Identification and Monitoring of Amomi Fructus and its Adulterants Based on DNA Barcoding Analysis and Designed DNA Markers
Abstract
1. Introduction
2. Results
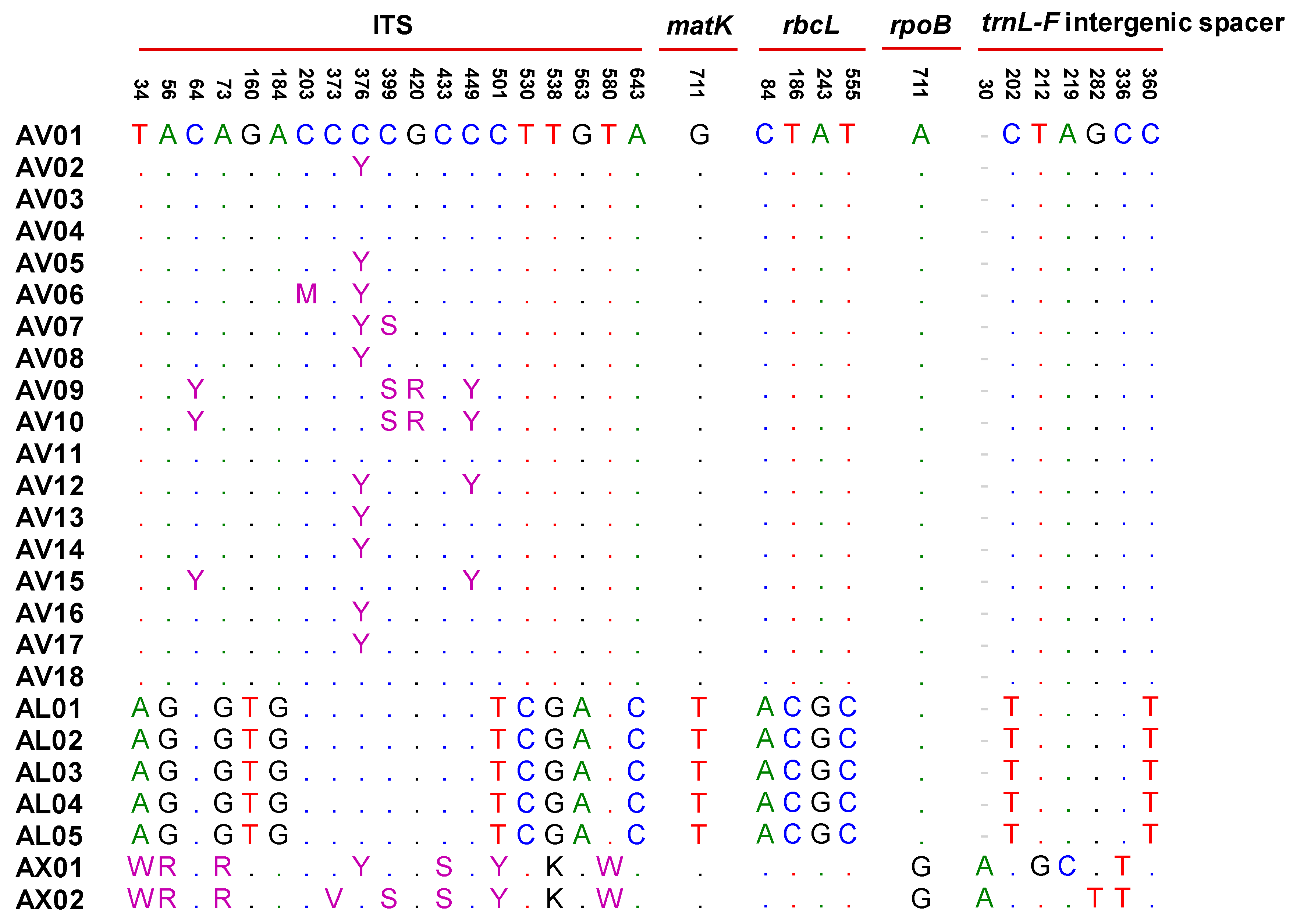
2.1. DNA Barcode Analysis
2.1.1. Internal Transcribed Spacer (ITS) Regions of Nuclear Ribosomal Cistron
2.1.2. Chloroplast Genome Based DNA Barcode Sequence Analysis
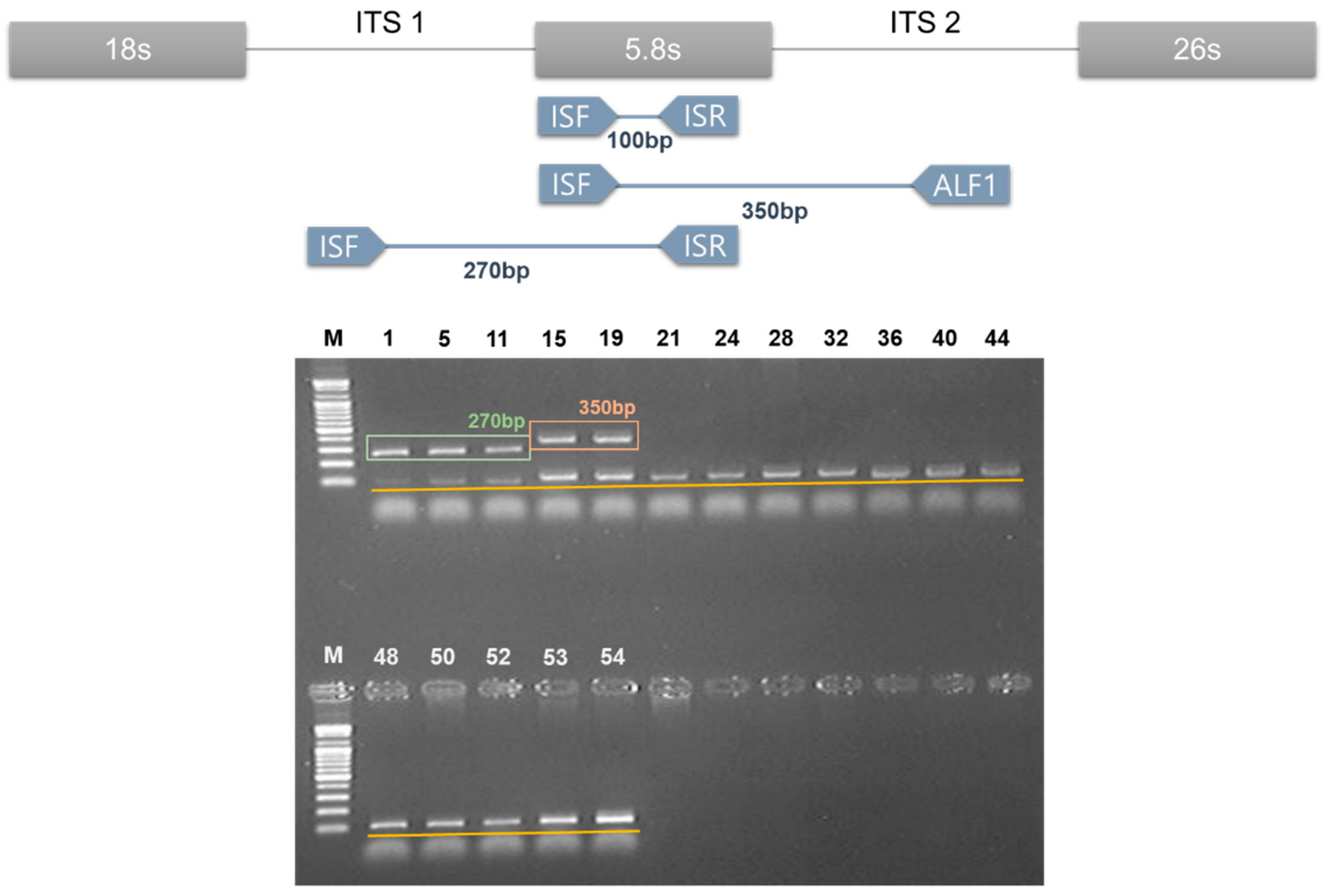
2.2. DNA Marker for Amomi Fructus Based on Discrepancy in the ITS Sequences
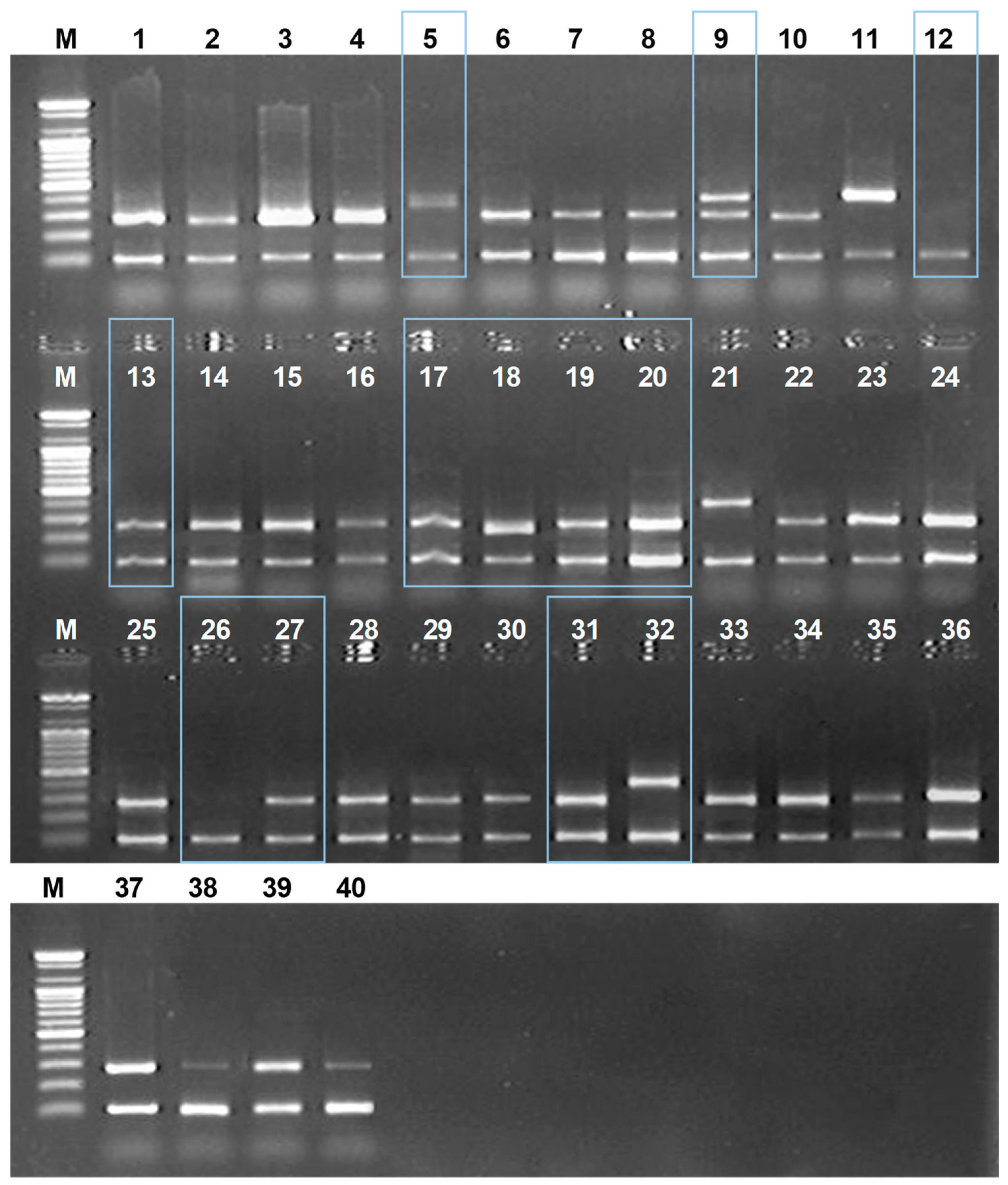
2.3. Monitoring Amomi Fructus Vouchers in Commercial Markets Using DNA Markers
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Preparation of Genomic DNA
4.3. PCR Amplification for DNA Barcode Analysis
4.4. Determination of DNA Sequences of PCR Product
4.5. Analysis of DNA Sequences and Preparation of Dendrogram
4.6. Multiplex PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Q.; Duan, Z.; Yang, J.; Ma, X.; Zhan, R. SNP Typing for Germplasm identification of Amomum villosum Lour. based on DNA barcoding markers. PLoS ONE 2014, 9, e114940. [Google Scholar] [CrossRef]
- Ju, Y.S.; Choi, G. Discrimination on Korean Medicinal Herbs for Clinicians; Korea Institute of Oriental Medicine: Daejeon, Korea, 2016; Volume 1, pp. 76–78. [Google Scholar]
- Korea Institute of Oriental Medicine. Defining dictionary for medicinal herbs [Korean, ‘Hanyak Giwon Sajeon′]. 2019. Published on the Internet. Available online: http://boncho.kiom.re.kr/codex/ (accessed on 10 October 2019).
- Ministry of Food and Drug Safety. The Korean Pharmacopoeia, 11th ed.; Ministry of Food and Drug Safety: Osong, Korea, 2017; Volume 2, the article of pharmaceutical drug; p. 50.
- Commission, C.P. Pharmacopoeia of the Peoples Republic of China; China Medical Science Press: Beijing, China, 2015. Available online: https://www.tsoshop.co.uk/Medical/Pharmacopoeia/Chinese-Pharmacopoeia/ (accessed on 18 August 2019).
- Ministry of Health and Welfare Taiwan. Taiwan Herbal Pharmacopeia, 2nd ed.; Ministry of Health and Welfare Taiwan: Taiwan, China, 2013; pp. 164–166.
- Tang, L.; He, G.; Su, J.; Xu, H. The strategy to promote the development of industry of genuine medicinal material of Amomum villosum. Chin. Agric. Sci. Bull. 2012, 28, 94–99. [Google Scholar]
- Quan, Q.; Lin, J.C.; Liang, J.M.; Hu, Z.L.; Huang, J.X. Compare on the content of ronyl Acetate and total volatile oil in Fructus Amomi from different producing area. Guid. J. Tradit. Chin. Med. Pharm. 2017, 23, 70–73. [Google Scholar]
- Chen, J.; Ding, P.; Xu, X.; Xu, H. A resource investigation and commodity identification of Fructus Amomi. J. Chin. Med. Mater. 2001, 24, 18. [Google Scholar]
- Han, J.P.; Li, M.N.; Shi, L.C. Identification of Amomi fructus and its adulterants based on ITS2 sequences. Glob. Tradit. Chin. Med. 2011, 4, 99–102. [Google Scholar]
- Choi, G. List of Fake Korean Medicinal Herbs; Korea Institute of Oriental Medicine: Daejeon, Korea, 2016; pp. 43–44. [Google Scholar]
- Cui, Y.; Chen, X.; Nie, L.; Sun, W.; Hu, H.; Lin, Y.; Li, H.; Zheng, X.; Song, J.; Yao, H. Comparison and phylogenetic analysis of chloroplast genomes of three medicinal and edible Amomum Species. Int. J. Mol. Sci. 2019, 20, 4040. [Google Scholar] [CrossRef]
- Huang, Q.I.; Yang, J.F.; Yan, P.; Zhan, R.T.; Xu, H.; Chen, W.W. Establishment and optimization of ISSR-PCR reaction system for Amomum villosum Lour. Lishizhen Med. Mater. Med. Res. 2010, 10, 2478–2480. [Google Scholar]
- Wang, P.; Huang, F.; Zhou, L.; Cao, L.; Liang, S.; Xu, H.; Liu, J. Analysis of Amomun villosum species and some adulterants of Zingiberaceae by RAPD. J. Chin. Med. Mater. 2000, 23, 71–74. [Google Scholar]
- Huaxin, P.; Feng, H.; Peixun, W.; Lian, Z.; Liuying, C.; Ruiyan, L. Identification of Amomum villosum, Amomum villosum var. xanthioides and Amomum longiligulare on ITS-1 sequence. Zhong Yao Cai 2001, 24, 481–483. [Google Scholar]
- Zhou, L.; Wang, P.; Huang, F.; Cao, L.; Liang, R. ITS sequence analysis of Amomum villosum. Chin. Tradit. Herb. Drugs. 2002, 33, 72–75. [Google Scholar]
- Vijayan, K.; Tsou, C. DNA barcoding in plants: Taxonomy in a new perspective. Curr. Sci. 2010, 99, 1530–1541. [Google Scholar]
- eFloras. Missouri Botanical Garden St. Louis, MO Harvard University Herbaria: Cambridge, MA, USA, 2008; Volume 24, pp. 333–346. Available online: http://www.efloras.org (accessed on 15 October 2019).
- Herbert, P.D.N.; Cywinska, A.; Ball, S.L.; de Waard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Herbert, P.D.N.; Gregory, T.R. The promise of DNA barcoding for taxonomy. Syst. Biol. 2005, 54, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, P.M.; Forrest, L.L.; Spouge, J.L.; Hajibabaei, M.; Ratnasingham, S. A DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2009, 106, 12794–12797. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, P.M.; Graham, S.W.; Little, D.P. Choosing and using a plant DNA barcode. PLoS ONE 2011, 6, e19254. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.S.; Ayres, K.L.; Toomey, N.; Haider, N.; Stahl, J.A.; Kelly, L.J.; Wikström, N.; Hollingsworth, P.M.; Duff, R.J.; Hoot, S.B.; et al. Selection of candidate coding DNA barcoding regions for use on land plants. Bot. Linn. Soc. 2009, 159, 1–11. [Google Scholar] [CrossRef]
- Kress, W.J.; Erickson, D.L. A two-locus global DNA barcode for land plants: The coding rbcL gene complements the noncoding trnH–psbA spacer region. PLoS ONE 2007, 2, e508. [Google Scholar] [CrossRef]
- Vinitha, M.R.; Kumar, U.S.; Aishwarya, K.; Sabu, M.; Thomas, G. Prospects for discriminating Zingiberaceae species in India using DNA barcodes. J. Integr Plant. Biol. 2014, 56, 760–773. [Google Scholar] [CrossRef]
- Wang, H.; Kim, M.K.; Kwon, W.S.; Jin, H.; Liang, Z. Molecular authentication of Panax ginseng and ginseng products using robust SNP markers in ribosomal external transcribed spacer region. J. Pharm. Biomed. Anal. 2011, 55, 972–976. [Google Scholar] [CrossRef]
- Wang, H.; Kim, M.K.; Kim, Y.J.; Lee, H.N.; Jin, H. Molecular authentication of the oriental medicines Pericarpium Citri Reticulatae and Citri Unshius Pericarpium using SNP markers. Gene 2012, 494, 92–95. [Google Scholar] [CrossRef]
- Kim, J.H.; Doh, E.J.; Lee, G.S. Evaluation of Medicinal Categorization of Atractylodes japonica Koidz. By using internal transcribed spacer sequencing analysis and HPLC fingerprinting combined with statistical tools. Evid. Based Complement. Alternat. Med. 2016. [Google Scholar] [CrossRef]
- Mishra, P.; Kumar, A.; Nagireddy, A.; Mani, D.N.; Shukla, A.K.; Tiwari, R. DNA barcoding: An efficient tool to overcome authentication challenges in the herbal market. Plant. Biotechnol. J. 2016, 14, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.S.; Zhang, L.X.; Peng, J.M.; Jie, M.A. Original report on investigation of Ammomum villosum germplasm resources in Xishuangbanna. Lishizhen Med. Mater. Med. Res. 2009, 20, 627–628. [Google Scholar]
- Zurawski, G.; Perrot, B.; Bottomley, W.; Whitefield, P.R. The structure of the gene for the large subunit of ribulose-1-5-bisphosphate carboxylase from spinach chloroplast DNA. Nucleic Acids Res. 1981, 9, 3251–3270. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wolfe, K.H. Protein-coding genes in chloroplast DNA: Compilation of nucleotide sequence, data base entries and rates of molecular evolution. In Cell Culture and Somatic Cell Genetics of Plant; Vasil, K., Ed.; Academic Press: San Diego, CA, USA, 1991; Volume 7BI, pp. 467–482. [Google Scholar]
- Hilu, K.W.; Liang, H. The matk gene: Sequence variation and application in plant systematics. Am. J. Bot. 1997, 84, 830–839. [Google Scholar] [CrossRef]
- Kelchner, S.A. The evolution of noncoding chloroplast DNA and its application in plant systematics. Ann. Mo. Bot. Gard. 2000, 87, 482–498. [Google Scholar] [CrossRef]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of the chloroplast DNA. Plant. Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef]
- Doh, E.J.; Paek, S.H.; Lee, G.S.; Lee, M.Y.; Oh, S.E. Application of partial internal transcribed spacer sequences for the discrimination of Artemisia capillaris from other Artemisia species. Evid. Based Complement. Alternat. Med. 2016. [Google Scholar] [CrossRef]
- Lee, O.R.; Kim, M.K.; Yang, D.C. Authentication of medicinal plants by SNP-based multiplex PCR. Methods Mol. Biol. 2012, 862, 135–147. [Google Scholar]
- Kim, W.J.; Moon, B.C.; Yang, S.; Han, K.S.; Choi, G.; Lee, A.Y. Rapid authentication of the herbal medicine plant species Aralia continentalis Kitag. and Angelica biserrata C.Q. Yuan and R.H. Shan using ITS2 sequences and Multiplex-SCAR markers. Molecules 2016, 21, 270. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, A.-Y.; Jo, A.; Choi, H.; Cho, S.-S.; Choi, C. Development of user-friendly method to distinguish subspecies of the Korean medicinal herb Perilla frutescens using multiplex-PCR. Molecules 2017, 22, 665. [Google Scholar] [CrossRef] [PubMed]
- Dechbumroong, P.; Aumnouypol, S.; Denduangboripant, J.; Sukrong, S. DNA barcoding of Aristoloc10hia plants and development of species-specific multiplex PCR to aid HPTLC in ascertainment of Aristolochia herbal materials. PLoS ONE 2018, 13, e0202625. [Google Scholar] [CrossRef] [PubMed]
- The Plant List, 1.1 ed. Available online: http://www.theplantlist.org/ (accessed on 1 January 2013).
- Thomas, V.P.; Sabu, M. Two species of Amomum (Zingiberaceae) from Western Ghats, India. Edinb. J. Bot. 2012, 69, 313–321. [Google Scholar] [CrossRef]
- Shetty, B.V.; Kaveriappa, K.M. An arboretum of endemic plants of Western Ghats at Mangalore University campus, Karnataka, India. Zoos′ Print J. 2001, 16, 431–438. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Fay, M.F.; Swensen, S.M.; Chase, M.W. Taxonomic affinities of Medusagyne oppositifolia (Medusagynaceae). Kew Bull. 1997, 52, 111–120. [Google Scholar] [CrossRef]
- Cuénoud, P.; Savolainen, V.; Chatrou, L.W.; Powell, M.; Grayer, R.J.; Chase, M.W. Molecular phylogenetics of caryophyllales based on nuclear 18S rDNA and plastid rbcL, atpB, and matK DNA sequences. Am. J. Bot. 2002, 89, 132–144. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI) [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; [1988]–[cited 2017 Apr 06]. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 19 November 2019).
Sample Availability: The nucleotide sequence data, besides those deposited in the NCBI GenBank, that support the findings of this study are available from the first or corresponding author upon request. |


| No. | Sample Code | Scientific Name | Medicinal Name |
|---|---|---|---|
| 1 | AV01 | Amomum villosum Lour. (=Wurfbainia villosa (Lour.) Skornick. A.D.Poulsen) | Amomi Fructus*,** |
| 2 | AV02 | ||
| 3 | AV03 | ||
| 4 | AV04 | ||
| 5 | AV05 | ||
| 6 | AV06 | ||
| 7 | AV07 | ||
| 8 | AV08 | ||
| 9 | AV09 | ||
| 10 | AV10 | ||
| 11 | AV11 | ||
| 12 | AV12 | ||
| 13 | AV13 | ||
| 14 | AV14 | ||
| 15 | AV15 | ||
| 16 | AV16 | ||
| 17 | AV17 | ||
| 18 | AV18 | ||
| 19 | AL01 | Amomum longiligulare T.L.Wu (=Wurfbainia longiligularis (T.L.Wu) Skornick. A.D. Poulsen) | Amomi Fructus** |
| 20 | AL02 | ||
| 21 | AL03 | ||
| 22 | AL04 | ||
| 23 | AL05 | ||
| 24 | AK01 | Amomum verum Blackw. (=Amomum krervanh Pierre ex Gagnep.) | Amomi Fructus Rotundus |
| 25 | AK02 | ||
| 26 | AK03 | ||
| 27 | AK04 | ||
| 28 | AC01 | Amomum compactum Sol. ex Maton | |
| 29 | AC02 | ||
| 30 | AC03 | ||
| 31 | AC04 | ||
| 32 | ATK01 | Amomum tsao-ko Crevost Lemarié (=Amomum tsaoko) | Amomi Tsao-ko Fructus |
| 33 | ATK02 | ||
| 34 | ATK03 | ||
| 35 | ATK04 | ||
| 36 | AH01 | Alpinia hainanensis K.Schum. (=Alpinia katsumadae Hayata) | Alpiniae Katsumadai Semen |
| 37 | AH02 | ||
| 38 | AH03 | ||
| 39 | AH04 | ||
| 40 | AO01 | Alpinia oxyphylla Miq. | Alpiniae Oxyphyllae Fructus |
| 41 | AO02 | ||
| 42 | AO03 | ||
| 43 | AO04 | ||
| 44 | AOR01 | Alpinia officinarum Hanc | Alpiniae Officinari Rhizoma |
| 45 | AOR02 | ||
| 46 | AOR03 | ||
| 47 | AOR04 | ||
| 48 | ACC01 | Alpinia conchigera Griff. | jie bian shan jiang*** |
| 49 | ACC02 | ||
| 50 | AZ01 | Alpinia zerumbet (Pers.) B.L.Burtt R.M.Sm. | yan shan jiang*** |
| 51 | AZ02 | ||
| 52 | AM01 | Alpinia malaccensis(n.l.Burman) Roscoe | mao ban shan jiang*** |
| 53 | AG01 | Alpinia galanga (l.) Willd. | Galangae Fructus |
| 54 | EC01 | Elettaria cardamomum (l.) Maton (=Amomum cardamomum l., Alpinia cardamomum (l.) Roxb.) | Cardamomi Fructus |
| 55 | EC02 |
| Barcode Target | Amplicon Size (~Bases) | Aligned Length (Bases) | Conserved Sites | Variable Sites | Parsimony Informative Sites | Singleton Sites |
|---|---|---|---|---|---|---|
| ITS | 645–665 | 670 | 516 | 154 | 132 | 22 |
| matk | 933 | 933 | 894 | 39 | 27 | 12 |
| rbcL | 743 | 743 | 657 | 86 | 12 | 74 |
| rpoB | 516 | 516 | 500 | 16 | 10 | 6 |
| trnL-F intergenic sapcer | 395–415 | 422 | 377 | 43 | 13 | 30 |
| matk + rbcL | 1676 | 1551 | 125 | 39 | 86 | |
| rpoB + trnL-F intergenic spacer | 938 | 877 | 59 | 23 | 36 | |
| mark + rbcL + rpoB | 2192 | 2051 | 141 | 49 | 92 | |
| mark + rbcL + trnL-F intergenic spacer | 2098 | 1928 | 168 | 52 | 116 | |
| Four plastid targets | 2614 | 2428 | 184 | 62 | 122 |
| No | Collected Species Name | Genetically Re-Identified Species | No | Collected Species Name | Genetically Re-Identified Species |
|---|---|---|---|---|---|
| 1 | A. villosum | A. villosum | 21 | A. longiligulare | A. longiligulare |
| 2 | A. villosum | A. villosum | 22 | A. villosum | A. villosum |
| 3 | A. villosum | A. villosum | 23 | A. villosum | A. villosum |
| 4 | A. villosum | A. villosum | 24 | A. villosum | A. villosum |
| 5 | A. villosum | A. longiligulare | 25 | A. villosum | A. villosum |
| 6 | A. villosum | A. villosum | 26 | A. longiligulare | Amomum ghaticum |
| 7 | A. villosum | A. villosum | 27 | A. longiligulare | A. villosum |
| 8 | A. villosum | A. villosum | 28 | A. villosum | A. villosum |
| 9 | A. villosum | A. villosum/A. longiligulare | 29 | A. villosum | A. villosum |
| 10 | A. villosum | A. villosum | 30 | A. villosum | A. villosum |
| 11 | A. longiligulare | A. longiligulare | 31 | A. villosum var. xanthioides | A. villosum |
| 12 | A. longiligulare | Amomum ghaticum | 32 | A. villosum var. xanthioides | A. longiligulare |
| 13 | A. longiligulare | A. villosum | 33 | A. villosum | A. villosum |
| 14 | A. villosum | A. villosum | 34 | A. villosum | A. villosum |
| 15 | A. villosum | A. villosum | 35 | A. villosum | A. villosum |
| 16 | A. villosum | A. villosum | 36 | A. villosum | A. villosum |
| 17 | A. villosum var. xanthioides | A. villosum | 37 | A. villosum | A. villosum |
| 18 | A. villosum var. xanthioides | A. villosum | 38 | A. villosum | A. villosum |
| 19 | A. villosum var. xanthioides | A. villosum | 39 | A. villosum | A. villosum |
| 20 | A. villosum var. xanthioides | A. villosum | 40 | A. villosum | A. villosum |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doh, E.J.; Kim, J.-H.; Lee, G. Identification and Monitoring of Amomi Fructus and its Adulterants Based on DNA Barcoding Analysis and Designed DNA Markers. Molecules 2019, 24, 4193. https://doi.org/10.3390/molecules24224193
Doh EJ, Kim J-H, Lee G. Identification and Monitoring of Amomi Fructus and its Adulterants Based on DNA Barcoding Analysis and Designed DNA Markers. Molecules. 2019; 24(22):4193. https://doi.org/10.3390/molecules24224193
Chicago/Turabian StyleDoh, Eui Jeong, Jung-Hoon Kim, and Guemsan Lee. 2019. "Identification and Monitoring of Amomi Fructus and its Adulterants Based on DNA Barcoding Analysis and Designed DNA Markers" Molecules 24, no. 22: 4193. https://doi.org/10.3390/molecules24224193
APA StyleDoh, E. J., Kim, J.-H., & Lee, G. (2019). Identification and Monitoring of Amomi Fructus and its Adulterants Based on DNA Barcoding Analysis and Designed DNA Markers. Molecules, 24(22), 4193. https://doi.org/10.3390/molecules24224193





