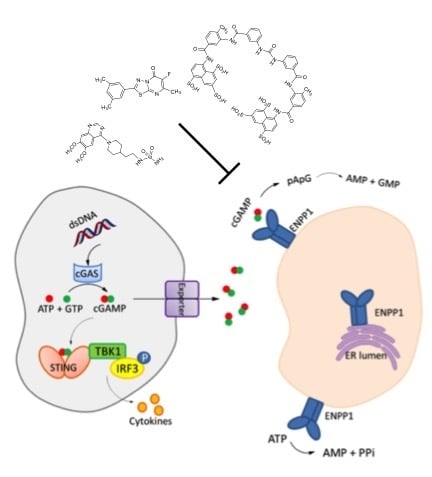
ENPP1, an Old Enzyme with New Functions, and Small Molecule Inhibitors—A STING in the Tale of ENPP1
Abstract
1. Introduction
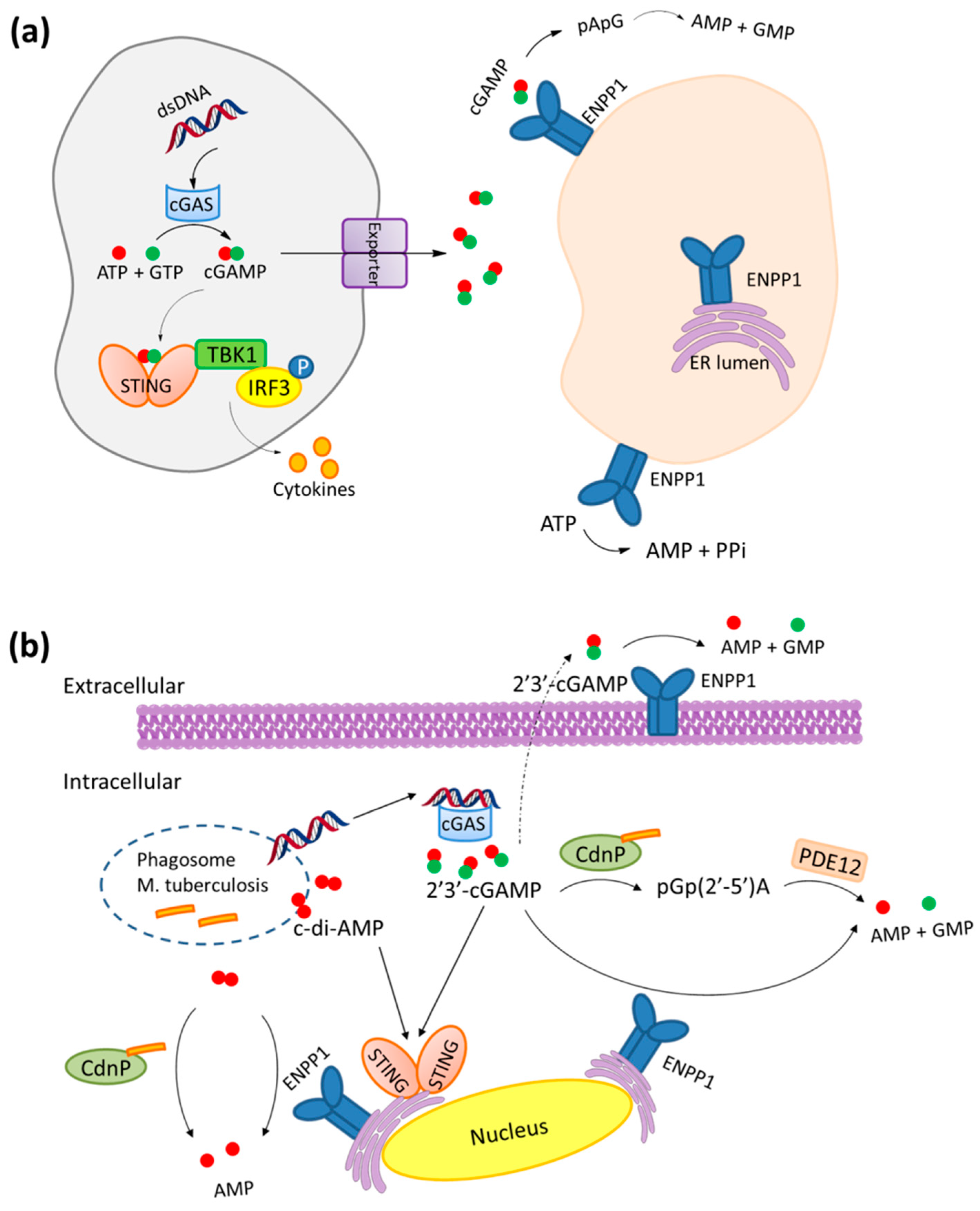
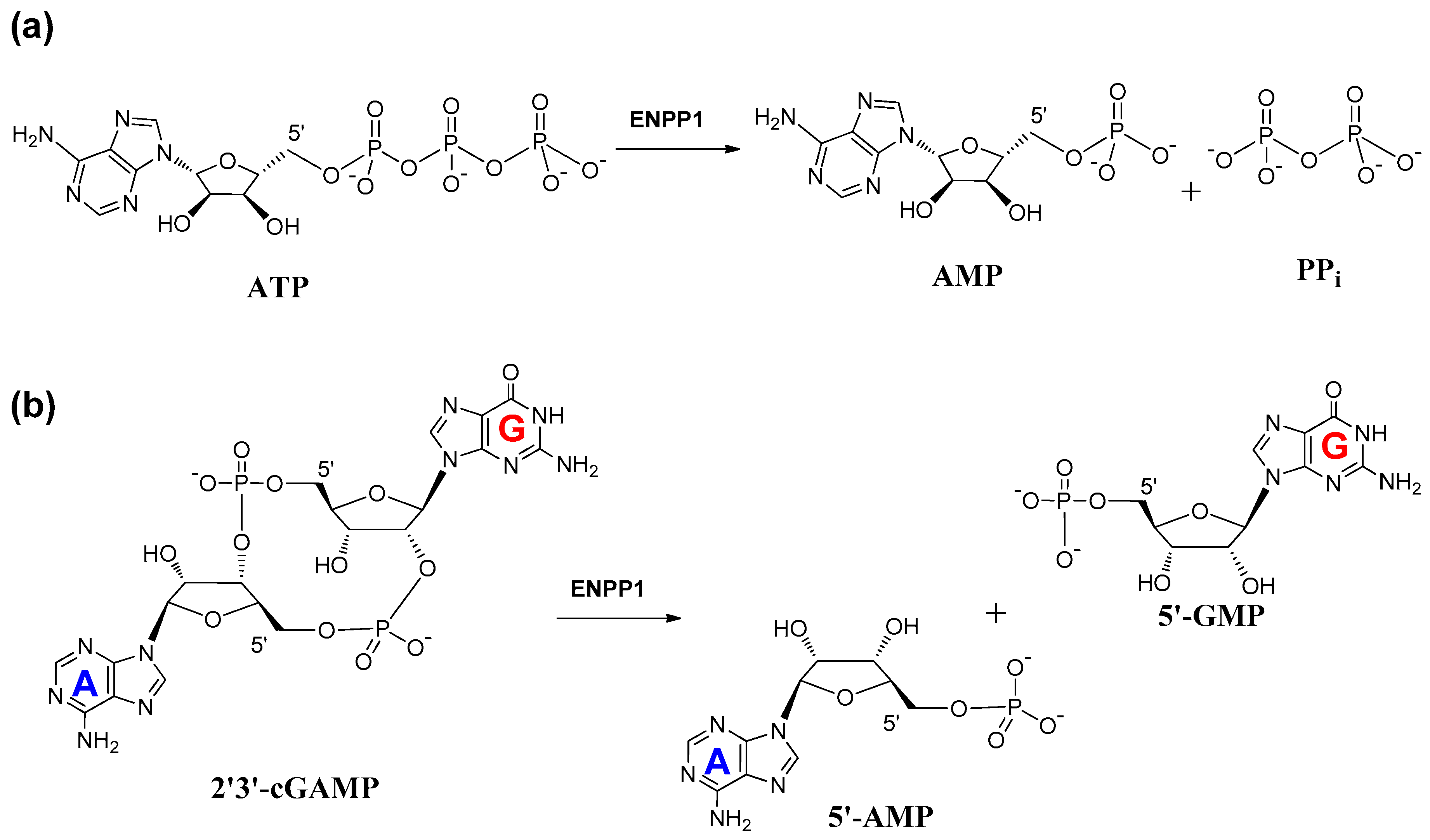
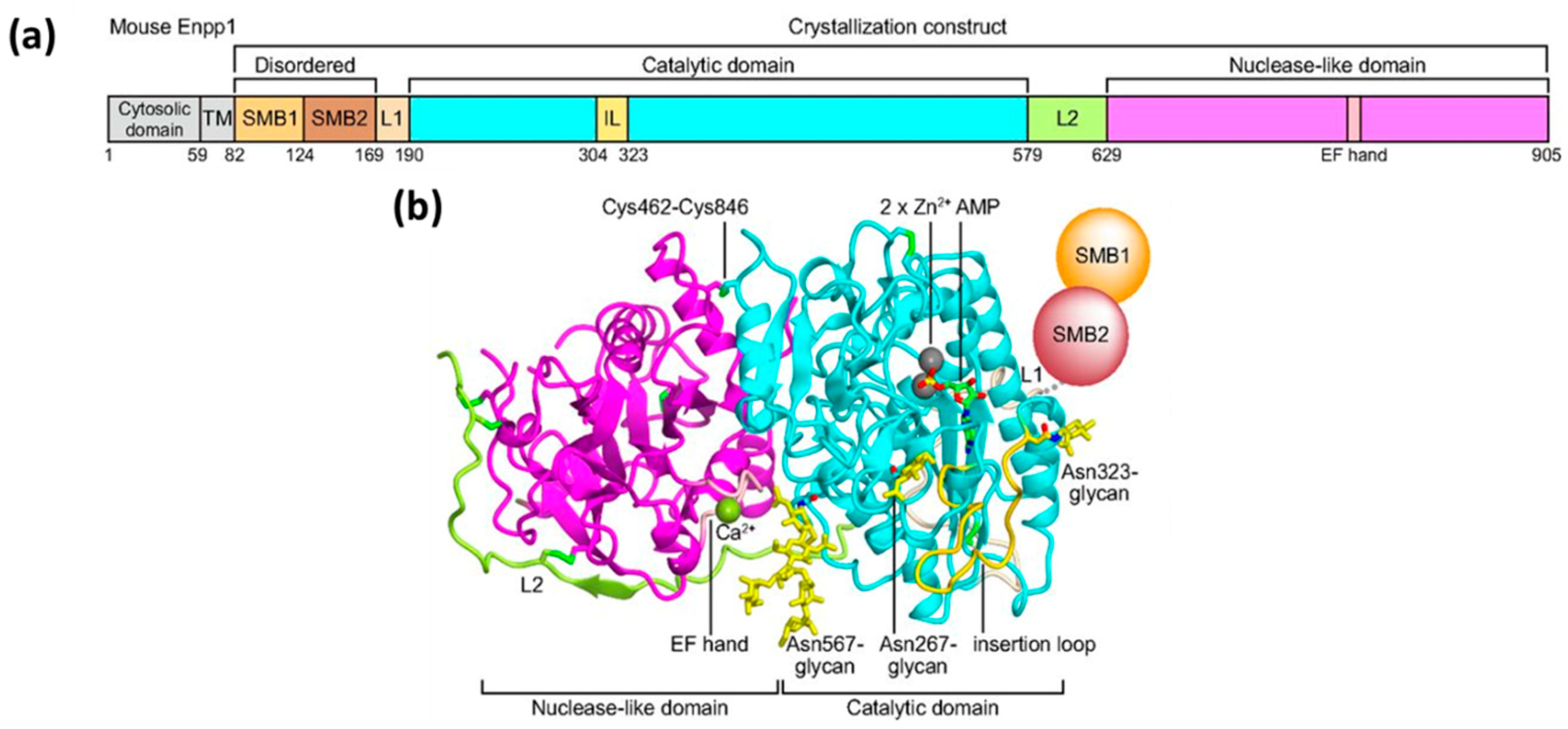
2. Mechanism of Hydrolysis of ATP and 2′3′-cGAMP by ENPP1
3. STING and ENPP1 in Cancer
4. Inhibitors of ENPP1
4.1. Nucleotide-Based Inhibitors of ENPP1
4.2. Non-Nucleotide-Based Inhibitors of ENPP1
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Evans:, W.H.; Hood, D.O.; Gurd, J.W. Purification and properties of a mouse liver plasma-membrane glycoprotein hydrolysing nucleotide pyrophosphate and phosphodiester bonds. Biochem. J. 1973, 135, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Old, L.J.; Boyse, E.A. Surface alloantigens of plasma cells. J. Exp. Med. 1970, 131, 1325–1341. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Rosenbach, M.; Vaughn, R.; Provvedini, D.; Rebbe, N.; Hickman, S.; Goding, J.; Terkeltaub, R. Expression of the murine plasma cell nucleotide pyrophosphohydrolase PC-1 is shared by human liver, bone, and cartilage cells. Regulation of PC-1 expression in osteosarcoma cells by transforming growth factor-beta. J. Clin. Invest. 1994, 94, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic signalling. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S172–S181. [Google Scholar] [CrossRef]
- Drury, A.N.; Szent-Györgyi, A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J. Physiol. 1929, 68, 213–237. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Ferrari, D.; Riegel, A.K.; Eltzschig, H.K. Extracellular nucleotide and nucleoside signaling in vascular and blood disease. Blood 2014, 124, 1029–1037. [Google Scholar] [CrossRef]
- Roberts, F.; Zhu, D.; Farquharson, C.; Macrae, V.E. ENPP1 in the Regulation of Mineralization and Beyond. Trends Biochem. Sci. 2019, 44, 616–628. [Google Scholar] [CrossRef]
- Stefan, C.; Jansen, S.; Bollen, M. NPP-type ectophosphodiesterases: Unity in diversity. Trends Biochem. Sci. 2005, 30, 542–550. [Google Scholar] [CrossRef]
- Cimpean, A.; Stefan, C.; Gijsbers, R.; Stalmans, W.; Bollen, M. Substrate-specifying determinants of the nucleotide pyrophosphatases/phosphodiesterases NPP1 and NPP2. Biochem. J. 2004, 381, 71–77. [Google Scholar] [CrossRef]
- Lorenz-Depiereux, B.; Schnabel, D.; Tiosano, D.; Häusler, G.; Strom, T.M. Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am. J. Hum. Genet. 2010, 86, 267–272. [Google Scholar] [CrossRef]
- Eytan, O.; Morice-Picard, F.; Sarig, O.; Ezzedine, K.; Isakov, O.; Li, Q.; Ishida-Yamamoto, A.; Shomron, N.; Goldsmith, T.; Fuchs-Telem, D.; et al. Cole Disease Results from Mutations in ENPP1. Am. J. Hum. Genet. 2013, 93, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Thumbigere-Math, V.; Alqadi, A.; Chalmers, N.I.; Chavez, M.B.; Chu, E.Y.; Collins, M.T.; Ferreira, C.R.; FitzGerald, K.; Gafni, R.I.; Gahl, W.A.; et al. Hypercementosis Associated with ENPP1 Mutations and GACI. J. Dent. Res. 2018, 97, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; van de Wetering, K.; Uitto, J. Pseudoxanthoma Elasticum as a Paradigm of Heritable Ectopic Mineralization Disorders: Pathomechanisms and Treatment Development. Am. J. Pathol. 2019, 189, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Van Gils, M.; Nollet, L.; Verly, E.; Deianova, N.; Vanakker, O.M. Cellular signaling in pseudoxanthoma elasticum: An update. Cell. Signal. 2019, 55, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Devriese, M.; Legrand, A.; Courtois, M.C.; Jeunemaitre, X.; Albuisson, J. Pseudoxanthoma elasticum with prominent arterial calcifications evoking CD73 deficiency. Vasc. Med. 2019, 24, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Staretz-Chacham, O.; Shukrun, R.; Barel, O.; Pode-Shakked, B.; Pleniceanu, O.; Anikster, Y.; Shalva, N.; Ferreira, C.R.; Ben-Haim Kadosh, A.; Richardson, J.; et al. Novel homozygous ENPP1 mutation causes generalized arterial calcifications of infancy, thrombocytopenia, and cardiovascular and central nervous system syndrome. Am. J. Med. Genet. 2019, 179, 2112–2118. [Google Scholar] [CrossRef]
- Quarles, L.D. Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat. Rev. Endocrinol. 2012, 8, 276–286. [Google Scholar] [CrossRef]
- Levy-Litan, V.; Hershkovitz, E.; Avizov, L.; Leventhal, N.; Bercovich, D.; Chalifa-Caspi, V.; Manor, E.; Buriakovsky, S.; Hadad, Y.; Goding, J.; et al. Autosomal-recessive hypophosphatemic rickets is associated with an inactivation mutation in the ENPP1 gene. Am. J. Hum. Genet. 2010, 86, 273–278. [Google Scholar] [CrossRef]
- Quarles, L.D. Evidence for a bone-kidney axis regulating phosphate homeostasis. J. Clin. Invest. 2003, 112, 642–646. [Google Scholar] [CrossRef]
- Chourabi, M.; Liew, M.S.; Lim, S.; H’mida-Ben Brahim, D.; Boussofara, L.; Dai, L.; Wong, P.M.; Foo, J.N.; Sriha, B.; Robinson, K.S.; et al. ENPP1 Mutation Causes Recessive Cole Disease by Altering Melanogenesis. J. Invest. Dermatol. 2018, 138, 291–300. [Google Scholar] [CrossRef]
- Kato, K.; Nishimasu, H.; Okudaira, S.; Mihara, E.; Ishitani, R.; Takagi, J.; Aoki, J.; Nureki, O. Crystal structure of Enpp1, an extracellular glycoprotein involved in bone mineralization and insulin signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 16876–16881. [Google Scholar] [CrossRef] [PubMed]
- Goldfine, I.D.; Maddux, B.A.; Youngren, J.F.; Reaven, G.; Accili, D.; Trischitta, V.; Vigneri, R.; Frittitta, L. The role of membrane glycoprotein plasma cell antigen 1/ectonucleotide pyrophosphatase phosphodiesterase 1 in the pathogenesis of insulin resistance and related abnormalities. Endocr. Rev. 2008, 29, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Ciociola, E.; Saraf, M.; Tumurbaatar, B.; Tuvdendorj, D.; Prasad, S.; Chandalia, M.; Abate, N. Metabolic consequences of ENPP1 overexpression in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E901–E911. [Google Scholar] [CrossRef] [PubMed]
- Huesa, C.; Zhu, D.; Glover, J.D.; Ferron, M.; Karsenty, G.; Milne, E.M.; Millan, J.L.; Ahmed, S.F.; Farquharson, C.; Morton, N.M.; et al. Deficiency of the bone mineralization inhibitor NPP1 protects mice against obesity and diabetes. Dis. Model. Mech. 2014, 7, 1341–1350. [Google Scholar] [CrossRef]
- Kato, K.; Nishimasu, H.; Oikawa, D.; Hirano, S.; Hirano, H.; Kasuya, G.; Ishitani, R.; Tokunaga, F.; Nureki, O. Structural insights into cGAMP degradation by Ecto-nucleotide pyrophosphatase phosphodiesterase 1. Nat. Commun. 2018, 9, 4424. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Wu, J.; Gao, D.; Wang, H.; Sun, L.; Chen, Z.J. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 2013, 341, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Wu, J.; Sun, L.; Chen, X.; Du, F.; Shi, H.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 2013, 339, 826–830. [Google Scholar] [CrossRef]
- Haag, S.M.; Gulen, M.F.; Reymond, L.; Gibelin, A.; Abrami, L.; Decout, A.; Heymann, M.; van der Goot, F.G.; Turcatti, G.; Behrendt, R.; et al. Targeting STING with covalent small-molecule inhibitors. Nature 2018, 559, 269–273. [Google Scholar] [CrossRef]
- Wang, J.; Lu, S.F.; Wan, B.; Ming, S.L.; Li, G.L.; Su, B.Q.; Liu, J.Y.; Wei, Y.S.; Yang, G.Y.; Chu, B.B. Maintenance of cyclic GMP-AMP homeostasis by ENPP1 is involved in pseudorabies virus infection. Mol. Immunol. 2018, 95, 56–63. [Google Scholar] [CrossRef]
- Dey, R.J.; Dey, B.; Zheng, Y.; Cheung, L.S.; Zhou, J.; Sayre, D.; Kumar, P.; Guo, H.; Lamichhane, G.; Sintim, H.O.; et al. Inhibition of innate immune cytosolic surveillance by an M. tuberculosis phosphodiesterase. Nat. Chem. Biol. 2017, 13, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yin, Q.; Kuss, P.; Maliga, Z.; Millán, J.L.; Wu, H.; Mitchison, T.J. Hydrolysis of 2′3′-cGAMP by ENPP1 and design of nonhydrolyzable analogs. Nat. Chem. Biol. 2014, 10, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Eaglesham, J.B.; Pan, Y.; Kupper, T.S.; Kranzusch, P.J. Viral and metazoan poxins are cGAMP-specific nucleases that restrict cGAS-STING signalling. Nature 2019, 566, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Danino, O.; Svetitsky, S.; Kenigsberg, S.; Levin, A.; Journo, S.; Gold, A.; Drexler, M.; Snir, N.; Elkayam, O.; Fischer, B.; et al. Inhibition of nucleotide pyrophosphatase/phosphodiesterase 1: Implications for developing a calcium pyrophosphate deposition disease modifying drug. Rheumatology 2018, 57, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, Y.; Hartmann, S.; Torsello, G.; Horstmann, R.; Seifarth, H.; Weissen--Plenz, G.; Rutsch, F. Expression of NPP1 is regulated during atheromatous plaque calcification. J. Cell. Mol. Med. 2011, 15, 220–231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharma, M.; Thode, T.; Weston, A.; Kaadige, M.R. Development of Enpp1 Inhibitors as a Strategy to Activate Stimulator of Interferon Genes (STING) in Cancers and Other Diseases. Int. J. Cell Sci. Mol. Biol. 2018, 5, 1–5. [Google Scholar]
- Abbasi, S.; Shin, D.M.; Beaty, N.; Masiuk, M.; Chen, S.; Gonzalez-Garcia, I.; Zhao, M.; Goding, J.; Morse, H.C.; Wang, H. Characterization of monoclonal antibodies to the plasma cell alloantigen ENPP1. Hybridoma (Larchmt) 2011, 30, 11–17. [Google Scholar] [CrossRef]
- Yoon, J.; Wang, H.; Kim, Y.C.; Yoshimoto, M.; Abbasi, S.; Morse Iii, H.C. Plasma cell alloantigen ENPP1 is expressed by a subset of human B cells with potential regulatory functions. Immunol. Cell. Biol. 2016, 94, 719–728. [Google Scholar] [CrossRef]
- Kaneda, M.M.; Messer, K.S.; Ralainirina, N.; Li, H.; Leem, C.J.; Gorjestani, S.; Woo, G.; Nguyen, A.V.; Figueiredo, C.C.; Foubert, P.; et al. PI3Kγ is a molecular switch that controls immune suppression. Nature 2016, 539, 437–442. [Google Scholar] [CrossRef]
- Grobben, B.; De Deyn, P.P.; Slegers, H. Rat C6 glioma as experimental model system for the study of glioblastoma growth and invasion. Cell Tissue Res. 2002, 310, 257–270. [Google Scholar] [CrossRef]
- Lau, W.M.; Doucet, M.; Stadel, R.; Huang, D.; Weber, K.L.; Kominsky, S.L. Enpp1: A potential facilitator of breast cancer bone metastasis. PLoS ONE 2013, 8, e66752. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Müller, C.E. Nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) and its inhibitors. MedChemComm 2017, 8, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, E.; Tran-Thi, T.-A.; Decker, K.F.A. Nucleotide Pyrophosphatase of Rat Liver. Eur. J. Biochem. 1975, 51, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in immunity and inflammation. Trends. Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef]
- Johnson, K.; Goding, J.; Van Etten, D.; Sali, A.; Hu, S.-I.; Farley, D.; Krug, H.; Hessle, L.; Millán, J.L.; Terkeltaub, R. Linked Deficiencies in Extracellular PPi and Osteopontin Mediate Pathologic Calcification Associated With Defective PC-1 and ANK Expression. J. Bone Miner. Res. 2003, 18, 994–1004. [Google Scholar] [CrossRef]
- Johnson, K.; Moffa, A.; Chen, Y.; Pritzker, K.; Goding, J.; Terkeltaub, R. Matrix Vesicle Plasma Cell Membrane Glycoprotein-1 Regulates Mineralization by Murine Osteoblastic MC3T3 Cells. J. Bone Miner. Res. 1999, 14, 883–892. [Google Scholar] [CrossRef]
- Horenstein, A.L.; Chillemi, A.; Zaccarello, G.; Bruzzone, S.; Quarona, V.; Zito, A.; Serra, S.; Malavasi, F. A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes. OncoImmunology 2013, 2, e26246. [Google Scholar] [CrossRef]
- Namasivayam, V.; Lee, S.-Y.; Müller, C.E. The promiscuous ectonucleotidase NPP1: Molecular insights into substrate binding and hydrolysis. Biochim. Biophys. Acta 2017, 1861, 603–614. [Google Scholar] [CrossRef]
- Wielinga, P.R.; van der Heijden, I.; Reid, G.; Beijnen, J.H.; Wijnholds, J.; Borst, P. Characterization of the MRP4- and MRP5-mediated Transport of Cyclic Nucleotides from Intact Cells. J. Biol. Chem. 2003, 278, 17664–17671. [Google Scholar] [CrossRef]
- Jedlitschky, G.; Burchell, B.; Keppler, D. The Multidrug Resistance Protein 5 Functions as an ATP-dependent Export Pump for Cyclic Nucleotides. J. Biol. Chem. 2000, 275, 30069–30074. [Google Scholar] [CrossRef]
- Guo, Y.; Kotova, E.; Chen, Z.-S.; Lee, K.; Hopper-Borge, E.; Belinsky, M.G.; Kruh, G.D. MRP8, ATP-binding Cassette C11 (ABCC11), Is a Cyclic Nucleotide Efflux Pump and a Resistance Factor for Fluoropyrimidines 2′,3′-Dideoxycytidine and 9′-(2′-Phosphonylmethoxyethyl)adenine. J. Biol. Chem. 2003, 278, 29509–29514. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.T.; Nhiep, N.T.H.; Vu, T.N.M.; Huynh, T.N.; Zhu, Y.; Huynh, A.L.D.; Chakrabortti, A.; Marcellin, E.; Lo, R.; Howard, C.B.; et al. Enhanced uptake of potassium or glycine betaine or export of cyclic-di-AMP restores osmoresistance in a high cyclic-di-AMP Lactococcus lactis mutant. PLoS Genet. 2018, 14, e1007574. [Google Scholar] [CrossRef] [PubMed]
- Conti, M. Phosphodiesterases and cyclic nucleotide signaling in endocrine cells. Mol. Endocrinol. 2000, 14, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Corrales, L.; McWhirter, S.M.; Dubensky, T.W.; Gajewski, T.F. The host STING pathway at the interface of cancer and immunity. J. Clin. Invest. 2016, 126, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Lemos, H.; Mohamed, E.; Huang, L.; Ou, R.; Pacholczyk, G.; Arbab, A.S.; Munn, D.; Mellor, A.L. STING Promotes the Growth of Tumors Characterized by Low Antigenicity via IDO Activation. Cancer Res. 2016, 76, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Lemos, H.; Huang, L.; McGaha, T.L.; Mellor, A.L. Cytosolic DNA sensing via the stimulator of interferon genes adaptor: Yin and Yang of immune responses to DNA. Eur. J. Immunol. 2014, 44, 2847–2853. [Google Scholar] [CrossRef]
- Huang, L.; Li, L.; Lemos, H.; Chandler, P.R.; Pacholczyk, G.; Baban, B.; Barber, G.N.; Hayakawa, Y.; McGaha, T.L.; Ravishankar, B.; et al. Cutting edge: DNA sensing via the STING adaptor in myeloid dendritic cells induces potent tolerogenic responses. J. Immunol. 2013, 191, 3509–3513. [Google Scholar] [CrossRef]
- Liu, M.; Wang, X.; Wang, L.; Ma, X.; Gong, Z.; Zhang, S.; Li, Y. Targeting the IDO1 pathway in cancer: From bench to bedside. J. Hematol. Oncol. 2018, 11, 100. [Google Scholar] [CrossRef]
- Munn, D.H.; Sharma, M.D.; Lee, J.R.; Jhaver, K.G.; Johnson, T.S.; Keskin, D.B.; Marshall, B.; Chandler, P.; Antonia, S.J.; Burgess, R.; et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 2002, 297, 1867–1870. [Google Scholar] [CrossRef]
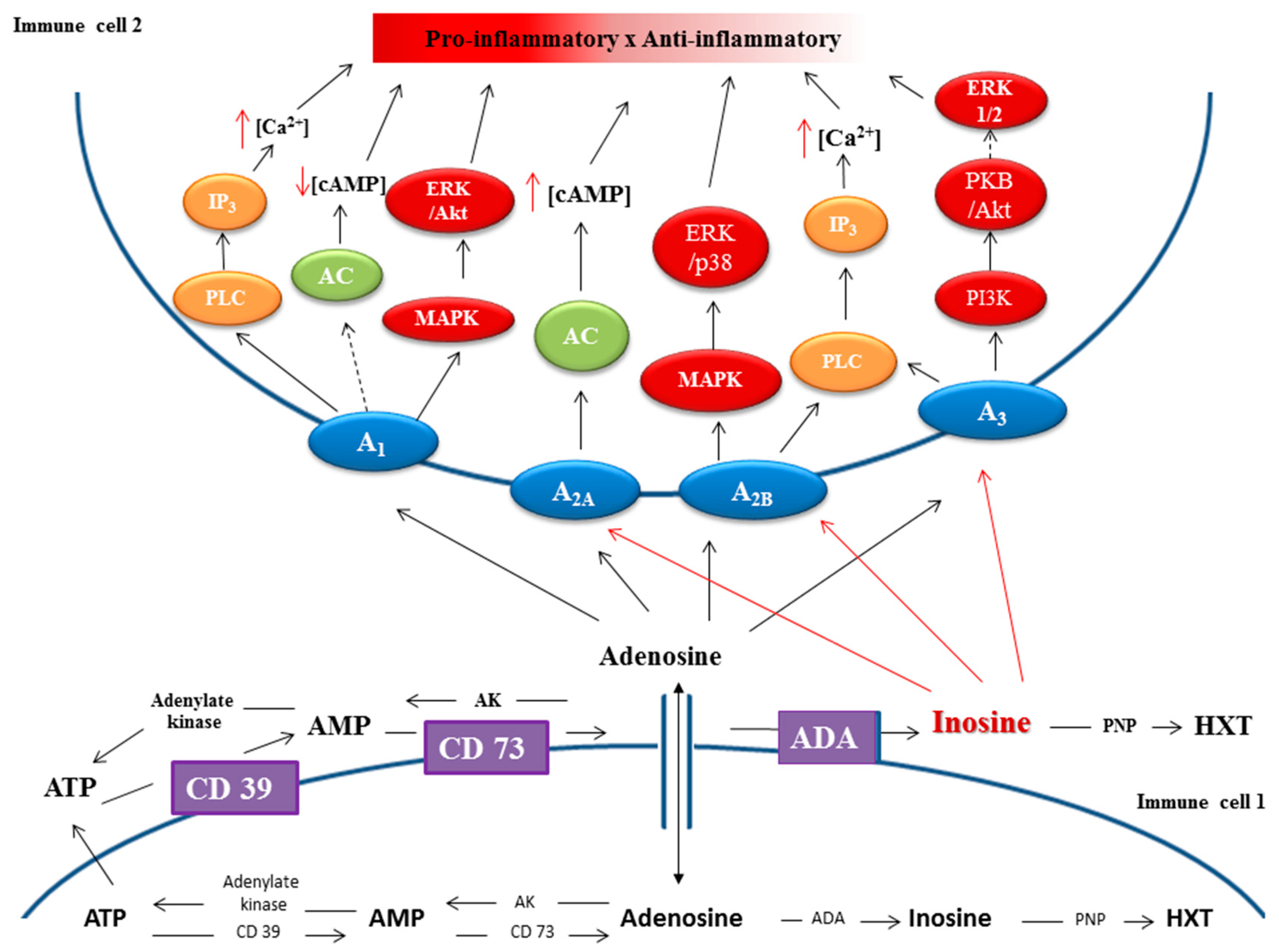
- Vijayan, D.; Young, A.; Teng, M.W.L.; Smyth, M.J. Targeting immunosuppressive adenosine in cancer. Nat. Rev. Cancer 2017, 17, 709–724. [Google Scholar] [CrossRef]
- Vigano, S.; Alatzoglou, D.; Irving, M.; Ménétrier-Caux, C.; Caux, C.; Romero, P.; Coukos, G. Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function. Front. Immunol. 2019, 10, 925. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.; Zebisch, M.; Sträter, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef] [PubMed]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.R.; Snaith, A.E.; Marino, D.; Cheng, X.; Lwin, S.T.; Orriss, I.R.; Hamdy, F.C.; Edwards, C.M. Tumour-derived alkaline phosphatase regulates tumour growth, epithelial plasticity and disease-free survival in metastatic prostate cancer. Br. J. Cancer 2017, 116, 227–236. [Google Scholar] [CrossRef]
- de Lourdes Mora-García, M.; García-Rocha, R.; Morales-Ramírez, O.; Montesinos, J.J.; Weiss-Steider, B.; Hernández-Montes, J.; Ávila-Ibarra, L.R.; Don-López, C.A.; Velasco-Velázquez, M.A.; Gutiérrez-Serrano, V.; et al. Mesenchymal stromal cells derived from cervical cancer produce high amounts of adenosine to suppress cytotoxic T lymphocyte functions. J. Transl. Med. 2016, 14, 302. [Google Scholar] [CrossRef]
- Montalbán Del Barrio, I.; Penski, C.; Schlahsa, L.; Stein, R.G.; Diessner, J.; Wöckel, A.; Dietl, J.; Lutz, M.B.; Mittelbronn, M.; Wischhusen, J.; et al. Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages—A self-amplifying, CD39- and CD73-dependent mechanism for tumor immune escape. J. Immunother. Cancer 2016, 4, 49. [Google Scholar] [CrossRef]
- García-Rocha, R.; Moreno-Lafont, M.; Mora-García, M.L.; Weiss-Steider, B.; Montesinos, J.J.; Piña-Sánchez, P.; Monroy-García, A. Mesenchymal stromal cells derived from cervical cancer tumors induce TGF-β1 expression and IL-10 expression and secretion in the cervical cancer cells, resulting in protection from cytotoxic T cell activity. Cytokine 2015, 76, 382–390. [Google Scholar] [CrossRef]
- Stagg, J.; Beavis, P.A.; Divisekera, U.; Liu, M.C.; Möller, A.; Darcy, P.K.; Smyth, M.J. CD73-deficient mice are resistant to carcinogenesis. Cancer Res. 2012, 72, 2190–2196. [Google Scholar] [CrossRef]
- Stagg, J.; Divisekera, U.; Duret, H.; Sparwasser, T.; Teng, M.W.; Darcy, P.K.; Smyth, M.J. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 2011, 71, 2892–2900. [Google Scholar] [CrossRef]
- Lapa, F.D.; Júnior, S.J.; Cerutti, M.L.; Santos, A.R. Pharmacology and Therapeutics. In Pharmacology of Adenosine Receptors and Their Signaling Role in Immunity and Inflammation; Gowder, S.J.T., Ed.; IntechOpen: London, UK, 2014; pp. 86–130. [Google Scholar]
- Lee, S.-Y.; Sarkar, S.; Bhattarai, S.; Namasivayam, V.; De Jonghe, S.; Stephan, H.; Herdewijn, P.; El-Tayeb, A.; Müller, C.E. Substrate-Dependence of Competitive Nucleotide Pyrophosphatase/Phosphodiesterase1 (NPP1) Inhibitors. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Müller, C.E. Large-volume sample stacking with polarity switching for monitoring of nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) reactions by capillary electrophoresis. Electrophoresis 2014, 35, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Nadel, Y.; Lecka, J.; Gilad, Y.; Ben-David, G.; Förster, D.; Reiser, G.; Kenigsberg, S.; Camden, J.; Weisman, G.A.; Senderowitz, H.; et al. Highly Potent and Selective Ectonucleotide Pyrophosphatase/Phosphodiesterase I Inhibitors Based on an Adenosine 5′-(α or γ)-Thio-(α,β- or β,γ)-methylenetriphosphate Scaffold. J. Med. Chem. 2014, 57, 4677–4691. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, S.A.; Lavoie, É.G.; Lecka, J.; Bigonnesse, F.; Sévigny, J. Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br. J. Pharmacol. 2007, 152, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Lecka, J.; Ben-David, G.; Simhaev, L.; Eliahu, S.; Oscar, J.; Luyindula, P.; Pelletier, J.; Fischer, B.; Senderowitz, H.; Sévigny, J. Nonhydrolyzable ATP Analogues as Selective Inhibitors of Human NPP1: A Combined Computational/Experimental Study. J. Med. Chem. 2013, 56, 8308–8320. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Lee, S.Y.; Leonczak, P.; Rozenski, J.; De Jonghe, S.; Hanck, T.; Müller, C.E.; Herdewijn, P. Imidazopyridine- and purine-thioacetamide derivatives: Potent inhibitors of nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1). J. Med. Chem. 2014, 57, 10080–10100. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, N.; Hoshino, S.I.; Kanda, Y.; Katada, T. Inhibition of phosphodiesterase/pyrophosphatase activity of PC-1 by its association with glycosaminoglycans. Eur. J. Biochem. 1999, 265, 763–770. [Google Scholar] [CrossRef]
- Lee, S.Y.; Perotti, A.; De Jonghe, S.; Herdewijn, P.; Hanck, T.; Müller, C.E. Thiazolo[3,2-a]benzimidazol-3(2H)-one derivatives: Structure-activity relationships of selective nucleotide pyrophosphatase/phosphodiesterase1 (NPP1) inhibitors. Bioorg. Med. Chem. 2016, 24, 3157–3165. [Google Scholar] [CrossRef]
- Grobben, B.; Claes, P.; Roymans, D.; Esmans, E.L.; Van Onckelen, H.; Slegers, H. Ecto-nucleotide pyrophosphatase modulates the purinoceptor-mediated signal transduction and is inhibited by purinoceptor antagonists. Br. J. Pharmacol. 2000, 130, 139–145. [Google Scholar] [CrossRef]
- Shayhidin, E.E.; Forcellini, E.; Boulanger, M.C.; Mahmut, A.; Dautrey, S.; Barbeau, X.; Lagüe, P.; Sévigny, J.; Paquin, J.F.; Mathieu, P. Quinazoline-4-piperidine sulfamides are specific inhibitors of human NPP1 and prevent pathological mineralization of valve interstitial cells. Br. J. Pharmacol. 2015, 172, 4189–4199. [Google Scholar] [CrossRef]
- Ausekle, E.; Ejaz, S.A.; Khan, S.U.; Ehlers, P.; Villinger, A.; Lecka, J.; Sévigny, J.; Iqbal, J.; Langer, P. New one-pot synthesis of N-fused isoquinoline derivatives by palladium-catalyzed C-H arylation: Potent inhibitors of nucleotide pyrophosphatase-1 and -3. Org. Biomol. Chem. 2016, 14, 11402–11414. [Google Scholar] [CrossRef]
- Semreen, M.H.; El-Gamal, M.I.; Ullah, S.; Jalil, S.; Zaib, S.; Anbar, H.S.; Lecka, J.; Sévigny, J.; Iqbal, J. Synthesis, biological evaluation, and molecular docking study of sulfonate derivatives as nucleotide pyrophosphatase/phosphodiesterase (NPP) inhibitors. Bioorg. Med. Chem. 2019, 27, 2741–2752. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, M.I.; Ullah, S.; Zaraei, S.O.; Jalil, S.; Zaib, S.; Zaher, D.M.; Omar, H.A.; Anbar, H.S.; Pelletier, J.; Sévigny, J.; et al. Synthesis, biological evaluation, and docking studies of new raloxifene sulfonate or sulfamate derivatives as inhibitors of nucleotide pyrophosphatase/phosphodiesterase. Eur. J. Med. Chem. 2019, 181, 111560. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.R.; Cervino, E.V.; Rentero, M.L.; Crespo, E.C.; Alvaro, A.O.; Casillas, M. Raloxifene: Mechanism of action, effects on bone tissue, and applicability in clinical traumatology practice. Open Orthop. J. 2009, 3, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Forcellini, E.; Boutin, S.; Lefebvre, C.-A.; Shayhidin, E.E.; Boulanger, M.-C.; Rhéaume, G.; Barbeau, X.; Lagüe, P.; Mathieu, P.; Paquin, J.-F. Synthesis and biological evaluation of novel quinazoline-4-piperidinesulfamide derivatives as inhibitors of NPP1. Eur. J. Med. Chem. 2018, 147, 130–149. [Google Scholar] [CrossRef]
- Weston, A.; Thode, T.; Munoz, R.; Daniel, S.; Soldi, R.; Kaadige, M.; Han, H.; Vankayalapti, H.; Sharma, S. Abstract 3077: Preclinical studies of SR-8314, a highly selective ENPP1 inhibitor and an activator of STING pathway. Cancer Res. 2019, 79, 3077. [Google Scholar] [CrossRef]
- Baird, J.; Dietsch, G.; Florio, V.; Gallatin, M.; Knox, C.; Odingo, J.; Crittenden, M.; Gough, M.J. MV-626, a potent and selective inhibitor of ENPP1 enhances STING activation and augments T-cell mediated anti-tumor activity in vivo. In Proceedings of the 33rd Annual Meeting of the Society for Immunotherapy of Cancer, Walter E. Washington Convention Center, Washington, DC, USA, 7–11 November 2018; p. 410. [Google Scholar]
- Asensio, A.C.; Rodríguez-Ferrer, C.R.; Castañeyra-Perdomo, A.; Oaknin, S.; Rotllán, P. Biochemical analysis of ecto-nucleotide pyrophosphatase phosphodiesterase activity in brain membranes indicates involvement of NPP1 isoenzyme in extracellular hydrolysis of diadenosine polyphosphates in central nervous system. Neurochem. Int. 2007, 50, 581–590. [Google Scholar] [CrossRef]
- Iqbal, J.; Lévesque, S.A.; Sévigny, J.; Müller, C.E. A highly sensitive CE-UV method with dynamic coating of silica-fused capillaries for monitoring of nucleotide pyrophosphatase/phosphodiesterase reactions. Electrophoresis 2008, 29, 3685–3693. [Google Scholar] [CrossRef]
- Jafari, B.; Yelibayeva, N.; Ospanov, M.; Ejaz, S.A.; Afzal, S.; Khan, S.U.; Abilov, Z.A.; Turmukhanova, M.Z.; Kalugin, S.N.; Safarov, S.; et al. Synthesis of 2-arylated thiadiazolopyrimidones by Suzuki–Miyaura cross-coupling: A new class of nucleotide pyrophosphatase (NPPs) inhibitors. RSC Adv. 2016, 6, 107556–107571. [Google Scholar] [CrossRef]
- Lee, S.Y.; Fiene, A.; Li, W.; Hanck, T.; Brylev, K.A.; Fedorov, V.E.; Lecka, J.; Haider, A.; Pietzsch, H.J.; Zimmermann, H.; et al. Polyoxometalates—Potent and selective ecto-nucleotidase inhibitors. Biochem. Pharmacol. 2015, 93, 171–181. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Fatima, N.; Khan, K.M.; Jalil, S.; Iqbal, S.; Atta-Ur-Rahman. New biscoumarin derivatives-cytotoxicity and enzyme inhibitory activities. Bioorg. Med. Chem. 2006, 14, 8066–8072. [Google Scholar] [CrossRef]
- Khan, K.M.; Fatima, N.; Rasheed, M.; Jalil, S.; Ambreen, N.; Perveen, S.; Choudhary, M.I. 1,3,4-Oxadiazole-2(3H)-thione and its analogues: A new class of non-competitive nucleotide pyrophosphatases/phosphodiesterases 1 inhibitors. Bioorg. Med. Chem. 2009, 17, 7816–7822. [Google Scholar] [CrossRef] [PubMed]
- Raza, R.; Akhtar, T.; Hameed, S.; Lecka, J.; Iqbal, J.; Sévigny, J. Identification of Potent and Selective Human Ecto-Nucleotide Pyrophosphatase/Phosphodiesterase-3 (hNPP3) Inhibitors. Open Enzym. Inhib. J. 2011, 4, 17–22. [Google Scholar]
- Patel, S.D.; Habeski, W.M.; Cheng, A.C.; de la Cruz, E.; Loh, C.; Kablaoui, N.M. Quinazolin-4-piperidin-4-methyl sulfamide PC-1 inhibitors: Alleviating hERG interactions through structure based design. Bioorg. Med. Chem. Lett. 2009, 19, 3339–3343. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Siddiqui, S.; Saleem, M.; Taha, M.; Saad, S.M.; Perveen, S.; Choudhary, M.I. Synthesis of triazole Schiff bases: Novel inhibitors of nucleotide pyrophosphatase/phosphodiesterase-1. Bioorg. Med. Chem. 2014, 22, 6509–6514. [Google Scholar] [CrossRef]



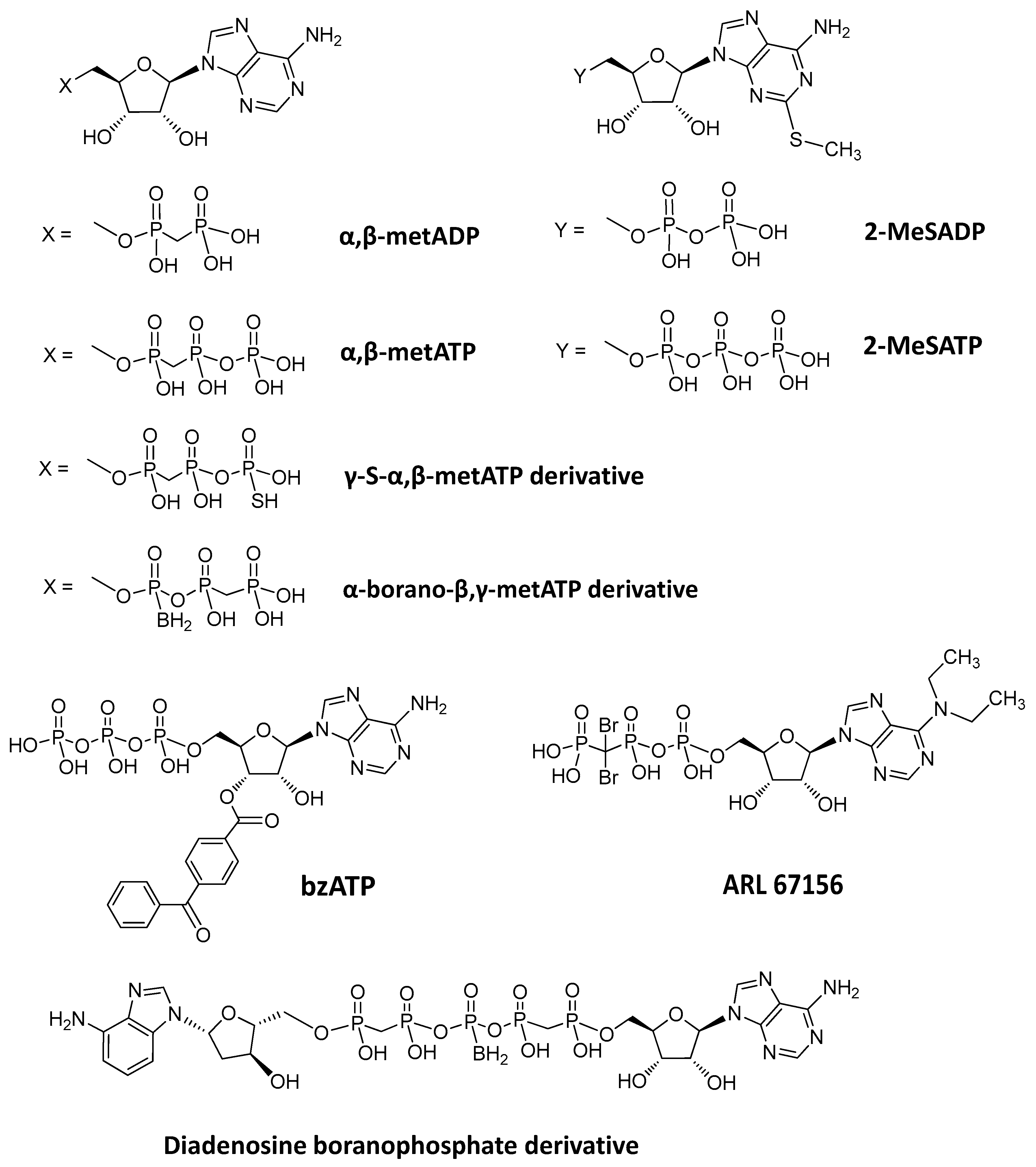
| Inhibitor (I) | Structure | Substrate | Enzyme | Ki/ IC50a | Inhibition | Ref |
|---|---|---|---|---|---|---|
| (µM) | Type | |||||
| Polysaccharides | 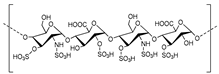 | |||||
| Heparin | ATP | Human, soluble | 100a | Competitive | [77] | |
| p-Nph-5′-TMP | Human, soluble | 1.0a | Competitive | [77] | ||
| εAP2A | Unspecified rat NPPs | 25 | Competitive | [88] | ||
| Polysulfonates | ||||||
| Suramin | 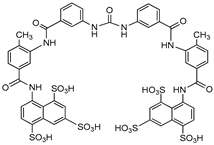 | P32-ATP | Unspecified rat NPPs | 61–83a | Not defined | [79] |
| ATP | Human, membrane-bound | 0.26 | Not defined | [89] | ||
| p-Nph-5′-TMP | Human, membrane-bound | 8.67a | Not defined | [90] | ||
| p-Nph-5′-TMP | Human, soluble | 1.07 | Uncompetitive | [71] | ||
| p-Nph-5′-AMP | Human, soluble | 1.03 | Uncompetitive | [71] | ||
| ATP | Human, soluble | 0.78 | Uncompetitive | [71] | ||
| Reactive blue 2 | 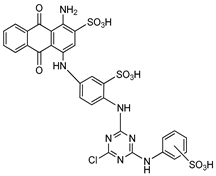 | P32-ATP | Unspecified rat NPPs | 9–15a | Not defined | [79] |
| ATP | Human, membrane-bound | 0.52 | Not defined | [89] | ||
| ATP | Human, soluble | 0.14 | Noncompetitive | [71] | ||
| p-Nph-5′-TMP | Human, soluble | 0.198 | Noncompetitive | [71] | ||
| p-Nph-5′-AMP | Human, soluble | 0.176 | Noncompetitive | [71] | ||
| Polyoxometalates | ||||||
| [TiW11CoO40]8− | ATP | Human, soluble | 0.00146 | Noncompetitive | [91] | |
| Other Heterocyclic compounds | ||||||
| PPADS | 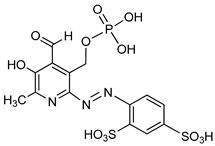 | P32-ATP | Unspecified rat NPPs | 9–15a | Not defined | [79] |
| Biscoumarin derivative | 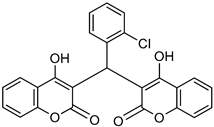 | p-NPPP | Human, soluble | 50–1000 | Noncompetitive | [92] |
| bis(p-NP)P | Snake venom | 8–1150 | Noncompetitive | [92] | ||
| Oxadiazole derivatives |  | p-NPPP | Human, soluble | 150–850 | Noncompetitive | [93] |
| bis(p-NP)P | Snake venom | 100–1400 | Noncompetitive | [93] | ||
| p-Nph-5′-TMP | Human, membrane-bound | 1.9–5.5a | Noncompetitive | [94] | ||
| Quinazoline derivative | 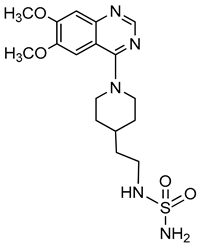 | ATP | Not determined | 0.036–5.98a | Not defined | [95] |
| p-Nph-5′-TMP | Human, membrane-bound | 0.059–0.110 | Noncompetitive | [80] | ||
| p-Nph-5′-TMP | Human, soluble | 0.06b | Competitive | [71] | ||
| p-Nph-5′-AMP | Human, soluble | 0.42b | Competitive | [71] | ||
| ATP | Human, soluble | 0.215 | Competitive | [71] | ||
| Triazole derivative | 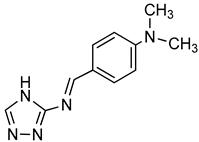 | bis(p-NP)P | Snake venom | 132–1164a | Not defined | [96] |
| Thioacetamide derivative | 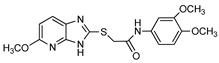 | ATP | Human, soluble | 5.34–89.7 | Competitive | [76] |
| p-Nph-5′-TMP | Human, soluble | 0.005–11.0 | Competitive | [76] | ||
| Isoquinoline derivative |  | p-Nph-5′-AMP | Human, soluble | 14.9 | Competitive | [71] |
| Thiadiazolopyrimidinone derivative | 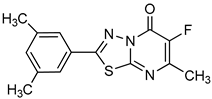 | p-Nph-5′-TMP | Human, membrane-bound | 0.36–2.81a | Competitive | [81] |
| p-Nph-5′-TMP | Human, membrane-bound | 0.31–2.26a | Competitive | [90] | ||
| Thiazolobenzimidazolon-e derivative | 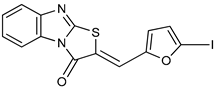 | ATP | Human, soluble | 0.467–0.981 | Uncompetitive | [78] |
| Sulfamate derivatives | 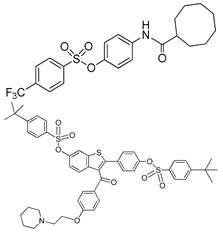 | p-Nph-5′-TMP | Human, soluble | 0.29a | [83] | |
| p-Nph-5′-TMP | 0.387a | Competitive | [82] | |||
| SR 8314 | Not disclosed | ATP | Human, soluble | 0.079 | Not determined | [86] |
| MV 626 | Not disclosed | ATP | 5–18 | [87] | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onyedibe, K.I.; Wang, M.; Sintim, H.O. ENPP1, an Old Enzyme with New Functions, and Small Molecule Inhibitors—A STING in the Tale of ENPP1. Molecules 2019, 24, 4192. https://doi.org/10.3390/molecules24224192
Onyedibe KI, Wang M, Sintim HO. ENPP1, an Old Enzyme with New Functions, and Small Molecule Inhibitors—A STING in the Tale of ENPP1. Molecules. 2019; 24(22):4192. https://doi.org/10.3390/molecules24224192
Chicago/Turabian StyleOnyedibe, Kenneth I., Modi Wang, and Herman O. Sintim. 2019. "ENPP1, an Old Enzyme with New Functions, and Small Molecule Inhibitors—A STING in the Tale of ENPP1" Molecules 24, no. 22: 4192. https://doi.org/10.3390/molecules24224192
APA StyleOnyedibe, K. I., Wang, M., & Sintim, H. O. (2019). ENPP1, an Old Enzyme with New Functions, and Small Molecule Inhibitors—A STING in the Tale of ENPP1. Molecules, 24(22), 4192. https://doi.org/10.3390/molecules24224192