared using D-proline (5) and methacryloyl chloride (4), was reacted with N-bromosuccinimide in DMF (N,N-dimethylformamide) to afford bromolactone 7 as a single enantiomer. Acidic hydrolysis with aqueous HBr then produced the key intermediate (R)-3-Bromo-2-hydroxy-2-methylpropanoic acid (8) [21]. Amide coupling with anilines 1,3 using thionyl chloride produced chiral anilides 9–10. Amide derivatives 9–10 were reacted with different commercial phenols (11a–g) using potassium carbonate in 2-propanol to give the desired (S)-meta-SF5 derivatives (12a–g, 13a–g).
Para-SF5 derivatives 16a–g were prepared, as illustrated in Scheme 2. Amide coupling with 4-(pentafluorosulfanyl)aniline (14) using thionyl chloride produced chiral anilide 15. Amide derivative 15 was reacted with different commercial phenols (11a–g) using potassium carbonate in 2-propanol to give the desired (S)-para-SF5 derivatives (16a–g).
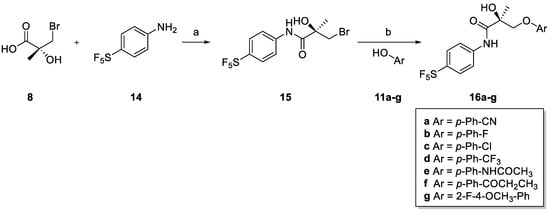
Scheme 2.
Synthesis of (S)-para-SF5 derivatives 16a–g. Reagents and conditions: (a) 8, SOCl2, DMAc, −10 oC, 3 h followed by 14, DMAc, rt, 16 h; (b) K2CO3, 2-Propanol, reflux, 4 h.
2.2. AR Activity In Vitro
All compounds listed in Table 1 and Table 2 were evaluated in vitro for their agonist activities using a luciferase reporter gene assay with CV-1 (Cercopithecus aethiops kidney cell line) and C2C12 cells (mouse myoblast cell line) expressing AR. The results of the reference compound (ostarine) are also shown for comparison in Table 1.

Table 1.
AR (androgen receptor) agonist activity of ostarine derivatives.

Table 2.
AR agonist activity of aryl propionamide derivatives.
Accordingly, with cyano and SF5 moieties, a series of para-substituted O-linked analogs were synthesized. All of these compounds displayed different degrees of AR agonist activity in CV-1 cells (Table 1). The introduction of the methoxy and N-amide groups as an electron-donating group on the para-phenyl ring (13e,g) resulted in significantly decreased AR agonist activity. When electron-withdrawing groups were introduced, compounds 13b, 13c, 13d, and 13f exhibited weak agonist activity (Table 1). The remaining compounds exhibited less than 66% efficacy of 1 μM dihydrotestosterone (DHT) at the concentrations tested. When the para-position of the phenyl ring was cyano moiety as an electron-withdrawing group, compound 13a showed potent agonist activity: 30 μM elicited 89.5% of the efficacy observed with 1 μM DHT, similar to that of ostarine (85.8% efficacy). The agonistic activity of the compounds in C2C12 cells was comparable.
To investigate the importance of the cyano moiety, compound series 12a–g and 16a–g in which the cyano is absent, were synthesized. Unfortunately, 16a–g in which the SF5 group as a bioisostere of the SF3 group is in the para position were completely inactive, and analogs 12a–g bearing only SF5 group at meta position lost AR agonist activity (Table 2). Thus, cyano moiety is required for AR agonism.
3. Materials and Methods
All reagents were commercially available and were used without further purification. The solvents used were of analytical grade. Melting points were taken on a Fisher Johns melting point apparatus, uncorrected, and reported in degrees centigrade. 1H NMR and 13C NMR spectra were scanned on a Bruker DRX-400 (400 MHz) using tetramethylsilane (TMS) as internal standard and one or two of the following solvents: DMSO-d6 and CDCl3. Chemical shifts were given in δ, ppm. Splitting patterns were designated as follows: s: singlet; d: doublet; t: triplet; q: quartet; m: multiplet. The mass spectra (MS) were recorded on a Finnigan MAT-95 mass spectrometer. The purity of all testing compounds was established by HPLC to be >95%. HPLC analysis was performed at room temperature using an Agilent Eclipse XDBC18 (250 mm × 4.6 mm) and as a mobile phase gradient from 5% MeCN/H2O (1‰ TFA (trifluoroethanoic acid)) for 1 min, 5% MeCN/H2O (1‰ TFA) to 95% MeCN/H2O (1‰ TFA) for 9 min, and 95% MeCN/H2O (1‰ TFA) for 5 min more, a flow rate of 1 mL/min, and plotted at 254 nm (Supplementary Materials).
3.1. General Synthesis
4-Bromo-3-(pentafluorosulfanyl)aniline (2). In a 100 mL round-bottomed flask, 3-(pentafluorosulfanyl)aniline (1) (2 g, 9.13 mmol, 1 eq) in 15 mL DMAc was added to give a yellow solution. The reaction mixture was cooled to −20 °C with stirring on. Then, 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione (1.435 g, 5.02 mmol, 0.55 eq) was added in 10 mL DMAc. The reaction mixture was held at −20 °C with stirring on for 16 h. The 100 mL water was added. The aq layer was backextracted with EA. The organic layers were combined and washed with water and brine. The organic was dried with Na2SO4, filtered, and concentrated to give crude product 3.3 g. The crude product was purified by column chromatography to give 4-Bromo-3-(pentafluorosulfanyl)aniline (2) (1.7 g, 62.5% yield) as an orange oil. 1H NMR (400 MHz, CDCl3) δ 7.47 (d, J = 8.8 Hz, 1H), 7.12 (s, 1H), 6.97 (d, J = 8.8 Hz, 1H), 4.30 (s, 2H). 19F NMR (376 MHz, CDCl3) δ 84.81–82.68 (m, 1F), 65.22 (d, J = 152 Hz, 4F). 13C NMR (101 MHz, CDCl3) δ 154.32, 154.15, 153.98, 145.52, 136.59, 118.89, 116.06, 116.01, 115.96, 115.90, 104.15. ESI-MS m/z: 297.97, 300.14 [M + H, M + 2H]+.
4-amino-2-(pentafluorosulfanyl)benzonitrile (3). In a 100 mL round-bottomed flask, 4-Bromo-3-(pentafluorosulfanyl)aniline (2) (1.7 g, 5.70 mmol, 1 eq) and cyanocopper (0.613 g, 6.84 mmol, 1.2 eq) were added in 30 mL NMP (1-Methyl-2-pyrrolidinone) to give a brown solution. The reaction vessel was purged with nitrogen. The reaction was heated to 180 °C with stirring on for 4 h. Then, 100 mL 25% EDA (ethylenediamine) (aq) was added. The aq layer was backextracted with EA. The organic layers were combined and washed with 25% EDA (aq) and brine. The organic was dried with Na2SO4, filtered, and concentrated to give crude product 1.5 g. The crude product was purified by column chromatography to give 4-amino-2-(pentafluorosulfanyl)benzonitrile (3) (1.05 g, 75% yield) as orange solid. m.p. 149–151 °C; 1H NMR (400 MHz, CDCl3) δ 7.54 (d, J = 8.4 Hz, 1H), 7.09 (s, 1H), 6.76 (d, J = 8.4 Hz, 1H), 4.46 (s, 2H). 19F NMR (376 MHz, CDCl3) δ 82.40–80.21 (m, 1F), 65.27 (d, J = 151.1 Hz, 4F). 13C NMR (101 MHz, CDCl3) δ 156.35, 150.29, 136.39, 117.38, 116.09, 113.80, 97.05. ESI-MS m/z: 245.25 [M + H]+.
methacryloyl-D-proline (6). A solution of methacryloyl chloride (4) (17.49 mL, 181 mmol, 1.04 eq) in 100 mL acetone was added to a solution of D-proline (5) (20 g, 174 mmol, 1 eq) in 2M NaOH (100 mL, 200 mmol, 1.15 eq) and 100 mL acetone dropwise at 0 °C for 30 min. The pH of the reaction mixture was maintained within 10~11 range via simultaneous dropwise addition of 5 mL 2M NaOH (aq) during the addition of methacryloyl chloride. The reaction mixture was warmed up to room temperature with stirring on for 16 h. The mixture was concentrated by rotovap. The reaction mixture was washed with Et2O. Then, 150 mL 2M HCl was added to adjust pH to 2. The aq layer was backextracted with EA. The organic layers were combined and washed with sat.NaCl (aq). The organic was dried with Na2SO4, filtered, and concentrated to give methacryloyl-D-proline (6) (31.5 g, 99% yield) as white solid. The crude product was used until the next step without further purification. m.p. 102–104 °C; 1H NMR (400 MHz, CDCl3) δ: 9.78 (s, 1H), 5.32 (d, J = 9 Hz, 1H), 5.25–5.03 (m, 1H), 4.57 (s, 1H), 3.63 (s, 2H), 2.22 (dd, J = 14.7, 7.7 Hz, 2H), 2.07–1.84 (m, 5H).
(3R,8aR)-3-(bromomethyl)-3-methyltetrahydro-1H-pyrrolo[2,1-c][1,4]oxazine-1,4(3H)-dione (7). In a 500 mL round-bottomed flask, methacryloyl-D-proline (6) (31.5 g, 172 mmol, 1 eq) in 150 mL anhydrous DMF was added to give a colorless solution. The reaction vessel was purged with nitrogen. The reaction mixture was cooled to 0 °C with stirring on for 30 min. 1-bromopyrrolidine-2,5-dione (61.2 g, 344 mmol, 2 eq) in 150 mL anhydrous DMF was added dropwise at 0 °C for 120 min. The reaction mixture was warmed up to room temperature with stirring on for overnight. The mixture was concentrated by rotovap. Then, 1000 mL EA (ethyl acetate) was added. The organic was washed with sat.NaHCO3, water, and brine. The organic was dried with Na2SO4, filtered, and concentrated to give the crude product. Then, 30 mL EA and 150 mL Et2O were added. The product was filtered through sintered glass funnel with 30 mL Et2O to give (3R,8aR)-3-(bromomethyl)-3-methyltetrahydro-1H-pyrrolo[2,1-c][1,4]oxazine-1,4(3H)-dione (7) (40 g, 89% yield) as white solid. m.p. 158–160 °C; 1H NMR (400 MHz, DMSO) δ 4.77–4.66 (m, 1H), 4.03 (d, J = 11.5 Hz, 1H), 3.87 (d, J = 11.3 Hz, 1H), 3.52 (dd, J = 18.7, 9.2 Hz, 1H), 3.40 (t, J = 10.3 Hz, 1H), 2.27 (dd, J = 17.3, 6.7 Hz, 1H), 1.95 (dt, J = 17.5, 8 Hz, 2H), 1.82 (dd, J = 19, 10.4 Hz, 1H), 1.58 (s, 3H). 13C NMR (101 MHz, DMSO) δ 167.26, 163.07, 83.88, 57.22, 45.41, 37.82, 29.02, 22.92, 21.58.
(R)-3-Bromo-2-hydroxy-2-methylpropanoic acid (8). In a 250 mL round-bottomed flask, (3R,8aR)-3-(bromomethyl)-3-methyltetrahydro-1H-pyrrolo[2,1-c][1,4]oxazine-1,4(3H)-dione (7) (25 g, 95 mmol, 1 eq) in HBr (250 mL, 921 mmol, 9.7 eq) was added to give an orange solution. The reaction was heated to 100°C with stirring on for 16 h. Then, 250 mL sat.NaCl was added. The aq layer was backextracted with EA. The organic layers were combined and washed with sat.NaCl (aq). The organic was washed with sat.NaHCO3. Later, 45 mL 12M HCl was added to adjust pH to 1. The aq layer was backextracted with EA. The organic layers were combined and washed with water and sat.NaCl (aq). The organic was dried with Na2SO4, filtered, and concentrated to give (R)-3-Bromo-2-hydroxy-2-methylpropanoic acid (8) (12.2 g, 69.9% yield) as white solid. m.p. 111–113 °C; 1H NMR (400 MHz, DMSO) δ 12.84 (s, 1H), 5.43 (s, 1H), 3.65 (d, J = 10.2 Hz, 1H), 3.54 (d, J = 10 Hz, 1H), 1.37 (s, 3H). 13C NMR (101 MHz, DMSO) δ 174.51, 73.11, 40.94, 24.45. [α]D20 = +9.9 (c = 1.1, MeOH).
3.2. General Procedure for the Synthesis of 9, 10, and 15
In a 100 mL round-bottomed flask, (R)-3-Bromo-2-hydroxy-2-methylpropanoic acid (1.7 eq) in anhydrous 25 mL DMAc was added to give a yellow solution. The reaction vessel was purged with nitrogen. The reaction mixture was cooled to −10 °C with stirring on. Thionyl chloride (1.85 eq) was added slowly. The reaction mixture was held at −5 °C with stirring on for 3 h. 1, 3, or 14 (1 eq) in 15 mL anhydous DMAc was added. The reaction mixture was warmed up to room temperaure with stirring on for 16 h. Then, 60 mL water was added. The aq layer was backextracted with EA. The organic layers were combined and washed with sat.NaHCO3 (aq), sat.NH4Cl (aq), and sat.NaCl (aq). The organic was dried with Na2SO4, filtered, and concentrated to give crude product. The crude product was purified by column chromatography to give 9, 10, and 15.
(R)-3-Bromo-2-hydroxy-2-methyl-N-(3-(pentafluorosulfanyl)phenyl)propanamide (9). Yellow solid (1.7 g, 96% yield). m.p. 105–107 °C; 1H NMR (400 MHz, CDCl3) δ 8.77 (s, 1H), 8.03 (s, 1H), 7.74 (d, J = 7.7 Hz, 1H), 7.51 (d, J = 8 Hz, 1H), 7.42 (t, J = 7.9 Hz, 1H), 4.01 (d, J = 10.4 Hz, 1H), 3.59 (d, J = 10.4 Hz, 1H), 3.01 (s, 1H), 1.61 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 84.94–82.68 (m, 1F), 62.66 (d, J = 150.2 Hz, 4F). 13C NMR (101 MHz, CDCl3) δ 171.12, 154.28, 137.42, 129.28, 122.63, 122.04, 117.46, 75.39, 41.50, 24.79. ESI-MS m/z: 409.14, 411.22 [M + H, M + 2H]+. [α]D20 = −28.1 (c = 1.1, MeOH).
(R)-3-Bromo-N-(4-cyano-3-(pentafluorosulfanyl)phenyl)-2-hydroxy-2-methylpropanamide (10). Light-yellow solid (450 mg, 49% yield). m.p. 125–127 °C; 1H NMR (400 MHz, CDCl3) δ 9.03 (s, 1H), 8.22 (s, 1H), 7.97 (d, J = 8.3 Hz, 1H), 7.81 (d, J = 7.9 Hz, 1H), 4.02 (d, J = 10.5 Hz, 1H), 3.60 (d, J = 10.5 Hz, 1H), 3.01 (s, 1H), 1.65 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 81.81–78.79 (m, 1F), 65.52 (d, J = 151.5 Hz, 4F). 13C NMR (101 MHz, CDCl3) δ 171.63, 155.37, 141.15, 136.09, 121.67, 119.13, 116.10, 104.70, 75.59, 40.98, 24.83. ESI-MS m/z: 384.24, 386.19 [M + H, M + 2H]+. [α]D20 = –23.3 (c = 0.1, MeOH).
(R)-3-Bromo-2-hydroxy-2-methyl-N-(4-(pentafluorosulfanyl)phenyl)propanamide (15). Light-yellow solid (1.1 g, 88% yield). m.p. 117–119 °C; 1H NMR (400 MHz, CDCl3) δ 8.85 (s, 1H), 7.73 (d, J = 9.4 Hz, 2H), 7.69 (d, J = 9.3 Hz, 2H), 4.02 (d, J = 10.4 Hz, 1H), 3.60 (d, J = 10.5 Hz, 1H), 3.21 (s, 1H), 1.63 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 85.87–83.96 (m, 1F), 63.38 (d, J = 150.3 Hz, 4F). 13C NMR (101 MHz, CDCl3) δ 171.30, 149.60, 139.66, 127.13, 127.09, 127.04, 119.10, 75.42, 41.38, 24.80. ESI-MS m/z: 384.24, 386.26 [M + H, M + 2H]+. [α]D20 = −28 (c = 1, MeOH).
3.3. General Procedure for the Synthesis of 12a–g, 13a–g, and 16a–g
In a 50 mL round-bottomed flask, 9, 10, or 15 (1 eq), different commercial phenols 11a–g (1.5 eq), and potassium carbonate (3 eq) were added in 20 mL 2-propanol to give a white suspension. The reaction vessel was purged with nitrogen. The reaction was heated to 85 °C with stirring on for 16 h. The reaction mixture was filtered through sintered glass funnel. The mixture was concentrated by rotovap. The crude product was purified by column chromatography to give 12a–g, 13a–g, and 16a–g.
(S)-3-(4-cyanophenoxy)-2-hydroxy-2-methyl-N-(3-(pentafluoro-l6-sulfanyl)phenyl)propanamide (12a). White solid (50 mg, 46% yield). m.p. 70–72 °C; 1H NMR (400 MHz, CDCl3) δ 8.92 (s, 1H), 8.09 (t, J = 2 Hz, 1H), 7.76 (d, J = 8 Hz, 1H), 7.57 (d, J = 8.8 Hz, 2H), 7.53 (dd, J = 8.4, 1.3 Hz, 1H), 7.44 (t, J = 8.2 Hz, 1H), 6.98 (d, J = 8.9 Hz, 2H), 4.50 (d, J = 9.2 Hz, 1H), 4.06 (d, J = 9.2 Hz, 1H), 3.43 (s, 1H), 1.62 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 84.99–82.98 (m, 1F), 62.66 (d, J = 150.2 Hz, 4F). 13C NMR (101 MHz, CDCl3) δ 171.80, 161.13, 154.29, 137.55, 134.12, 129.30, 122.52, 122, 118.80, 117.40, 116.33, 115.48, 105.10, 75.58, 72.74, 23.03. HRMS(ESI)m/z calcd for C17H15F5N2O3S [M + H]+: 423.0802, Found: 423.0818. [α]D20 = +3 (c = 0.1, MeOH).
(S)-3-(4-fluorophenoxy)-2-hydroxy-2-methyl-N-(3-(pentafluoro-l6-sulfanyl)phenyl)propanamide (12b). White solid (95 mg, 88% yield). m.p. 91–93 °C; 1H NMR (400 MHz, CDCl3) δ 8.94 (s, 1H), 8.08 (t, J = 1.9 Hz, 1H), 7.74 (d, J = 8.2 Hz, 1H), 7.50 (dd, J = 8.3, 1.2 Hz, 1H), 7.40 (t, J = 8.2 Hz, 1H), 6.94 (dt, J = 11.9, 3 Hz, 2H), 6.87–6.78 (m, 2H), 4.40 (d, J = 9 Hz, 1H), 3.94 (d, J = 9 Hz, 1H), 3.58 (s, 1H), 1.57 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 85.25–82.86 (m, 1F), 62.63 (d, J = 150.1 Hz, 4F), –122.19 (s, 1F). 13C NMR (101 MHz, CDCl3) δ 172.39, 159.06, 156.67, 153.98, 137.67, 129.23, 122.54, 121.85, 117.38, 116.14, 116.06, 115.98, 115.90, 75.62, 73.32, 22.94. HRMS(ESI)m/z calcd for C16H15F6NO3S [M + H]+: 416.0755, Found: 416.0744. [α]D20 = –3.3 (c = 0.3, MeOH).
(S)-3-(4-chlorophenoxy)-2-hydroxy-2-methyl-N-(3-(pentafluoro-l6-sulfanyl)phenyl)propanamide (12c). White solid (110 mg, 98% yield). m.p. 82–84 °C; 1H NMR (400 MHz, CDCl3) δ 8.93 (s, 1H), 8.08 (t, J = 2 Hz, 1H), 7.72 (d, J = 8 Hz, 1H), 7.50 (dd, J = 8.3, 1.4 Hz, 1H), 7.40 (t, J = 8.2 Hz, 1H), 7.23 – 7.17 (m, 2H), 6.86–6.78 (m, 2H), 4.39 (d, J = 9 Hz, 1H), 3.94 (d, J = 9.2 Hz, 1H), 3.58 (s, 1H), 1.57 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 84.97–82.86 (m, 1F), 62.65 (d, J = 150.2 Hz, 4F). 13C NMR (101 MHz, CDCl3) δ 172.30, 156.50, 154.25, 137.63, 129.50, 129.24, 126.84, 122.56, 121.90, 117.39, 116.11, 75.59, 72.91, 22.95. HRMS(ESI)m/z calcd for C16H15ClF5NO3S [M + H]+: 432.0460, Found: 432.0455. [α]D20 = +1.2 (c = 0.2, MeOH).
(S)-2-hydroxy-2-methyl-N-(3-(pentafluoro-l6-sulfanyl)phenyl)-3-(4-(trifluoromethyl)phenoxy)propanamide (12d). White solid (70 mg, 58% yield). m.p. 96–98 °C; 1H NMR (400 MHz, CDCl3) δ 8.93 (s, 1H), 8.09 (s, 1H), 7.74 (d, J = 8 Hz, 1H), 7.53 (t, J = 6.8 Hz, 3H), 7.42 (t, J = 8.2 Hz, 1H), 6.97 (d, J = 8.4 Hz, 2H), 4.49 (d, J = 9 Hz, 1H), 4.03 (d, J = 9 Hz, 1H), 3.44 (s, 1H), 1.60 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 84.86 – 82.96 (m, 1F), 62.64 (d, J = 150.1 Hz, 4F), –61.69 (s, 3F). 13C NMR (101 MHz, CDCl3) δ 172.03, 160.21, 154.26, 137.55, 129.28, 127.10, 127.06, 125.54, 124.23, 123.90, 122.85, 122.51, 121.96, 117.37, 114.72, 75.59, 72.57, 23. HRMS(ESI)m/z calcd for C17H15F8NO3S [M + Na]+: 488.0543, Found: 488.0521. [α]D20 = –2.5 (c = 0.2, MeOH).
(S)-3-(4-acetamidophenoxy)-2-hydroxy-2-methyl-N-(3-(pentafluoro-l6-sulfanyl)phenyl)propanamide (12e). White solid (100 mg, 85% yield). m.p. 79–81 °C; 1H NMR (400 MHz, DMSO) δ 10.19 (s, 1H), 9.76 (s, 1H), 8.53 (s, 1H), 8.06 (d, J = 7.5 Hz, 1H), 7.57 (p, J = 8.3 Hz, 2H), 7.45 (d, J = 8.9 Hz, 2H), 6.86 (d, J = 8.9 Hz, 2H), 6.13 (s, 1H), 4.17 (d, J = 9.5 Hz, 1H), 3.93 (d, J = 9.5 Hz, 1H), 2 (s, 3H), 1.43 (s, 3H). 19F NMR (376 MHz, DMSO) δ 88.61–86.49 (m, 1F), 63.66 (d, J = 150.6 Hz, 4F). 13C NMR (101 MHz, DMSO) δ 173.81, 167.68, 154.23, 152.87, 139.30, 132.74, 129.49, 123.32, 120.33, 116.73, 114.55, 74.67, 73.73, 23.76, 23.04. HRMS(ESI)m/z calcd for C18H19F5N2O4S [M + H]+: 455.1064, Found: 455.1046. [α]D20 = –2.5 (c = 1.2, MeOH).
(S)-2-hydroxy-2-methyl-N-(3-(pentafluoro-l6-sulfanyl)phenyl)-3-(4-propionylphenoxy)propanamide (12f). White solid (95 mg, 80% yield). m.p. 76–78 °C; 1H NMR (400 MHz, CDCl3) δ 9.11 (s, 1H), 8.12 (s, 1H), 7.85 (d, J = 8.7 Hz, 2H), 7.74 (d, J = 8 Hz, 1H), 7.49 (d, J = 8.3 Hz, 1H), 7.39 (t, J = 8.2 Hz, 1H), 6.86 (d, J = 8.5 Hz, 2H), 4.47 (d, J = 9.3 Hz, 1H), 4.26 (s, 1H), 4.05 (d, J = 9.2 Hz, 1H), 2.91 (q, J = 7.2 Hz, 2H), 1.60 (s, 3H), 1.17 (t, J = 7.3 Hz, 3H). 19F NMR (376 MHz, CDCl3) δ 84.90–82.96 (m, 1F), 62.65 (d, J = 150.2 Hz, 4F). 13C NMR (101 MHz, CDCl3) δ 200.16, 172.43, 161.78, 154.17, 137.71, 130.52, 130.24, 129.21, 122.60, 121.82, 117.38, 114.38, 75.55, 72.78, 31.48, 22.94, 8.36. HRMS(ESI)m/z calcd for C19H20F5NO4S [M + Na]+: 476.0931, Found: 476.0923. [α]D20 = +1 (c = 0.2, MeOH).
(S)-3-(2-fluoro-4-methoxyphenoxy)-2-hydroxy-2-methyl-N-(3-(pentafluoro-l6-sulfanyl)phenyl)propanamide (12g). White solid (100 mg, 86% yield). m.p. 92–94 °C; 1H NMR (400 MHz, CDCl3) δ 8.98 (s, 1H), 8.09 (t, J = 2 Hz, 1H), 7.71 (d, J = 8.2 Hz, 1H), 7.49 (dd, J = 8.3, 1.4 Hz, 1H), 7.39 (t, J = 8.2 Hz, 1H), 6.93 (t, J = 9.2 Hz, 1H), 6.64 (dd, J = 12.6, 2.9 Hz, 1H), 6.56 (ddd, J = 9, 2.9, 1.5 Hz, 1H), 4.43 (d, J = 9.3 Hz, 1H), 3.97 (d, J = 9.2 Hz, 1H), 3.90 (s, 1H), 3.72 (s, 3H), 1.56 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 85.16 – 82.99 (m, 1F), 62.64 (d, J = 150.1 Hz, 4F), –131.31 (s, 1F). 13C NMR (101 MHz, CDCl3) δ 172.62, 155.42, 155.33, 154.62, 154.20, 152.18, 139.77, 139.65, 137.74, 129.15, 122.63, 121.77, 118, 117.98, 117.46, 109.15, 109.12, 103.35, 103.13, 75.61, 75.38, 55.74, 22.94. HRMS(ESI)m/z calcd for C17H17F6NO4S [M + Na]+: 468.0680, Found: 468.0692. [α]D20 = −7 (c = 0.1, MeOH).
(S)-N-(4-cyano-3-(pentafluoro-l6-sulfanyl)phenyl)-3-(4-cyanophenoxy)-2-hydroxy-2-methylpropanamide (13a). White solid (260 mg, 60% yield). m.p. 77–79 °C; 1H NMR (400 MHz, CDCl3) δ 9.20 (s, 1H), 8.27 (s, 1H), 7.96 (d, J = 8.4 Hz, 1H), 7.78 (d, J = 8.5 Hz, 1H), 7.57 (d, J = 8 Hz, 2H), 6.98 (d, J = 8.2 Hz, 2H), 4.50 (d, J = 9.2 Hz, 1H), 4.07 (d, J = 9 Hz, 1H), 3.53 (s, 1H), 1.63 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 81.19–79.05 (m, 1F), 65.53 (d, J = 151.3 Hz, 4F). 13C NMR (101 MHz, CDCl3) δ 172.36, 161.02, 155.55, 141.42, 136.05, 134.13, 121.61, 119.10, 118.76, 116.13, 115.46, 105.18, 104.46, 75.79, 72.60, 22.99. HRMS(ESI)m/z calcd for C18H14F5N3O3S [M + Na]+: 470.0574, Found: 470.0566. [α]D20 = −5.8 (c = 1, MeOH).
(S)-N-(4-cyano-3-(pentafluoro-l6-sulfanyl)phenyl)-3-(4-fluorophenoxy)-2-hydroxy-2-methylpropanamide (13b). White solid (40 mg, 62% yield). m.p. 85–87 °C; 1H NMR (400 MHz, CDCl3) δ 9.16 (s, 1H), 8.25 (s, 1H), 7.96 (d, J = 8.5 Hz, 1H), 7.79 (d, J = 8.4 Hz, 1H), 6.98 (t, J = 8.4 Hz, 2H), 6.86 (dd, J = 8.8, 4.1 Hz, 2H), 4.43 (d, J = 9 Hz, 1H), 3.95 (d, J = 9 Hz, 1H), 3.46 (s, 1H), 1.59 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 81.37–79.25 (m, 1F), 65.53 (d, J = 151.2 Hz, 4F), –121.89 (s, 1F). 13C NMR (101 MHz, CDCl3) δ 172.76, 159.14, 156.76, 155.58, 153.75, 141.41, 136.07, 121.55, 119.04, 116.25, 116.15, 116.04, 116.02, 115.96, 104.48, 75.89, 73.05, 22.92. HRMS(ESI)m/z calcd for C17H14F6N2O3S [M + H]+: 441.0708, Found: 441.0723. [α]D20 = −0.7 (c = 0.2, MeOH).
(S)-3-(4-chlorophenoxy)-N-(4-cyano-3-(pentafluoro-l6-sulfanyl)phenyl)-2-hydroxy-2-methylpropanamide (13c). White solid (51 mg, 76% yield). m.p. 65–67 °C; 1H NMR (400 MHz, CDCl3) δ 9.14 (s, 1H), 8.24 (s, 1H), 7.95 (d, J = 8.5 Hz, 1H), 7.79 (d, J = 8.5 Hz, 1H), 7.25 (d, J = 9 Hz, 2H), 6.84 (d, J = 8.8 Hz, 2H), 4.44 (d, J = 9 Hz, 1H), 3.97 (d, J = 9 Hz, 1H), 3.40 (s, 1H), 1.59 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 81.55 – 78.71 (m, 1F), 65.49 (d, J = 151.3 Hz, 4F). 13C NMR (101 MHz, CDCl3) δ 172.61, 156.23, 155.58, 141.36, 136.07, 129.60, 127.10, 121.55, 119.04, 116.14, 116.07, 104.53, 75.85, 72.60, 22.96. HRMS(ESI)m/z calcd for C17H14ClF5N2O3S [M + H]+: 457.0412, Found: 457.0408. [α]D20 = −3 (c = 0.1, MeOH).
(S)-N-(4-cyano-3-(pentafluoro-l6-sulfanyl)phenyl)-2-hydroxy-2-methyl-3-(4 (trifluoromethyl)phenoxy)propanamide (13d). White solid (30 mg, 42% yield). m.p. 69–71 °C; 1H NMR (400 MHz, CDCl3) δ 9.19 (s, 1H), 8.27 (s, 1H), 7.95 (d, J = 8.3 Hz, 1H), 7.77 (d, J = 8.5 Hz, 1H), 7.55 (d, J = 8.4 Hz, 2H), 6.98 (d, J = 8.5 Hz, 2H), 4.51 (d, J = 9.2 Hz, 1H), 4.05 (d, J = 9 Hz, 1H), 3.49 (s, 1H), 1.62 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 80.94 – 79.24 (m, 1F), 65.52 (d, J = 151.2 Hz, 4F), –61.72 (s, 3F). 13C NMR (101 MHz, CDCl3) δ 172.54, 160.05, 155.73, 155.55, 155.36, 141.42, 136.05, 131.62, 127.13, 127.10, 127.06, 125.50, 124.69, 124.36, 124.03, 123.71, 122.80, 121.58, 119.07, 116.17, 114.72, 104.41, 75.84, 72.43, 22.96. HRMS(ESI)m/z calcd for C18H14F8N2O3S [M + Na]+: 513.0495, Found: 513.0483. [α]D20 = −3 (c = 0.1, MeOH).
(S)-3-(4-acetamidophenoxy)-N-(4-cyano-3-(pentafluoro-l6-sulfanyl)phenyl)-2-hydroxy-2-methylpropanamide (13e). White solid (40 mg, 57% yield). m.p. 112–114 °C; 1H NMR (400 MHz, DMSO) δ 10.69 (s, 1H), 9.78 (s, 1H), 8.80 (s, 1H), 8.35 (d, J = 8.7 Hz, 1H), 8.12 (d, J = 8.7 Hz, 1H), 7.45 (d, J = 9 Hz, 2H), 6.85 (d, J = 9 Hz, 2H), 6.29 (s, 1H), 4.18 (d, J = 9.7 Hz, 1H), 3.95 (d, J = 9.6 Hz, 1H), 1.99 (s, 3H), 1.44 (s, 3H). 19F NMR (376 MHz, DMSO) δ 83.68 (m, 1F), 66.17 (d, J = 152 Hz, 4F). 13C NMR (101 MHz, DMSO) δ 174.78, 167.68, 154.14, 143.36, 136.56, 132.80, 122.40, 120.33, 118.99, 116.37, 114.59, 101.46, 74.86, 73.74, 23.76, 22.97. HRMS(ESI)m/z calcd for C19H18F5N3O4S [M + Na]+: 502.0836, Found: 502.0829. [α]D20 = −5.2 (c = 0.3, MeOH).
(S)-N-(4-cyano-3-(pentafluoro-l6-sulfanyl)phenyl)-2-hydroxy-2-methyl-3-(4-propionylphenoxy)propanamide (13f). White solid (37 mg, 53% yield). m.p. 66–68 °C; 1H NMR (400 MHz, DMSO) δ 10.71 (s, 1H), 8.79 (d, J = 1.9 Hz, 1H), 8.35 (d, J = 8.7 Hz, 1H), 8.12 (d, J = 8.7 Hz, 1H), 7.91 (d, J = 8.9 Hz, 2H), 7.03 (d, J = 8.8 Hz, 2H), 6.36 (s, 1H), 4.33 (d, J = 9.9 Hz, 1H), 4.09 (d, J = 9.8 Hz, 1H), 2.96 (q, J = 7.2 Hz, 2H), 1.46 (s, 3H), 1.06 (t, J = 7.2 Hz, 3H). 19F NMR (376 MHz, DMSO) δ 83.81 (p, J = 153.6 Hz, 1F), 66.16 (d, J = 151.9 Hz, 4F). 13C NMR (101 MHz, DMSO) δ 198.75, 174.59, 162.06, 143.31, 136.56, 129.99, 129.71, 122.45, 119.04, 116.34, 114.41, 101.52, 74.75, 73.59, 30.75, 22.92, 8.23. HRMS(ESI)m/z calcd for C20H19F5N2O4S [M + Na]+: 501.0883, Found: 501.0883. [α]D20 = −1 (c = 0.1, MeOH).
(S)-N-(4-cyano-3-(pentafluoro-l6-sulfanyl)phenyl)-3-(2-fluoro-4-methoxyphenoxy)-2-hydroxy-2-methylpropanamide (13g). White solid (55 mg, 80% yield). m.p. 58–60 °C; 1H NMR (400 MHz, CDCl3) δ 9.24 (s, 1H), 8.26 (s, 1H), 7.93 (d, J = 8.5 Hz, 1H), 7.76 (d, J = 8.4 Hz, 1H), 6.93 (t, J = 9.1 Hz, 1H), 6.64 (d, J = 12.7 Hz, 1H), 6.59 (d, J = 8.9 Hz, 1H), 4.45 (d, J = 9.2 Hz, 1H), 3.97 (d, J = 9.2 Hz, 1H), 3.84 (s, 1H), 3.74 (s, 3H), 1.57 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 81.73–79.26 (m, 1F), 65.50 (d, J = 151.3 Hz, 4F), –131.34 (s, 1F). 13C NMR (101 MHz, CDCl3) δ 173.15, 155.56, 155.47, 155.28, 154.60, 152.16, 141.55, 139.54, 139.43, 135.97, 121.66, 119.13, 118.08, 118.06, 116.22, 109.20, 109.16, 104.26, 103.35, 103.14, 75.68, 75.54, 55.79, 22.85. HRMS(ESI)m/z calcd for C18H16F6N2O4S [M + Na]+: 493.0633, Found: 493.0601. [α]D20 = −2.5 (c = 0.1, MeOH).
(S)-3-(4-cyanophenoxy)-2-hydroxy-2-methyl-N-(4-(pentafluoro-l6-sulfanyl)phenyl)propanamide (16a). White solid (140 mg, 71% yield). m.p. 73–75 °C; 1H NMR (400 MHz, CDCl3) δ 8.87 (s, 1H), 7.68 – 7.59 (m, 4H), 7.49 (d, J = 8.5 Hz, 2H), 6.90 (d, J = 8.5 Hz, 2H), 4.42 (d, J = 9.2 Hz, 1H), 3.99 (d, J = 9.2 Hz, 1H), 3.34 (s, 1H), 1.54 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 84.93 (p, J = 150.4 Hz, 1F), 63.41 (d, J = 150.1 Hz, 4F). 13C NMR (101 MHz, CDCl3) δ 170.80, 160.11, 138.79, 133.11, 126.10, 117.97, 117.76, 114.47, 104.13, 74.61, 71.75, 22.02. HRMS(ESI)m/z calcd for C17H15F5N2O3S [M + H]+: 423.0802, Found: 423.0818. [α]D20 = +0.2 (c = 0.9, MeOH).
(S)-3-(4-fluorophenoxy)-2-hydroxy-2-methyl-N-(4-(pentafluoro-l6-sulfanyl)phenyl)propanamide (16b). White solid (90 mg, 83% yield). m.p. 87–89 °C; 1H NMR (400 MHz, CDCl3) δ 8.93 (s, 1H), 7.73 (d, J = 9.5 Hz, 2H), 7.70 (d, J = 9.8 Hz, 2H), 6.98 (t, J = 8.5 Hz, 2H), 6.86 (dd, J = 9.1, 4.1 Hz, 2H), 4.42 (d, J = 9 Hz, 1H), 3.95 (d, J = 8.9 Hz, 1H), 3.40 (s, 1H), 1.58 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 86.09–84.13 (m, 1F), 63.41 (d, J = 150.1 Hz, 4F), –122.12 (s, 1F). 13C NMR (101 MHz, CDCl3) δ 172.27, 159.09, 156.71, 153.90, 153.88, 139.90, 127.14, 127.10, 127.05, 118.92, 116.20, 116.05, 115.97, 75.65, 73.21, 23. HRMS(ESI)m/z calcd for C16H15F6NO3S [M + H]+: 416.0755, Found: 416.0744. [α]D20 = −6.2 (c = 0.2, MeOH).
(S)-3-(4-chlorophenoxy)-2-hydroxy-2-methyl-N-(4-(pentafluoro-l6-sulfanyl)phenyl)propanamide (16c). White solid (88 mg, 78% yield). m.p. 65–68 °C; 1H NMR (400 MHz, CDCl3) δ 8.94 (s, 1H), 7.70 (q, J = 9.5 Hz, 4H), 7.22 (d, J = 8.5 Hz, 2H), 6.83 (d, J = 8.5 Hz, 2H), 4.41 (d, J = 8.9 Hz, 1H), 3.95 (d, J = 9 Hz, 1H), 3.48 (s, 1H), 1.58 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 84.98 (d, J = 150.4 Hz,, 1F), 63.42 (d, J = 150.3 Hz, 4F). 13C NMR (101 MHz, CDCl3) δ 172.25, 156.41, 149.45, 139.85, 129.54, 127.08, 126.91, 118.95, 116.08, 75.63, 72.81, 23. HRMS(ESI)m/z calcd for C16H15ClF5NO3S [M + H]+: 432.0460, Found: 432.0455. [α]D20 = −1.7 (c = 0.5, MeOH).
(S)-2-hydroxy-2-methyl-N-(4-(pentafluorosulfanyl)phenyl)-3-(4-(trifluoromethyl)phenoxy)propanamide (16d). White solid (20 mg, 17% yield). m.p. 78–80 °C; 1H NMR (400 MHz, CDCl3) δ 8.93 (s, 1H), 7.73 (d, J = 9.5 Hz, 2H), 7.69 (d, J = 9.5 Hz, 2H), 7.55 (d, J = 8.8 Hz, 2H), 6.98 (d, J = 8.7 Hz, 2H), 4.49 (d, J = 9 Hz, 1H), 4.04 (d, J = 9 Hz, 1H), 3.37 (s, 1H), 1.61 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 84.90 (p, J = 150.4 Hz, 1F), 63.39 (d, J = 150.2 Hz, 4F), –61.70 (s, 3F). 13C NMR (101 MHz, CDCl3) δ 171.98, 160.17, 149.53, 139.81, 127.15, 127.12, 127.08, 127.06, 125.52, 124.33, 124, 122.83, 118.96, 114.75, 75.63, 72.59, 23.03. HRMS(ESI)m/z calcd for C17H15F8NO3S [M + Na]+: 488.0543, Found: 488.0521. [α]D20 = −3.7 (c = 0.3, MeOH).
(S)-3-(4-acetamidophenoxy)-2-hydroxy-2-methyl-N-(4-(pentafluorosulfanyl)phenyl)propanamide (16e). White solid (98 mg, 83% yield). m.p. 156–157 °C; 1H NMR (400 MHz, DMSO) δ 10.17 (s, 1H), 9.77 (s, 1H), 8.02 (d, J = 8.9 Hz, 2H), 7.85 (d, J = 9.2 Hz, 2H), 7.45 (d, J = 8.9 Hz, 2H), 6.85 (d, J = 8.9 Hz, 2H), 6.17 (s, 1H), 4.17 (d, J = 9.5 Hz, 1H), 3.94 (d, J = 9.5 Hz, 1H), 2 (s, 3H), 1.43 (s, 3H). 19F NMR (376 MHz, DMSO) δ 89.74–87.61 (m, 1F), 64.86 (d, J = 150.7 Hz, 5F). 13C NMR (101 MHz, DMSO) δ 173.79, 167.68, 154.24, 141.77, 132.74, 126.47, 120.36, 119.57, 114.57, 74.73, 73.78, 23.75, 22.99. HRMS(ESI)m/z calcd for C18H19F5N2O4S [M + Na]+: 477.0883, Found: 477.0859. [α]D20 = −0.6 (c = 0.5, MeOH).
(S)-2-hydroxy-2-methyl-N-(4-(pentafluorosulfanyl)phenyl)-3-(4-propionylphenoxy)propanamide (16f). White solid (100 mg, 85% yield). m.p. 100–102 °C; 1H NMR (400 MHz, CDCl3) δ 8.99 (s, 1H), 7.91 (d, J = 8.8 Hz, 2H), 7.71 (s, 4H), 6.91 (d, J = 8.8 Hz, 2H), 4.49 (d, J = 9.2 Hz, 1H), 4.06 (d, J = 9.2 Hz, 1H), 3.66 (s, 1H), 2.94 (q, J = 7.2 Hz, 2H), 1.61 (s, 3H), 1.20 (t, J = 7.2 Hz, 3H). 19F NMR (376 MHz, CDCl3) δ 86.14–83.94 (m, 1F), 63.41 (d, J = 150.2 Hz, 4F). 13C NMR (101 MHz, CDCl3) δ 199.69, 172.11, 161.50, 149.46, 139.89, 130.88, 130.28, 127.07, 118.96, 114.40, 75.60, 72.59, 31.51, 23.04, 8.35. HRMS(ESI)m/z calcd for C19H20F5NO4S [M + Na]+: 476.0931, Found: 476.0923. [α]D20 = +0.2 (c = 0.7, MeOH).
(S)-3-(2-fluoro-4-methoxyphenoxy)-2-hydroxy-2-methyl-N-(4-(pentafluorosulfanyl)phenyl)propanamide (16g). White solid (100 mg, 86% yield). m.p. 91–93 °C; 1H NMR (400 MHz, CDCl3) δ 9 (s, 1H), 7.76 – 7.62 (m, 4H), 6.92 (t, J = 9.2 Hz, 1H), 6.64 (dd, J = 12.7, 2.9 Hz, 1H), 6.57 (ddd, J = 9, 2.9, 1.4 Hz, 1H), 4.42 (d, J = 9.3 Hz, 1H), 3.97 (d, J = 9.2 Hz, 1H), 3.90 (s, 1H), 3.72 (s, 3H), 1.56 (s, 3H). 19F NMR (376 MHz, CDCl3) δ 86.57–84.10 (m, 1F), 63.39 (d, J = 150.3 Hz, 3F), –131.31 (s, 1F). 13C NMR (101 MHz, CDCl3) δ 172.68, 155.45, 155.36, 154.62, 152.18, 149.38, 139.98, 139.73, 139.62, 127.02, 126.97, 126.93, 119.04, 117.99, 117.97, 109.17, 109.15, 103.36, 103.14, 75.59, 75.39, 55.74, 22.97. HRMS(ESI)m/z calcd for C17H17F6NO4S [M + H]+: 446.0861, Found: 446.0818. [α]D20 = –3.9 (c = 0.8, MeOH).
3.4. Cell Preparation
CV-1 cells and C2C12 cells were both provided and certified by the Cell Bank at Shanghai Institutes for Biological Sciences, the Chinese Academy of Sciences, and confirmed as negative for mycoplasma contamination. CV-1 cells were cultured in phenol red-free LG-DMEM medium (Life Technologies, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum, 50 IU/mL penicillin, and 50 μg/mL streptomycin. C2C12 were cultured in phenol red-free HG-DMEM medium (Life Technologies, Carlsbad, CA, USA) supplemented with 10% (v/v) fetal bovine serum, 50 IU/mL penicillin, 50 μg/mL streptomycin, and 1% sodium pyruvate. Cells were maintained at 37 °C in a 5% CO2 incubator and seeded onto a 10 cm cell culture dish before transfection. After overnight culture, the cells were transiently co-transfected with AR expressing plasmid (pSVAR0) and luciferase reporter gene vector (MMTV-Luc) using FuGENE® HD Transfection Reagent (Promega, Madison, WI, USA) with cells reaching 80–90% confluence. After 18 h, the transfected cells were distributed to 384-well plates at a density of 15,000 cells per well and incubated for further 6 h at 37 °C before compound treatment.
3.5. Agonist Assay
DHT (Sigma, St. Louis, MO, USA) and ostarine were used as positive controls. The agonist activity of compounds was measured by Steadylite plus Reporter Gene Assay System (PerkinElmer, Boston, MA, USA) according to the manufacturer’s instructions. In brief, after incubation for 6 h, as mentioned above, 10 μL testing compounds diluted in culture medium with eight different working concentrations (384 pM to 30 μM; DHT (1 fM–1 μM); ostarine (32 pM–2.5 μM)) were added to the cell well (40 μL). After 24 h incubation, Steadylite reagent (50 μL, equal volume) was introduced, gently shook for 2 min, and kept at room temperature for 15 min before luminescence 384 measurement on an EnSpire multilabel plate reader (PerkinElmer, Boston, MA, USA).
3.6. Cytotoxicity
CellTiter-Glo® 2.0 assay (Promega, Madison, WI, USA) was applied to assess cytotoxicity. In brief, cells were seeded onto 384-well plates at a density of 1500 cells per well and incubated for 24 h. A total of 10 μL testing compounds diluted in culture medium were added and reacted for 24 h. CellTiter-Glo reagent was then introduced, and luminescence 384 was measured as above.
4. Conclusions
In summary, a small library of SF5-containing ostarine analogs instead of the CF3 group was synthesized, and their biological activity and SAR (structure-activity relationship), as SARMs, were studied. A series of para-SF5, meta-CN derivatives (13a–g) displayed different degrees of AR agonist activity. It appears that mono-SF5 substitution in the phenyl ring (12a–g, 16a–g) resulted in a total loss of AR agonist activity. The derivatives containing the SF5 group maintained fewer AR agonist activities than the derivatives containing the CF3 group. The results pointed to the potential of using this derivative containing the SF5 group scaffold to develop new AR agonists.
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/24/23/4227/s1, 1H, 13C, 19F NMR spectra, HPLC and HRMS of the synthesized compounds can be found in the Supporting Information.
Author Contributions
P.S. and J.J. collaborated in the synthesis and purification of compounds; P.S., W.L. and J.J. collaborated in the discussion of structure-activity relationship, and writing of the manuscript; Y.Z., D.Y. and M.-W.W. supervised the biological work and wrote the biological component of this manuscript; Y.Z., D.Y. and M.-W.W. revised the manuscript; All the authors discussed, edited, and approved the final version.
Funding
We thank Yang Feng for technical assistance. This work was partially supported by the “Personalized Medicines‒Molecular Signature-based Drug Discovery and Development Strategic Priority Research Program” of Chinese Academy of Sciences grants (XDA12020347 to M.-W.W.; XDA12040308 to D.Y.), the Thousand Talents Program in China (to M.-W.W.), and the financial support for Shanghai Science and Technology Council (16DZ2280100).
Conflicts of Interest
There are no conflicts to declare.
Abbreviations
| SF5 | Pentafluorosulfane |
| CF3 | Trifluoromethyl |
| SARMs | Selective androgen receptor modulators |
| AR | Androgen receptor |
| DMAc | N,N-dimethylacetamide |
| DMF | N,N-dimethylformamide |
| DHT | Dihydrotestosterone |
| SAR | Structure-activity relationship |
| TMS | Tetramethylsilane |
References
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef]
- Sowaileh, M.F.; Hazlitt, R.A.; Colby, D.A. Application of the Pentafluorosulfanyl Group as a Bioisosteric Replacement. ChemMedChem 2017, 12, 1481–1490. [Google Scholar] [CrossRef]
- Savoie, P.R.; Welch, J.T. Preparation and utility of organic pentafluorosulfanyl-containing compounds. Chem. Rev. 2015, 115, 1130–1190. [Google Scholar] [CrossRef]
- Bassetto, M.; Ferla, S.; Pertusati, F. Polyfluorinated groups in medicinal chemistry. Future Med. Chem. 2015, 7, 527–546. [Google Scholar] [CrossRef]
- Altomonte, S.; Zanda, M. Synthetic chemistry and biological activity of pentafluorosulphanyl (SF5) organic molecules. J. Fluor. Chem. 2012, 143, 57–93. [Google Scholar] [CrossRef]
- Saethre, L.J.; Berrah, N.; Bozek, J.D.; Borve, K.J.; Carroll, T.X.; Kukk, E.; Gard, G.L.; Winter, R.; Thomas, T.D. Chemical insights from high-resolution X-ray photoelectron spectroscopy and ab initio theory: Propyne, trifluoropropyne, and ethynylsulfur pentafluoride. J. Am. Chem. Soc. 2001, 123, 10729–10737. [Google Scholar] [CrossRef]
- Bowden, R.D.; Comina, P.J.; Greenhall, M.P.; Kariuki, B.M.; Loveday, A.; Philp, D. A new method for the synthesis of aromatic sulfurpentafluorides and studies of the stability of the sulfurpentafluoride group in common synthetic transformations. Tetrahedron 2000, 56, 3399–3408. [Google Scholar] [CrossRef]
- Umemoto, T.; Garrick, L.M.; Saito, N. Discovery of practical production processes for arylsulfur pentafluorides and their higher homologues, bis- and tris(sulfur pentafluorides): Beginning of a new era of “super-trifluoromethyl” arene chemistry and its industry. Beilstein J. Org. Chem. 2012, 8, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Gujjar, R.; El Mazouni, F.; White, K.L.; White, J.; Creason, S.; Shackleford, D.M.; Deng, X.Y.; Charman, W.N.; Bathurst, I.; Burrows, J.; et al. Lead Optimization of Aryl and Aralkyl Amine-Based Triazolopyrimidine Inhibitors of Plasmodium falciparum Dihydroorotate Dehydrogenase with Antimalarial Activity in Mice. J. Med. Chem. 2011, 54, 3935–3949. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Jasuja, R. Selective androgen receptor modulators as function promoting therapies. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Mohler, M.L.; Bohl, C.E.; Miller, D.D.; Dalton, J.T. Selective androgen receptor modulators in preclinical and clinical development. Nucl. Recept. Signal. 2008, 6, e010. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Calof, O.M.; Storer, T.W.; Lee, M.L.; Mazer, N.A.; Jasuja, R.; Montori, V.M.; Gao, W.Q.; Dalton, J.T. Drug Insight: Testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nat. Clin. Pract. Endoc. 2006, 2, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Marhefka, C.A.; Gao, W.; Chung, K.; Kim, J.; He, Y.; Yin, D.; Bohl, C.; Dalton, J.T.; Miller, D.D. Design, synthesis, and biological characterization of metabolically stable selective androgen receptor modulators. J. Med. Chem. 2004, 47, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Gao, W.; Kearbey, J.D.; Xu, H.; Chung, K.; He, Y.; Marhefka, C.A.; Veverka, K.A.; Miller, D.D.; Dalton, J.T. Pharmacodynamics of selective androgen receptor modulators. J. Pharmacol. Exp. Ther. 2003, 304, 1334–1340. [Google Scholar] [CrossRef]
- Clark, R.V.; Walker, A.C.; Andrews, S.; Turnbull, P.; Wald, J.A.; Magee, M.H. Safety, pharmacokinetics and pharmacological effects of the selective androgen receptor modulator, GSK2881078, in healthy men and postmenopausal women. Br. J. Clin. Pharmacol. 2017, 83, 2179–2194. [Google Scholar] [CrossRef]
- Basaria, S.; Collins, L.; Dillon, E.L.; Orwoll, K.; Storer, T.W.; Miciek, R.; Ulloor, J.; Zhang, A.Q.; Eder, R.; Zientek, H.; et al. The Safety, Pharmacokinetics, and Effects of LGD-4033, a Novel Nonsteroidal Oral, Selective Androgen Receptor Modulator, in Healthy Young Men. J. Gerontol. Ser. a-Biol. Sci. Med. Sci. 2013, 68, 87–95. [Google Scholar] [CrossRef]
- Zilbermint, M.F.; Dobs, A.S. Nonsteroidal selective androgen receptor modulator Ostarine (TM) in cancer cachexia. Future Oncol. 2009, 5, 1211–1220. [Google Scholar] [CrossRef]
- Ferla, S.; Bassetto, M.; Pertusati, F.; Kandil, S.; Westwell, A.D.; Brancale, A.; McGuigan, C. Rational design and synthesis of novel anti-prostate cancer agents bearing a 3,5-bis-trifluoromethylphenyl moiety. Bioorg. Med. Chem. Lett. 2016, 26, 3636–3640. [Google Scholar] [CrossRef]
- Guo, C.X.; Linton, A.; Kephart, S.; Ornelas, M.; Pairish, M.; Gonzalez, J.; Greasley, S.; Nagata, A.; Burke, B.J.; Edwards, M.; et al. Discovery of Aryloxy Tetramethylcyclobutanes as Novel Androgen Receptor Antagonists. J. Med. Chem. 2011, 54, 7693–7704. [Google Scholar] [CrossRef]
- Jung, M.E.; Ouk, S.; Yoo, D.; Sawyers, C.L.; Chen, C.; Tran, C.; Wongvipat, J. Structure-Activity Relationship for Thiohydantoin Androgen Receptor Antagonists for Castration-Resistant Prostate Cancer (CRPC). J. Med. Chem. 2010, 53, 2779–2796. [Google Scholar] [CrossRef]
- Piatnitski Chekler, E.L.; Unwalla, R.; Khan, T.A.; Tangirala, R.S.; Johnson, M.; St Andre, M.; Anderson, J.T.; Kenney, T.; Chiparri, S.; McNally, C.; et al. 1-(2-Hydroxy-2-methyl-3-phenoxypropanoyl)indoline-4-carbonitrile derivatives as potent and tissue selective androgen receptor modulators. J. Med. Chem. 2014, 57, 2462–2471. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 3–16g are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).