Cu(II)-Catalyzed C-N Coupling of (Hetero)aryl Halides and N-Nucleophiles Promoted by α-Benzoin Oxime
Abstract
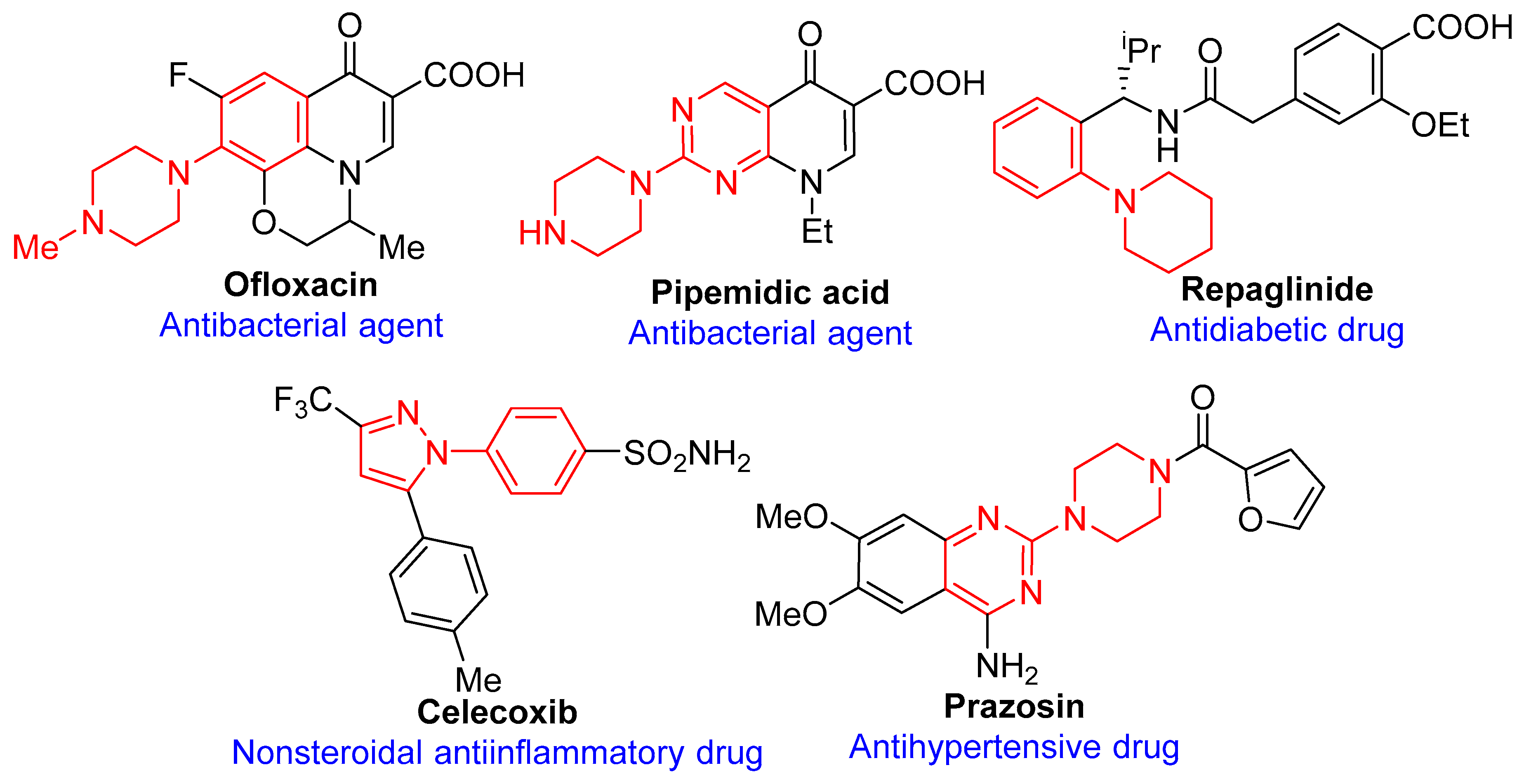
:1. Introduction
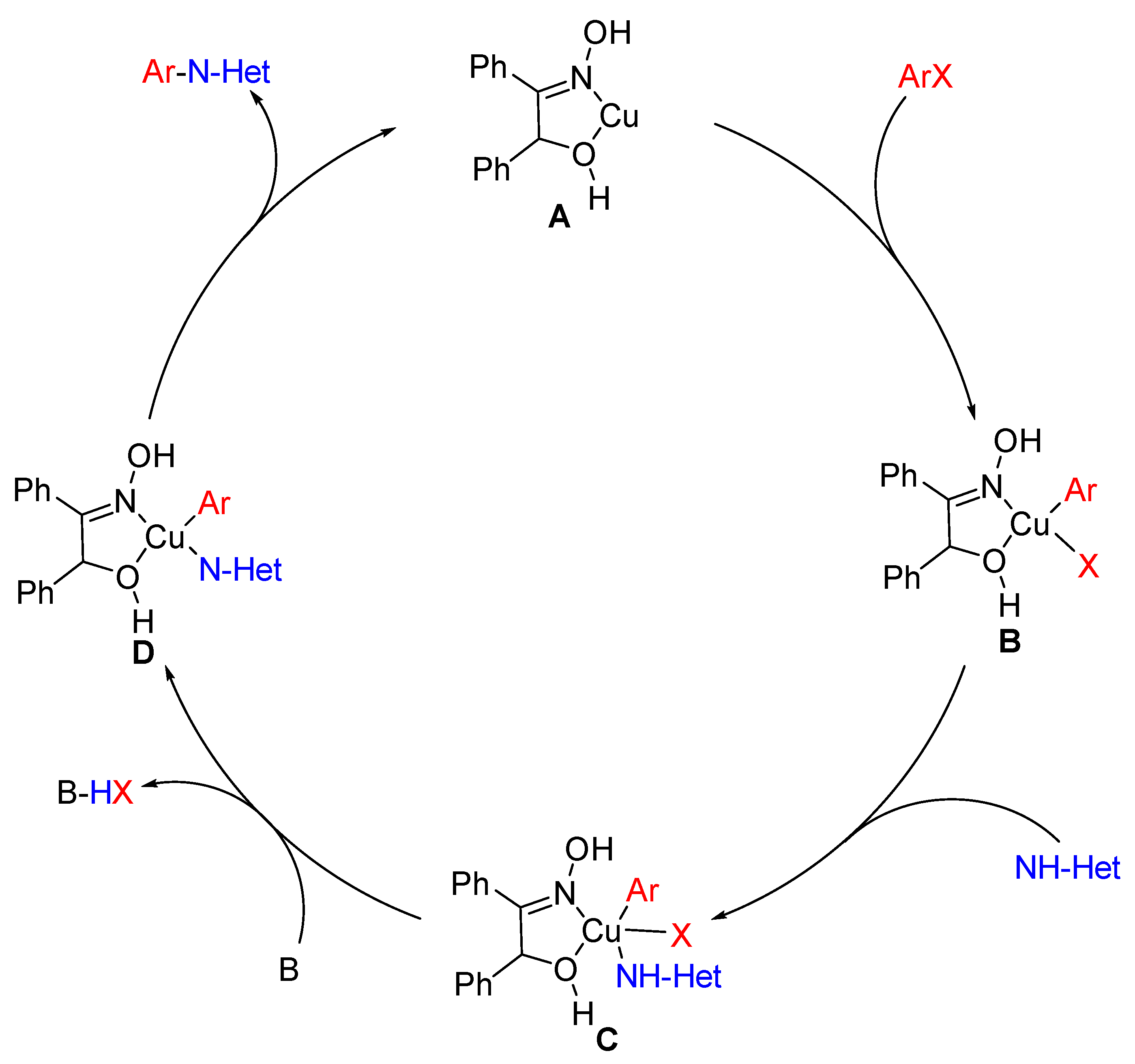
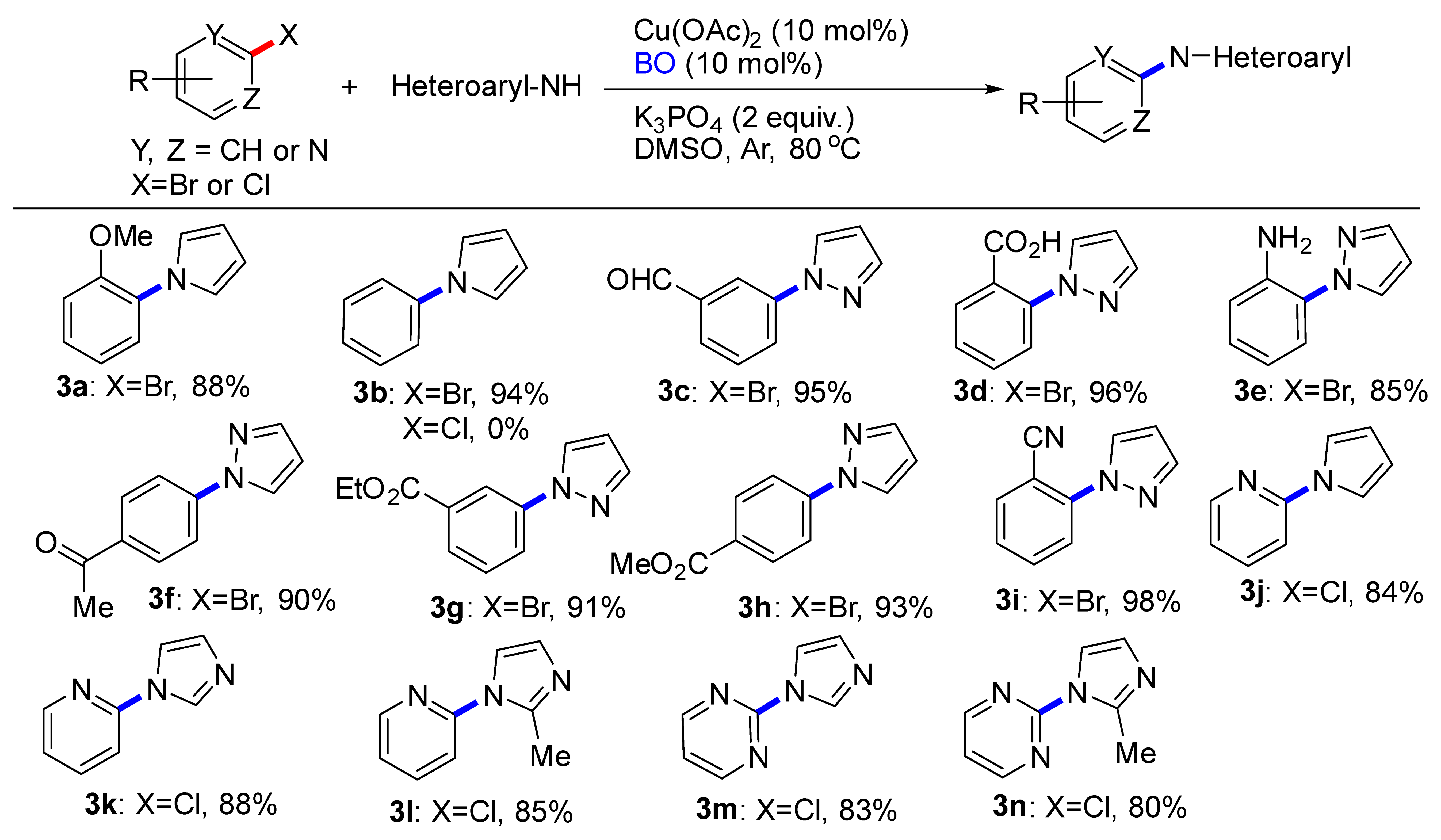
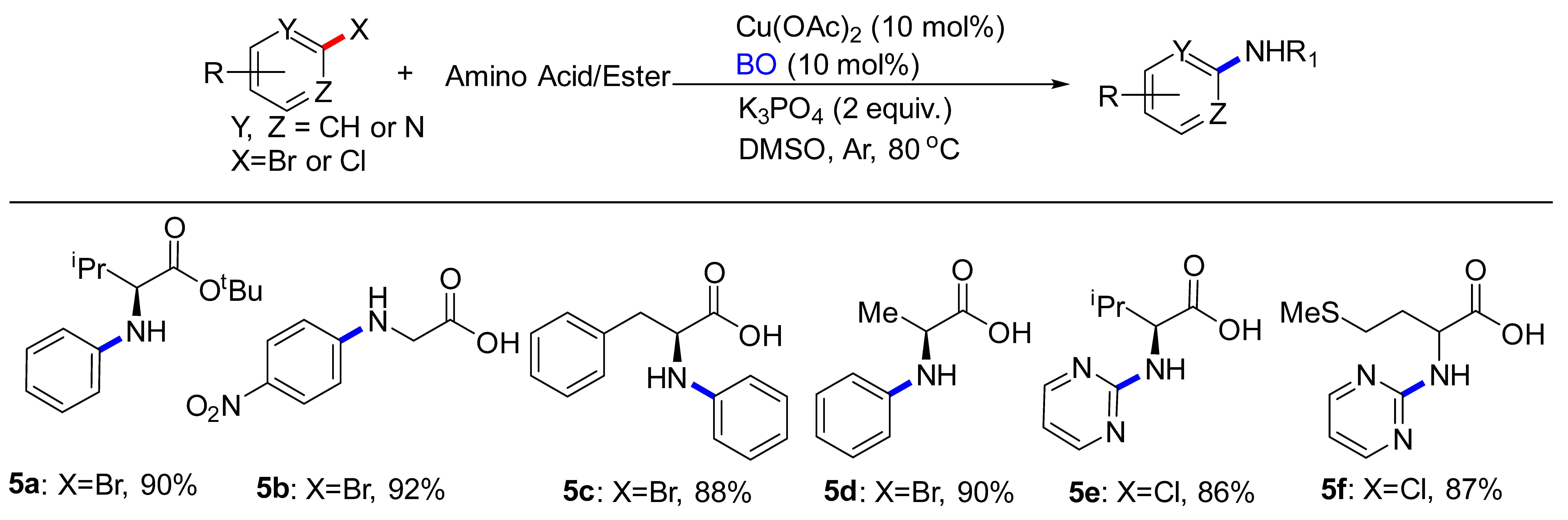
2. Results and Discussions
3. Materials and Methods
General Procedure for Catalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Roughley, S.D.; Jordan, A.M. The medicinal chemist’s toolbox: An analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 2011, 54, 3451–3479. [Google Scholar] [CrossRef] [PubMed]
- Enders, D.; Niemeier, O.; Henseler, A. Organocatalysis by N-heterocyclic carbenes. Chem. Rev. 2007, 107, 5606–5655. [Google Scholar] [CrossRef] [PubMed]
- Olcay, E.; Beytemur, O.; Kaleagasioglu, F.; Gulmez, T.; Mutlu, Z.; Olgac, V. Oral toxicity of pefloxacin, norfloxacin, ofloxacin and ciprofloxacin: Comparison of biomechanical and histopathological effects on Achilles tendon in rats. J. Toxicol. Sci. 2011, 36, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Iacovino, R.; Rapuano, F.; Caso, J.V.; Russo, A.; Lavorgna, M.; Russo, C.; Isidori, M.; Russo, L.; Malgieri, G.; Isernia, C. β-Cyclodextrin inclusion complex to improve physicochemical properties of pipemidic acid: Characterization and bioactivity evaluation. Int. J. Mol. Sci. 2013, 14, 13022–13041. [Google Scholar] [CrossRef]
- Owens, D.R. Repaglinide-prandial glucose regulator: A new class of oral antidiabetic drugs. Diabet. Med. 1998, 15, S28–S36. [Google Scholar] [CrossRef]
- Liu, C.; Liu, L.; Zhu, H.; Sheng, J.; Wu, X.; He, X.; Tian, D.; Liao, J.; Li, P. Celecoxib alleviates nonalcoholic fatty liver disease by restoring autophagic flux. Sci. Rep. 2018, 8, 4108. [Google Scholar] [CrossRef]
- Yu, C.; Zhu, C.; Xu, S.; Cao, X.; Wu, G. Selective MT2 melatonin receptor antagonist blocks melatonin-induced antinociception in rats. Neurosci. Lett. 2000, 282, 161–164. [Google Scholar] [CrossRef]
- Kwan, E.E.; Zeng, Y.; Besser, H.A.; Jacobsen, E.N. Concerted nucleophilic aromatic substitutions. Nat. Chem. 2018, 10, 917–923. [Google Scholar] [CrossRef]
- Monnier, F.; Taillefer, M. Catalytic C-C, C-N, and C-O Ullmann-type coupling reactions. Angew. Chem. Int. Ed. 2009, 48, 6954–6971. [Google Scholar] [CrossRef]
- Ma, D.; Cai, Q. Copper/amino acid catalyzed cross-couplings of aryl and vinyl halides with nucleophiles. Acc. Chem. Res. 2008, 41, 1450–1460. [Google Scholar] [CrossRef]
- Surry, D.S.; Buchwald, S.L. Diamine ligands in copper-catalyzed reactions. Chem. Sci. 2010, 1, 13–31. [Google Scholar] [CrossRef]
- Hartwig, J.F. Carbon-heteroatom bond-forming reductive eliminations of amines, ethers, and sulfides. Acc. Chem. Res. 1998, 31, 852–860. [Google Scholar] [CrossRef]
- Hartwig, J.F. Transition metal catalyzed synthesis of arylamines and aryl ethers from aryl halides and triflates: Scope and mechanism. Angew. Chem. Int. Ed. 1998, 37, 2046–2067. [Google Scholar]
- Wolfe, J.P.; Wagaw, S.; Marcoux, J.F.; Buchwald, S.L. Rational development of practical catalysts for aromatic carbon-nitrogen bond formation. Acc. Chem. Res. 1998, 31, 805–818. [Google Scholar] [CrossRef]
- Avila-Sorrosa, A.; Estudiante-Negrete, F.; Hernandez-Ortega, S.; Toscano, R.A.; Morales-Morales, D. Buchwald-Hartwig C-N cross coupling reactions catalyzed by a pseudo-pincer Pd(II) compound. Inorg. Chim. Acta. 2010, 363, 1262–1268. [Google Scholar] [CrossRef]
- Guram, A.S.; Rennels, R.A.; Buchwald, S.L. A simple catalytic method for the conversion of aryl bromides to arylamines. Angew. Chem. Int. Ed. 1995, 34, 1348–1350. [Google Scholar] [CrossRef]
- Louie, J.; Hartwig, J.F. Palladium-catalyzed synthesis of arylamines from aryl halides. Mechanistic studies lead to coupling in the absence of tin reagents. Tetrahedron Lett. 2010, 36, 3609–3612. [Google Scholar]
- Yang, B.H.; Buchwald, S.L. Palladium-catalyzed amination of aryl halides and sulfonates. J. Organomet. Chem. 1999, 576, 125–146. [Google Scholar] [CrossRef]
- Zhou, W.; Fan, M.; Yin, J.; Jiang, Y.; Ma, D. CuI/oxalic diamide catalyzed coupling reaction of (hetero)aryl chlorides and amines. J. Am. Chem. Soc. 2015, 137, 11942–11945. [Google Scholar] [CrossRef]
- Fan, M.; Zhou, W.; Jiang, Y.; Ma, D. CuI/oxalamide catalyzed couplings of (hetero)aryl chlorides and phenols for diaryl ether formation. Angew. Chem. Int. Ed. 2016, 55, 6211–6215. [Google Scholar] [CrossRef]
- Xia, S.; Gan, L.; Wang, K.; Li, Z.; Ma, D. Copper-catalyzed hydroxylation of (hetero)aryl halides under mild conditions. J. Am. Chem. Soc. 2016, 138, 13493–13496. [Google Scholar] [CrossRef]
- Pawar, G.G.; Wu, H.; De, S.; Ma, D. Copper(I) oxide/n,n’-bis[(2-furyl)methyl]oxalamide-catalyzed coupling of (hetero)aryl halides and nitrogen heterocycles at low catalytic loading. Adv. Synth. Catal. 2017, 359, 1631–1636. [Google Scholar] [CrossRef]
- Gao, J.; Bhunia, S.; Wang, K.; Gan, L.; Xia, S.; Ma, D. Discovery of N-(naphthalen-1-yl)-n′-alkyl oxalamide ligands enables Cu-catalyzed aryl amination with high turnovers. Org. Lett. 2017, 19, 2809–2812. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jiang, Y.; Zhang, L.; Guo, Y.; Ma, D. Oxalic diamides and tert-butoxide: Two types of ligands enabling practical access to alkyl aryl ethers via Cu-catalyzed coupling reaction. J. Am. Chem. Soc. 2019, 141, 3541–3549. [Google Scholar] [CrossRef] [PubMed]
- Korob, R.O.; Cohen, I.M.; Agatiello, O.E. Tungsten and molybdenum co-precipitation by α-benzoin oxime for activation analysis of tungsten: Use of molybdenum as tracer. J. Radioanal. Chem. 1976, 34, 329–333. [Google Scholar] [CrossRef]
- Gao, Z.; Siow, K.S. Adsorptive stripping voltammetric determination of traces of molybdenum in natural water in the presence of α-benzoinoxime. Mikrochimica Acta. 1996, 124, 211–218. [Google Scholar] [CrossRef]
- Fan, F.; Bai, J.; Fan, F.; Yin, X.; Wang, Y.; Tian, W.; Wu, X.; Qin, Z. Solvent extraction of uranium from aqueous solutions by α-benzoinoxime. J. Radioanal. Nucl. Ch. 2014, 300, 1039–1043. [Google Scholar] [CrossRef]
- Joshi, S.R.; Habib, S.I. Synthesis, characterization and antimicrobial evaluation of benzoinoxime transition metal complexes. J. Chem. Pharm. Res. 2014, 6, 1085–1088. [Google Scholar]
- Ribas, X.; Güell, I. Cu(I)/Cu(III) catalytic cycle involved in Ullmann-type cross-coupling reactions. Pure Appl. Chem. 2014, 86, 345–360. [Google Scholar] [CrossRef]
- Reddy, V.P.; Kumar, A.V.; Rao, K.R. New strategy for the synthesis of N-aryl pyrroles: Cu-catalyzed C-N cross-coupling reaction of trans-4-hydroxy-l-proline with aryl halides. Tetrahedron Lett. 2011, 52, 777–780. [Google Scholar] [CrossRef]
- Taillefer, M.; Xia, N.; Ouali, A. Efficient iron/copper co-catalyzed arylation of nitrogen nucleophiles. Angew. Chem. Int. Ed. 2007, 46, 934–936. [Google Scholar] [CrossRef]
- Zhou, Q.; Du, F.; Chen, Y.; Fu, Y.; Sun, W.; Wu, Y.; Chen, G. l-(−)-Quebrachitol as a ligand for selective copper(0)-catalyzed N-arylation of nitrogen-containing heterocycles. J. Org. Chem. 2018, 84, 8160–8167. [Google Scholar] [CrossRef] [PubMed]
- Young, M.B.; Barrow, J.C.; Glass, K.L.; Lundell, G.F.; Newton, C.L.; Pellicore, J.M.; Rittle, K.E.; Selnick, H.G.; Stauffer, K.J.; Vacca, J.P.; et al. Discovery and evaluation of potent P1 aryl heterocycle-based thrombin inhibitors. J. Med. Chem. 2004, 47, 2995. [Google Scholar] [CrossRef] [PubMed]
- de La, H.A.; Diaz-Ortiz, A.; Elguero, J.; Martinez, L.J.; Moreno, A.; Sanchez-Migallon, A. Solvent-free preparation of tris-pyrazolyl-1,3,5-triazines. Tetrahedron 2001, 57, 4397–4403. [Google Scholar] [CrossRef]
- Li, L.; Zhu, L.; Chen, D.; Hu, X.; Wang, R. Use of acylhydrazine- and acylhydrazone-type ligands to promote CuI-catalyzed C-N cross-coupling reactions of aryl bromides with N-heterocycles. Eur. J. Org. Chem. 2011, 2692–2696. [Google Scholar] [CrossRef]
- Cristau, H.-J.; Cellier, P.P.; Spindler, J.-F.; Taillefer, M. Mild conditions for copper-catalyzed N-arylation of pyrazoles. Eur. J. Org. Chem. 2004, 695–709. [Google Scholar] [CrossRef]
- Taywade, A.; Chavan, S.; Ulhe, A.; Berad, B. Unique CuI-pyridine based ligands catalytic systems for N-arylation of indoles and other heterocycles. Synth. Commun. 2018, 48, 1443–1453. [Google Scholar] [CrossRef]
- Jia, X.F.; Yang, D.P.; Wang, W.H.; Luo, F.; Cheng, J. Chelation-assisted palladium-catalyzed cascade bromination/cyanation reaction of 2-arylpyridine and 1-arylpyrazole C-H bonds. J. Org. Chem. 2009, 74, 9470–9474. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, J. Unique chemoselective Clauson-Kass reaction of substituted aniline catalyzed by MgI2 etherate. Tetrahedron 2011, 67, 898–903. [Google Scholar] [CrossRef]
- Zhu, L.; Guo, P.; Li, G.; Lan, J.; Xie, R.; You, J. Simple copper salt-catalyzed N-arylation of nitrogen-containing heterocycles with aryl and heteroaryl halides. J. Org. Chem. 2007, 72, 8535–8538. [Google Scholar] [CrossRef]
- Liang, L.; Li, Z.; Zhou, X. Pyridine N-oxides as ligands in Cu-catalyzed N-arylation of imidazoles in water. Org. Lett. 2009, 11, 3294–3297. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Y.; Zhu, L.; Lan, J.; Xie, R.; You, J. A concept of supported amino acid ionic liquids and their application in metal scavenging and heterogeneous catalysis. J. Am. Chem. Soc. 2007, 129, 13879–13886. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.X.; Guo, S.M.; Zhang, M.B.; Li, J.H. N-Arylations of nitrogen-containing heterocycles with aryl and heteroaryl halides using a copper(I) oxide nanoparticle/1,10-phenanthroline catalytic system. Synthesis 2008, 1707–1716. [Google Scholar] [CrossRef]
- Pedersen, E.B.; Carlsen, D. Phosphoramides; VIII. Phosphorus pentoxide/amine mixtures as reagents in a facile synthesis of dialkylaminopyridines. Synthesis 1978, 844–845. [Google Scholar] [CrossRef]
- Prokopcova, H.; Bergman, S.D.; Aelvoet, K.; Smout, V.; Herrebout, W.; Van der Veken, B.; Meerpoel, L.; Maes, B.U.W. C-2 Arylation of piperidines through directed transition-metal-catalyzed sp3 c-h activation. Chem-Eur. J. 2010, 16, 13063–13067. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Yoshimoto, Y.; Ghorai, S.K.; Nakamura, M. Transition-metal-free electrophilic amination between aryl grignard reagents and N-chloroamines. Org. Lett. 2010, 12, 1516–1519. [Google Scholar] [CrossRef]
- Narayan, S.; Seelhammer, T.; Gawley, R.E. Microwave-assisted solvent-free amination of halo-(pyridine or pyrimidine) without transition metal catalyst. Tetrahedron Lett. 2004, 45, 757–759. [Google Scholar] [CrossRef]
- Shim, S.C.; Huh, K.T.; Park, W.H. A new and facile synthesis of N-substituted pyrrolidines from amines with aqueous succinaldehyde using tetracarbonylhydridoferrate, HFe(CO)4-, as a highly selective reducing agent. Tetrahedron 1986, 42, 259–263. [Google Scholar] [CrossRef]
- Lund, H.J. Electroanal. Some reactions of electrochemically prepared anions of DMF and DMSO. Chem. 2005, 584, 174–181. [Google Scholar]
- Fattorusso, C.; Campiani, G.; Kukreja, G.; Persico, M.; Butini, S.; Romano, M.P.; Altarelli, M.; Ros, S.; Brindisi, M.; Savini, L.; et al. Design, synthesis, and structure-activity relationship studies of 4-quinolinyl- and 9-acrydinylhydrazones as potent antimalarial agents. J. Med. Chem. 2008, 51, 1333–1343. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; Abdel-Wahab, B.F.; El-Sharief, M.A.M.S.; Abdulla, M.M. Synthesis and anti-arrhythmic activity of some piperidine-based 1,3-thiazole, 1,3,4-thiadiazole, and 1,3-thiazolo [2,3-c]-1,2,4-triazole derivatives. Mon. Fuer Chem. 2009, 140, 431–437. [Google Scholar] [CrossRef]
- Nacario, R.; Kotakonda, S.; Fouchard, D.M.D.; Tillekeratne, L.M.V.; Hudson, R.A. Reductive monoalkylation of aromatic and aliphatic nitro compounds and the corresponding amines with nitriles. Org. Lett. 2005, 7, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Snyder, S.A.; Longbottom, D.A.; Nalbandian, A.Z.; Huang, X. New uses for the burgess reagent in chemical synthesis: Methods for the facile and stereoselective formation of sulfamidates, glycosylamines, and sulfamides. Chem-Eur. J. 2004, 10, 5581–5606. [Google Scholar] [CrossRef] [PubMed]
- King, S.M.; Buchwald, S.L. Development of a method for the N-arylation of amino acid esters with aryl triflates. Org. Lett. 2016, 18, 4128–4131. [Google Scholar] [CrossRef] [PubMed]
- Azarifar, D.; Bosra, H.G.; Zolfigol, M.-A.; Tajbaksh, M. Microwave-assisted synthesis of N-arylglycines: Improvement of sydnone synthesis. Heterocycles 2006, 68, 175–181. [Google Scholar] [CrossRef]
- Ma, F.; Xie, X.; Ding, L.; Gao, J.; Zhang, Z. Palladium-catalyzed coupling reaction of amino acids (esters) with aryl bromides and chlorides. Tetrahedron 2011, 67, 9405–9410. [Google Scholar] [CrossRef]
- Xu, H.; Wolf, C. Copper catalyzed coupling of aryl chlorides, bromides and iodides with amines and amides. Chem. Commun. 2009, 1715–1717. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
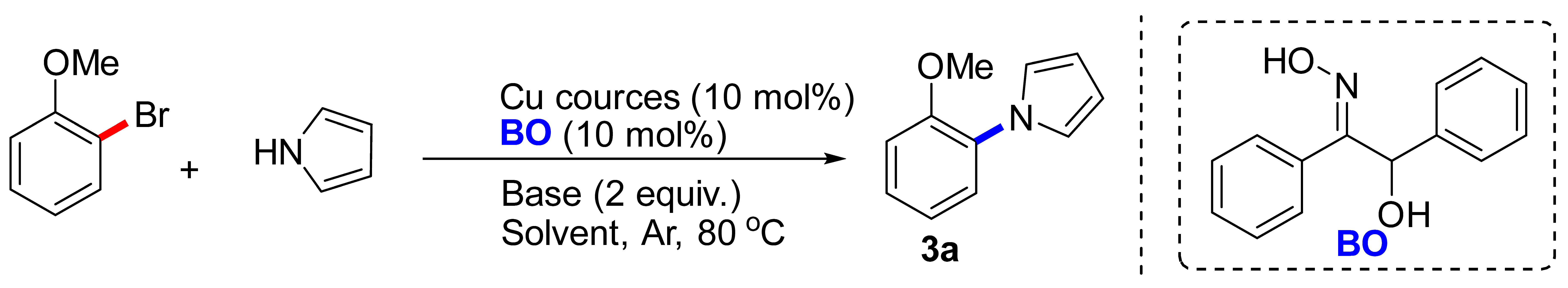
| Entry | Cu Sources | Base | Solvent | Yield (%) b |
|---|---|---|---|---|
| 1 | CuI | K2CO3 | DMF | 12 |
| 2 | Cu powder | K2CO3 | DMF | 15 |
| 3 | Cu(OTf)2 | K2CO3 | DMF | 22 |
| 4 | Cu(II) gluconate | K2CO3 | DMF | Trace |
| 5 | Cu2(OH)2CO3 | K2CO3 | DMF | Trace |
| 6 | Cu(OAc)2 | K2CO3 | DMF | 53 |
| 7 | Cu(OAc)2 | Cs2CO3 | DMF | 60 |
| 8 | Cu(OAc)2 | K3PO4 | DMF | 72 |
| 9 | Cu(OAc)2 | NaHCO3 | DMF | 0 c |
| 10 | Cu(OAc)2 | Et3N | DMF | 0 c |
| 11 | Cu(OAc)2 | t-BuOK | DMF | Trace |
| 12 | Cu(OAc)2 | K3PO4 | DMSO | 90 |
| 13 | Cu(OAc)2 | K3PO4 | Dioxane | 43 |
| 14 | Cu(OAc)2 | K3PO4 | DCE | Trace |
| 15 | Cu(OAc)2 | K3PO4 | Toluene | 0 c |
| 16 | Cu(OAc)2 | K3PO4 | H2O | 0 c |
| 17 | Cu(OAc)2 | K3PO4 | DMSO | 70 d |
| 18 | Cu(OAc)2 | K3PO4 | DMSO | 15 e |
| 19 | Cu(OAc)2 | K3PO4 | DMSO | 90 f |
| 20 | Cu(OAc)2 | K3PO4 | DMSO | 61 g |
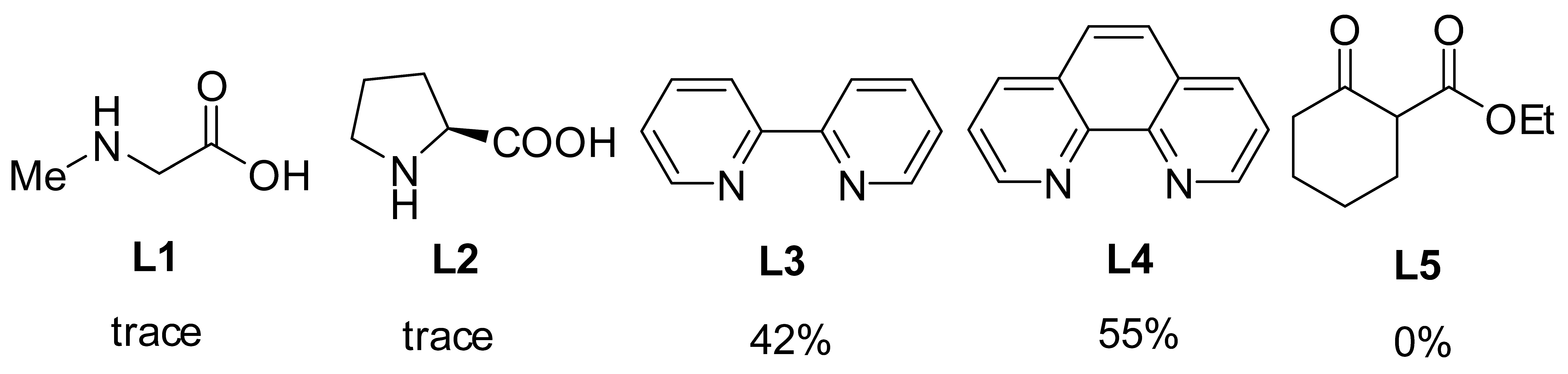 | ||||
 |
 |
 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, C.; Zhang, L.; Zhao, Y. Cu(II)-Catalyzed C-N Coupling of (Hetero)aryl Halides and N-Nucleophiles Promoted by α-Benzoin Oxime. Molecules 2019, 24, 4177. https://doi.org/10.3390/molecules24224177
Yuan C, Zhang L, Zhao Y. Cu(II)-Catalyzed C-N Coupling of (Hetero)aryl Halides and N-Nucleophiles Promoted by α-Benzoin Oxime. Molecules. 2019; 24(22):4177. https://doi.org/10.3390/molecules24224177
Chicago/Turabian StyleYuan, Chunling, Lei Zhang, and Yingdai Zhao. 2019. "Cu(II)-Catalyzed C-N Coupling of (Hetero)aryl Halides and N-Nucleophiles Promoted by α-Benzoin Oxime" Molecules 24, no. 22: 4177. https://doi.org/10.3390/molecules24224177
APA StyleYuan, C., Zhang, L., & Zhao, Y. (2019). Cu(II)-Catalyzed C-N Coupling of (Hetero)aryl Halides and N-Nucleophiles Promoted by α-Benzoin Oxime. Molecules, 24(22), 4177. https://doi.org/10.3390/molecules24224177





