Curcumin Nicotinate Selectively Induces Cancer Cell Apoptosis and Cycle Arrest through a P53-Mediated Mechanism
Abstract
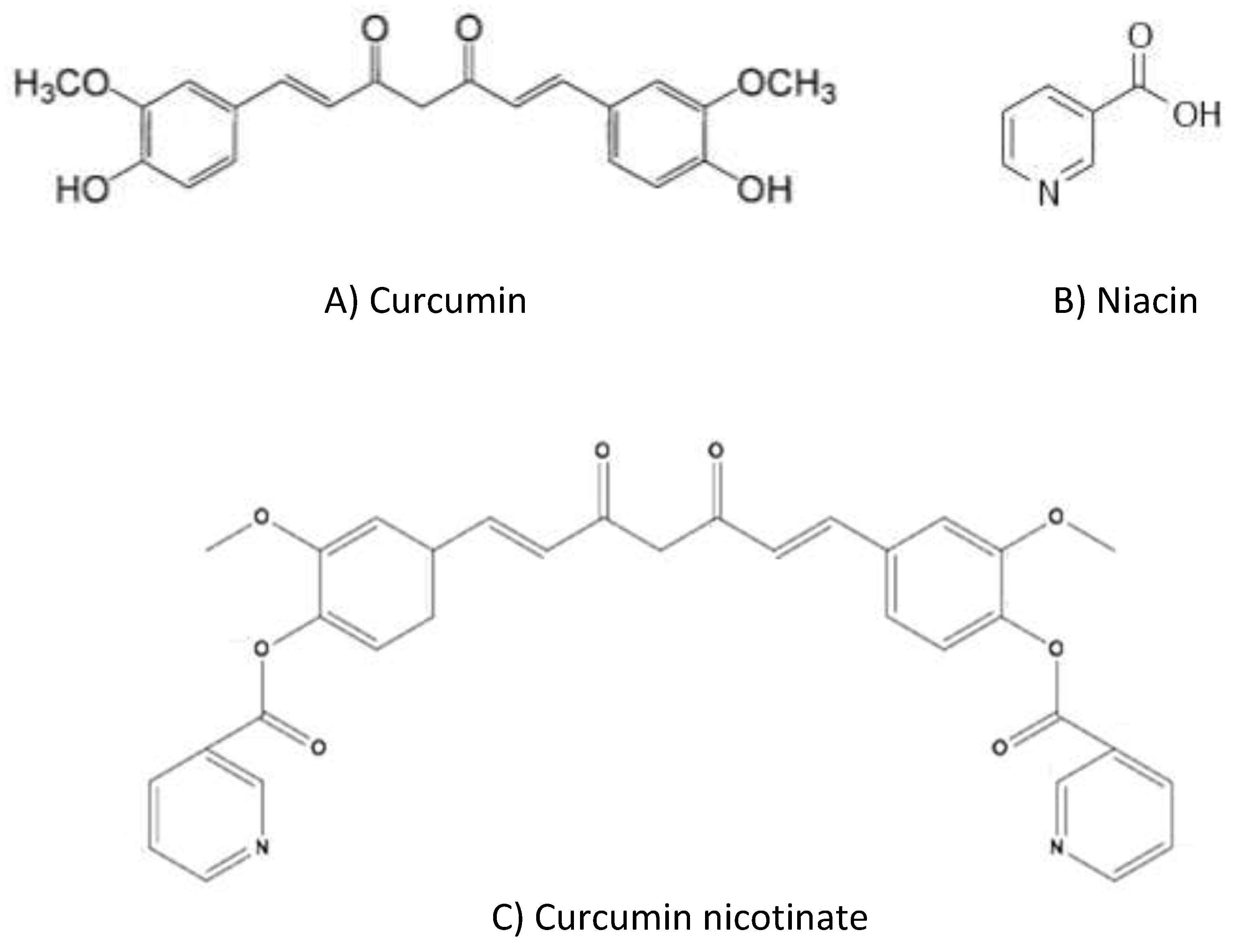
1. Introduction
2. Results
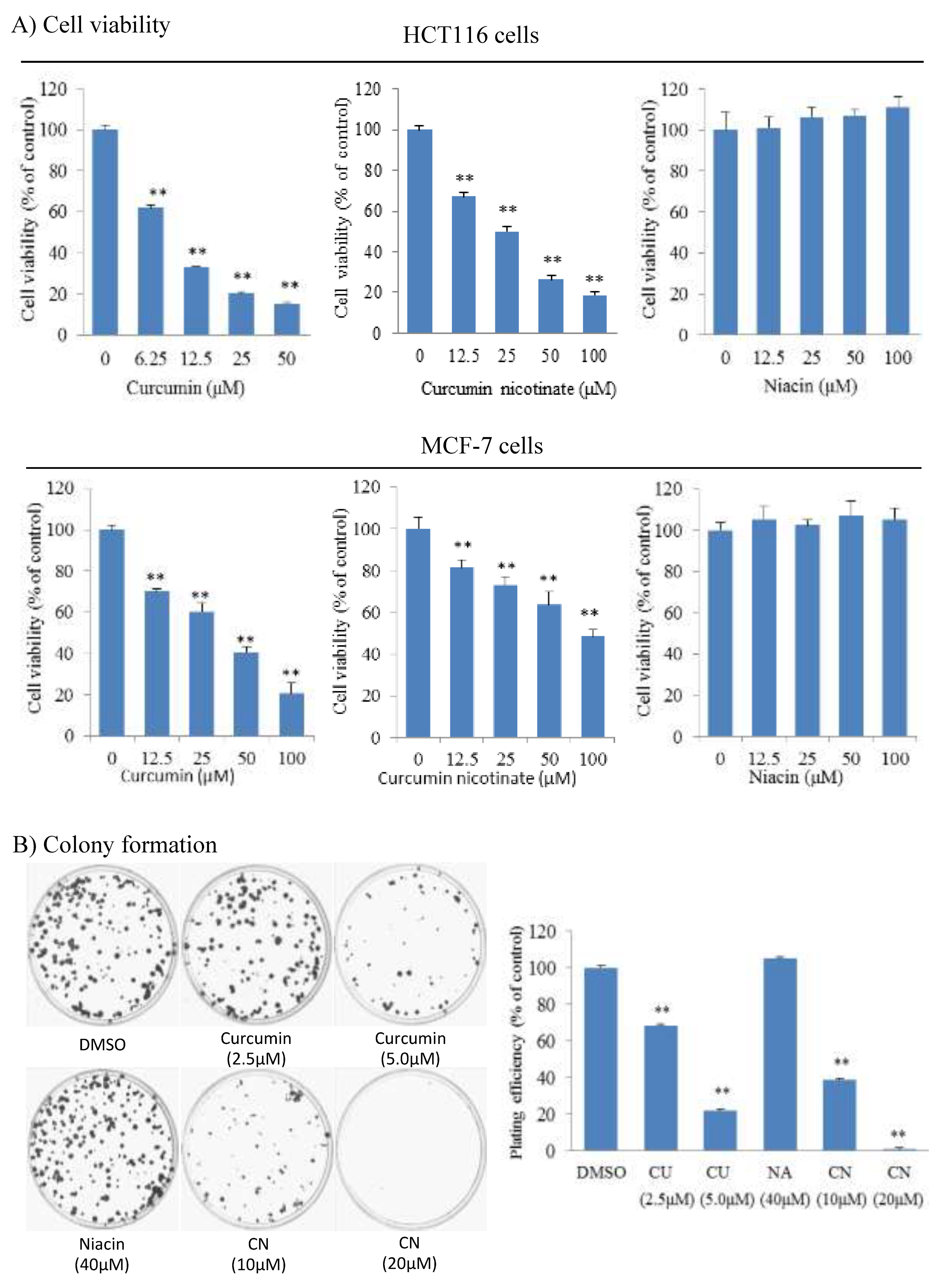
2.1. Curcumin Nicotinate Has Antiproliferative Activity in Multiple Cancer Cell Lines
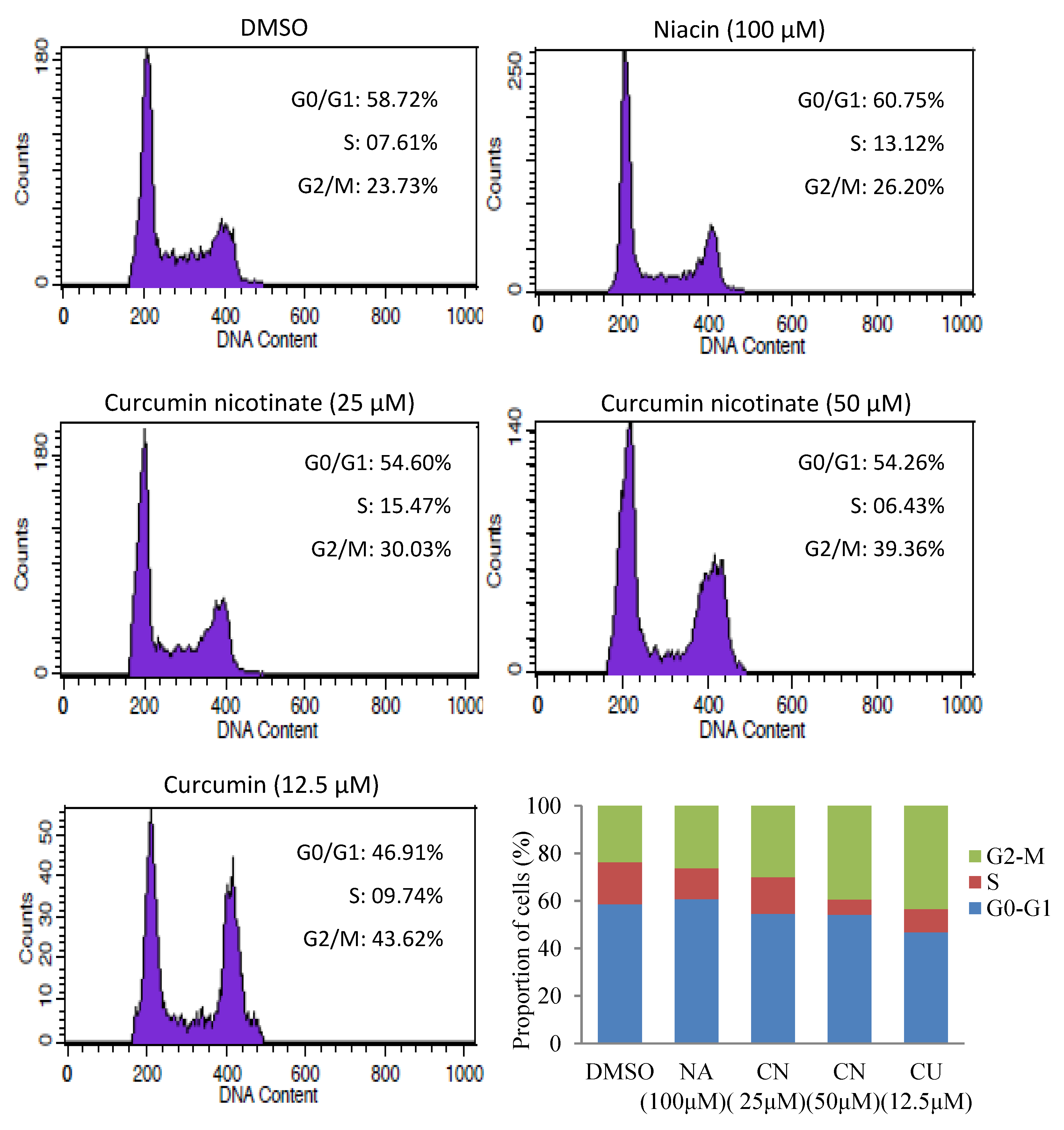
2.2. Curcumin Nicotinate Induces Apoptosis and Cell Cycle Arrest
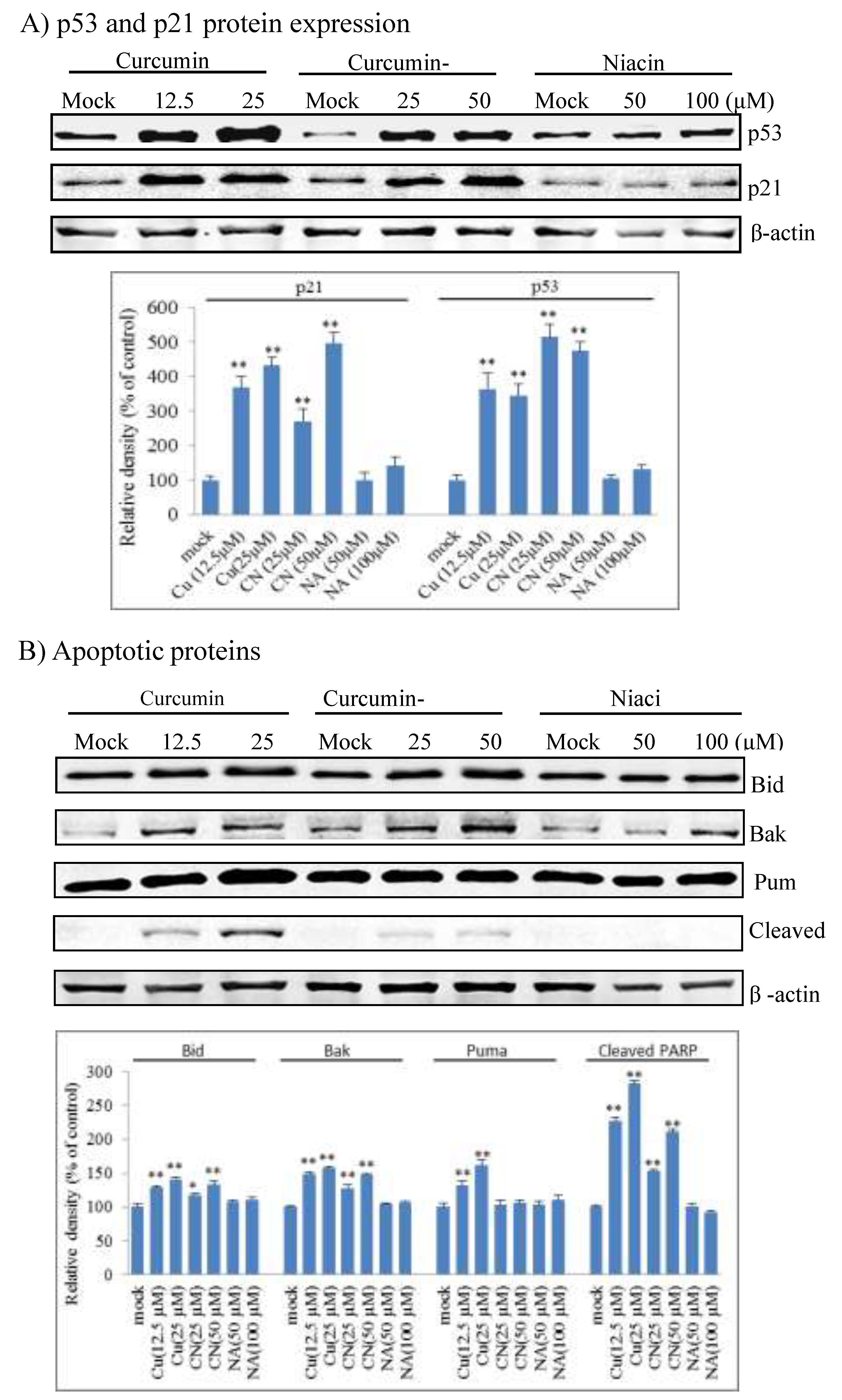
2.3. Curcumin Nicotinate Induces Cell Cycle Arrest and Apoptosis Through a p53-Mediated Mechanism
2.4. Curcumin Nicotinate Demonstrates Minimal Antiproliferative Activity in Non-Transformed MCF10A Cells
3. Discussion
4. Materials and Methods
4.1. Antibody, Drugs and Chemicals
4.2. Cell Lines and Culture Conditions
4.3. Cell Viability Assay
4.4. Colony Formation Assay
4.5. Apoptosis Assay
4.6. Cell Cycle Analysis
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations Used
References
- Luo, Q.; Zhang, L.; Luo, C.; Jiang, M. Emerging strategies in cancer therapy combining chemotherapy with immunotherapy. Cancer Lett. 2019, 454, 191–203. [Google Scholar] [CrossRef]
- Egan, M.E.; Pearson, M.; Weiner, S.A.; Rajendran, V.; Rubin, D.; Glockner-Pagel, J.; Canny, S.; Du, K.; Lukacs, G.L.; Caplan, M.J. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science 2004, 304, 600–602. [Google Scholar] [CrossRef] [PubMed]
- Rajitha, B.; Belalcazar, A.; Nagaraju, G.P.; Shaib, W.L.; Snyder, J.P.; Shoji, M.; Pattnaik, S.; Alam, A.; El-Rayes, B.F. Inhibition of NF-kappaB translocation by curcumin analogs induces G0/G1 arrest and downregulates thymidylate synthase in colorectal cancer. Cancer Lett. 2016, 373, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Chan, J.Y.; Wei, W.I.; Wong, T.S. Anti-cancer effects of curcumin on head and neck cancers. Anticancer Agents Med. Chem. 2012, 12, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, D. Farmer to pharmacist: Curcumin as an anti-invasive and antimetastatic agent for the treatment of cancer. Front. Chem. 2014, 2, 113. [Google Scholar] [CrossRef] [PubMed]
- Abouzied, M.M.; Eltahir, H.M.; Abdel Aziz, M.A.; Ahmed, N.S.; Abd El-Ghany, A.A.; Abd El-Aziz, E.A.; Abd El-Aziz, H.O. Curcumin ameliorate DENA-induced HCC via modulating TGF-beta, AKT, and caspase-3 expression in experimental rat model. Tumour Biol. 2015, 36, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Duan, X.; Cai, H.; Wang, L.; Li, M.; Qu, J.; Li, W.; Wang, Y.; Wang, J. Curcumin inhibits the invasion of lung cancer cells by modulating the PKCalpha/Nox-2/ROS/ATF-2/MMP-9 signaling pathway. Oncol. Rep. 2015, 34, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Abuelba, H.; Cotrutz, C.E.; Stoica, B.A.; Stoica, L.; Olinici, D.; Petreus, T. In vitro evaluation of curcumin effects on breast adenocarcinoma 2D and 3D cell cultures. Rom. J. Morphol. Embryol. 2015, 56, 71–76. [Google Scholar]
- Bimonte, S.; Barbieri, A.; Palma, G.; Rea, D.; Luciano, A.; D’Aiuto, M.; Arra, C.; Izzo, F. Dissecting the role of curcumin in tumour growth and angiogenesis in mouse model of human breast cancer. BioMed Res. Int. 2015, 2015, 878134. [Google Scholar] [CrossRef]
- Lee, Y.H.; Song, N.Y.; Suh, J.; Kim, D.H.; Kim, W.; Ann, J.; Lee, J.; Baek, J.H.; Na, H.K.; Surh, Y.J. Curcumin suppresses oncogenicity of human colon cancer cells by covalently modifying the cysteine 67 residue of SIRT1. Cancer Lett. 2018, 431, 219–229. [Google Scholar] [CrossRef]
- Seo, J.A.; Kim, B.; Dhanasekaran, D.N.; Tsang, B.K.; Song, Y.S. Curcumin induces apoptosis by inhibiting sarco/endoplasmic reticulum Ca2+ ATPase activity in ovarian cancer cells. Cancer Lett. 2016, 371, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Cao, Y.; Mo, N.; Li, J.; Mo, X. Curcurmin inhibits on proliferation in A549 cells through P53/P21/PCNA/elF4E signaling pathway. J. Third Mil. Med. Univ. 2012, 34, 49–53. [Google Scholar]
- El-Azab, M.; Hishe, H.; Moustafa, Y.; El-Awady el, S. Anti-angiogenic effect of resveratrol or curcumin in Ehrlich ascites carcinoma-bearing mice. Eur. J. Pharm. 2011, 652, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Sreenivasan, S.; Ravichandran, S.; Vetrivel, U.; Krishnakumar, S. Modulation of multidrug resistance 1 expression and function in retinoblastoma cells by curcumin. J. Pharm. Pharm. 2013, 4, 103–109. [Google Scholar]
- Lu, W.D.; Qin, Y.; Yang, C.; Li, L.; Fu, Z.X. Effect of curcumin on human colon cancer multidrug resistance in vitro and in vivo. Clinics 2013, 68, 694–701. [Google Scholar] [CrossRef]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Gescher, A.J.; Steward, W.P. Curcumin: The story so far. Eur. J. Cancer 2005, 41, 1955–1968. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Epelbaum, R.; Schaffer, M.; Vizel, B.; Badmaev, V.; Bar-Sela, G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer 2010, 62, 1137–1141. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Yang, K.Y.; Lin, L.C.; Tseng, T.Y.; Wang, S.C.; Tsai, T.H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 853, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Garcea, G.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J.; Berry, D.P. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br. J. Cancer 2004, 90, 1011–1015. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar] [PubMed]
- Kennedy, D.O. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef]
- Jacobson, M.K.; Jacobson, E.L. Vitamin B3 in Health and Disease: Toward the Second Century of Discovery. In ADP-Ribosylation and NAD+ Utilizing Enzymes; Chang, P., Ed.; Humana Press: New York, NY, USA, 2018; Volume 1813, pp. 3–8. [Google Scholar]
- Kirkland, J.B. Niacin requirements for genomic stability. Mutat. Res. 2012, 733, 14–20. [Google Scholar] [CrossRef]
- Surjana, D.; Halliday, G.M.; Damian, D.L. Role of nicotinamide in DNA damage, mutagenesis, and DNA repair. J. Nucleic Acids 2010, 2010. [Google Scholar] [CrossRef]
- Kiesewetter, B.; Mayerhoefer, M.E.; Lukas, J.; Zielinski, C.C.; Mullauer, L.; Raderer, M. Rituximab plus bendamustine is active in pretreated patients with extragastric marginal zone B cell lymphoma of the mucosa-associated lymphoid tissue (MALT lymphoma). Ann. Hematol. 2014, 93, 249–253. [Google Scholar] [CrossRef]
- El Weshi, A.; Memon, M.; Raja, M.; Bazarbashi, S.; Rahal, M.; El Foudeh, M.; Pai, C.; Allam, A.; El Hassan, I.; Ezzat, A. VIP (etoposide, ifosfamide, cisplatin) in adult patients with recurrent or refractory Ewing sarcoma family of tumors. Am. J. Clin. Oncol. 2004, 27, 529–534. [Google Scholar] [CrossRef]
- He, L.; Wang, X.; Luo, D. Synthesis of Curcumin Niconate. China J. Chin. Mater. Med. 2011, 52, 175–177. [Google Scholar]
- Guo, J.; He, Q.; Guo, Y.; Tuo, Q.; Liao, D.; Yan, J. Determination of equilibrium solubility and apparent oil-water partition coefficient of nicotinate-curcumin ester. Zhongguo Yao Shi 2018, 21, 197–201. [Google Scholar]
- Mortezaee, K.; Salehi, E.; Mirtavoos-Mahyari, H.; Motevaseli, E.; Najafi, M.; Farhood, B.; Rosengren, R.J.; Sahebkar, A. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J. Cell. Physiol. 2019, 234, 12537–12550. [Google Scholar] [CrossRef] [PubMed]
- Mou, S.; Zhou, Z.; He, Y.; Liu, F.; Gong, L. Curcumin inhibits cell proliferation and promotes apoptosis of laryngeal cancer cells through Bcl-2 and PI3K/Akt, and by upregulating miR-15a. Oncol. Lett. 2017, 14, 4937–4942. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bu, S. Curcumin Induces Autophagy, Apoptosis, and Cell Cycle Arrest in Human Pancreatic Cancer Cells. Evid. Based Complement. Altern. Med. 2017, 2017, 5787218. [Google Scholar] [CrossRef] [PubMed]
- El-Deiry, W.S. p21(WAF1) Mediates Cell-Cycle Inhibition, Relevant to Cancer Suppression and Therapy. Cancer Res. 2016, 76, 5189–5191. [Google Scholar] [CrossRef]
- Cao, A.L.; Tang, Q.F.; Zhou, W.C.; Qiu, Y.Y.; Hu, S.J.; Yin, P.H. Ras/ERK signaling pathway is involved in curcumin-induced cell cycle arrest and apoptosis in human gastric carcinoma AGS cells. J. Asian Nat. Prod. Res. 2015, 17, 56–63. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, C.; Xi, H.; Gao, Y.; Xu, D. Curcumin induces apoptosis in pancreatic cancer cells through the induction of forkhead box O1 and inhibition of the PI3K/Akt pathway. Mol. Med. Rep. 2015, 12, 5415–5422. [Google Scholar] [CrossRef]
- Catania, A.; Barrajon-Catalan, E.; Nicolosi, S.; Cicirata, F.; Micol, V. Immunoliposome encapsulation increases cytotoxic activity and selectivity of curcumin and resveratrol against HER2 overexpressing human breast cancer cells. Breast Cancer Res. Treat. 2013, 141, 55–65. [Google Scholar] [CrossRef]
- Safavy, A.; Raisch, K.P.; Mantena, S.; Sanford, L.L.; Sham, S.W.; Krishna, N.R.; Bonner, J.A. Design and development of water-soluble curcumin conjugates as potential anticancer agents. J. Med. Chem. 2007, 50, 6284–6288. [Google Scholar] [CrossRef]
- Sun, M.; Su, X.; Ding, B.; He, X.; Liu, X.; Yu, A.; Lou, H.; Zhai, G. Advances in nanotechnology-based delivery systems for curcumin. Nanomedicine 2012, 7, 1085–1100. [Google Scholar] [CrossRef]
- Mohanty, C.; Acharya, S.; Mohanty, A.K.; Dilnawaz, F.; Sahoo, S.K. Curcumin-encapsulated MePEG/PCL diblock copolymeric micelles: A novel controlled delivery vehicle for cancer therapy. Nanomedicine 2010, 5, 433–449. [Google Scholar] [CrossRef]
- Yan, R.; Zu, X.; Ma, J.; Liu, Z.; Adeyanju, M.; Cao, D. Aldo-keto reductase family 1 B10 gene silencing results in growth inhibition of colorectal cancer cells: Implication for cancer intervention. Int. J. Cancer J. Int. Du Cancer 2007, 121, 2301–2306. [Google Scholar] [CrossRef] [PubMed]
- Zu, X.; Yan, R.; Robbins, S.; Krishack, P.A.; Liao, D.F.; Cao, D. Reduced 293T cell susceptibility to acrolein due to aldose reductase-like-1 protein expression. Toxicol. Sci. 2007, 97, 562–568. [Google Scholar] [CrossRef]
- Bu, Y.; Li, X.; He, Y.; Huang, C.; Shen, Y.; Cao, Y.; Huang, D.; Cai, C.; Wang, Y.; Wang, Z.; et al. A phosphomimetic mutant of RelA/p65 at Ser536 induces apoptosis and senescence: An implication for tumor-suppressive role of Ser536 phosphorylation. Int. J. Cancer 2016, 138, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yan, R.; Luo, D.; Watabe, K.; Liao, D.F.; Cao, D. Aldo-keto reductase family 1 member B10 promotes cell survival by regulating lipid synthesis and eliminating carbonyls. J. Biol. Chem. 2009, 284, 26742–26748. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.-c.; He, L.; Khoshaba, R.; Lu, F.-g.; Cai, C.; Zhou, F.-l.; Liao, D.-f.; Cao, D. Curcumin Nicotinate Selectively Induces Cancer Cell Apoptosis and Cycle Arrest through a P53-Mediated Mechanism. Molecules 2019, 24, 4179. https://doi.org/10.3390/molecules24224179
He Y-c, He L, Khoshaba R, Lu F-g, Cai C, Zhou F-l, Liao D-f, Cao D. Curcumin Nicotinate Selectively Induces Cancer Cell Apoptosis and Cycle Arrest through a P53-Mediated Mechanism. Molecules. 2019; 24(22):4179. https://doi.org/10.3390/molecules24224179
Chicago/Turabian StyleHe, Ying-chun, Lan He, Ramina Khoshaba, Fang-guo Lu, Chuan Cai, Fang-liang Zhou, Duan-fang Liao, and Deliang Cao. 2019. "Curcumin Nicotinate Selectively Induces Cancer Cell Apoptosis and Cycle Arrest through a P53-Mediated Mechanism" Molecules 24, no. 22: 4179. https://doi.org/10.3390/molecules24224179
APA StyleHe, Y.-c., He, L., Khoshaba, R., Lu, F.-g., Cai, C., Zhou, F.-l., Liao, D.-f., & Cao, D. (2019). Curcumin Nicotinate Selectively Induces Cancer Cell Apoptosis and Cycle Arrest through a P53-Mediated Mechanism. Molecules, 24(22), 4179. https://doi.org/10.3390/molecules24224179




