Experimental Data and Modelling of the Solubility of High-Carotenoid Paprika Extract in Supercritical Carbon Dioxide
Abstract
:1. Introduction
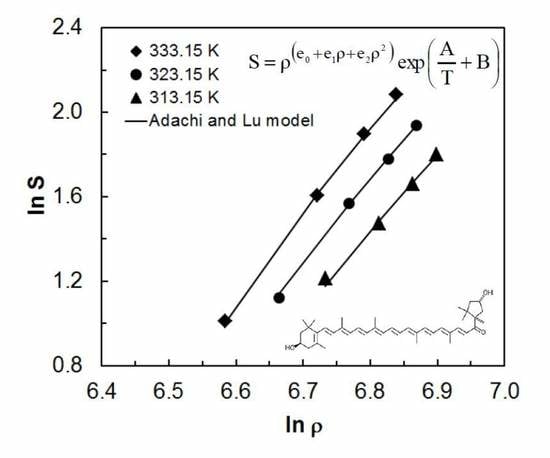
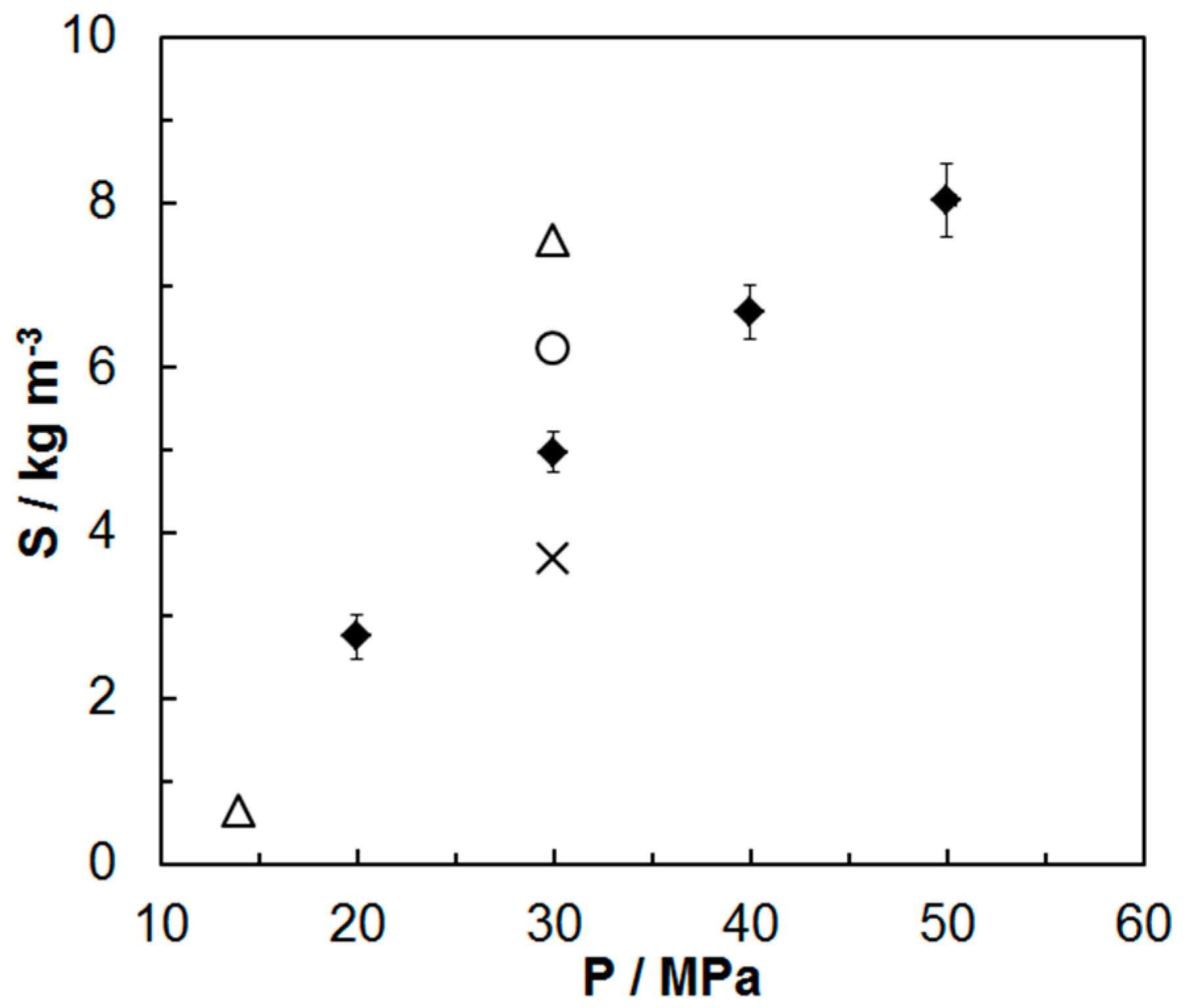
2. Results and Discussion
2.1. Experimental Solubility Results
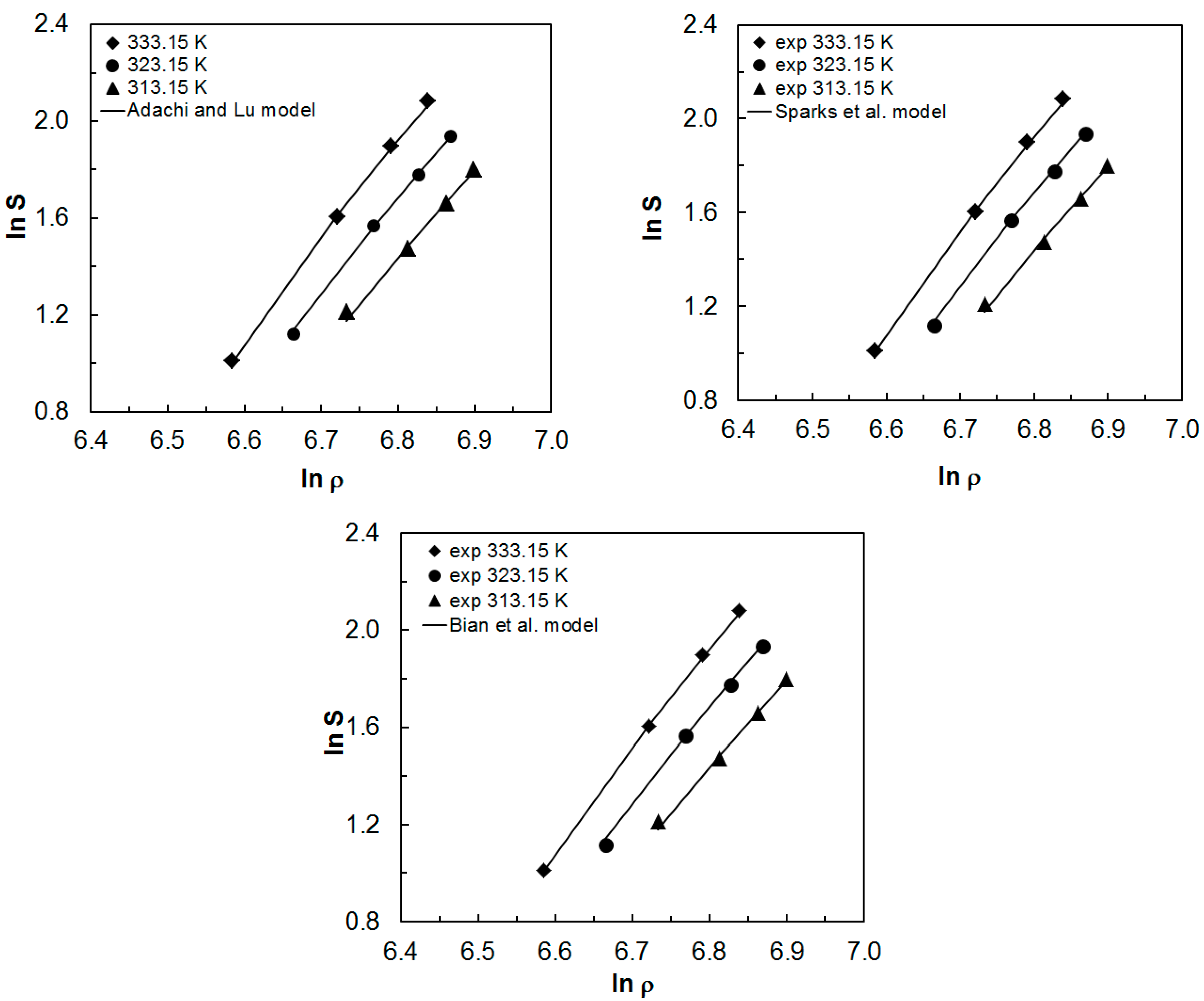
2.2. Correlation Results with Experimental Solubility Data
3. Materials and Methods
3.1. Materials
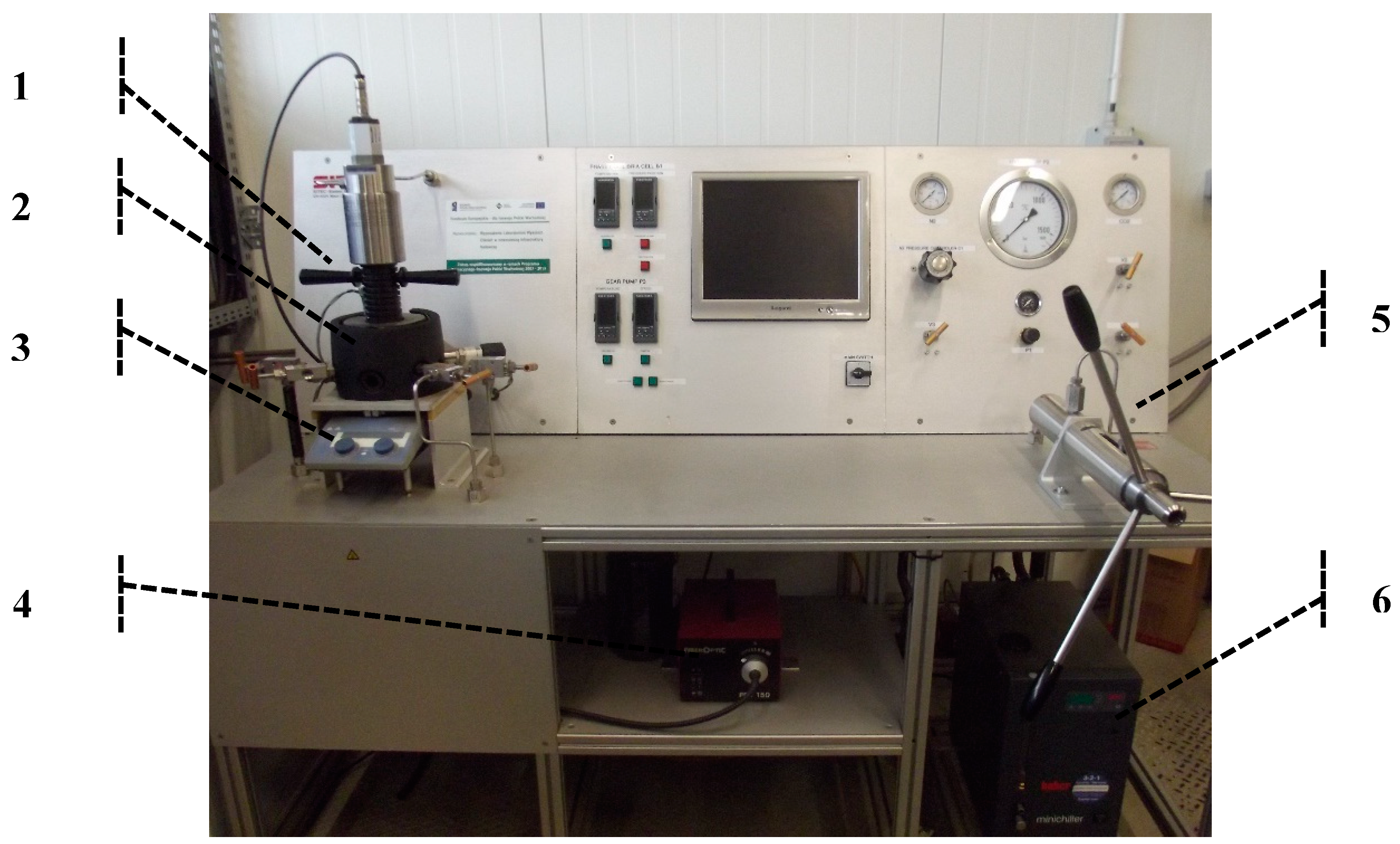
3.2. Measuring Equipment and Experimental Methods
3.3. Analysis Methods
3.3.1. Determination of Total Carotenoid Content
3.3.2. Determination of Extract Color Value
3.3.3. Determination of Carotenoids Concentration
3.3.4. Determination of Fatty Acid Content
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tepić, A.; Zeković, Z.; Kravić, S.; Mandić, A. Pigment content and fatty acid composition of paprika oleoresins obtained by conventional and supercritical carbon dioxide extraction. CyTA J. Food 2009, 7, 95–102. [Google Scholar] [CrossRef]
- Matsufuji, H.; Nakamura, H.; Chino, M.; Takeda, M. Antioxidant activity of capsanthin and the fatty acid esters in paprika (Capsicum annuum). J. Agric. Food Chem. 1998, 46, 3468–3472. [Google Scholar] [CrossRef]
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability—a review. J. Food Sci. Technol. 2015, 52, 1258–1271. [Google Scholar] [CrossRef]
- Aizawa, K.; Inakuma, T. Dietary capsanthin, the main carotenoid in paprika (Capsicum annuum), alters plasma high-density lipoprotein-cholesterol levels and hepatic gene expression in rats. Br. J. Nutr. 2009, 102, 1760–1766. [Google Scholar] [CrossRef]
- Maoka, T.; Mochida, K.; Kozuka, M.; Ito, Y.; Fujiwara, Y.; Hashimoto, K.; Enjo, F.; Ogata, M.; Nobukuni, Y.; Tokuda, H.; et al. Cancer chemopreventive activity of carotenoids in the fruits of red paprika Capsicum annuum L. Cancer Lett. 2001, 172, 103–109. [Google Scholar] [CrossRef]
- Leo, L.; Rescio, L.; Ciurlia, L.; Zacheo, G. Supercritical carbon dioxide extraction of oil and α-tocopherol from almond seeds. J. Sci. Food Agric. 2005, 85, 2167–2174. [Google Scholar] [CrossRef]
- Elizalde-Solis, O.; Arenas-Quevedo, M.G.; Verónico-Sánchez, F.J.; García-Morales, R.; Zúñiga-Moreno, A. Solubilities of Binary Systems α-Tocopherol + Capsaicin and α-Tocopherol + Palmitic Acid in Supercritical Carbon Dioxide. J. Chem. Eng. Data 2019, 64, 1948–1955. [Google Scholar] [CrossRef]
- Uddin, M.S.; Sarker, M.Z.I.; Ferdosh, S.; Akanda, M.J.H.; Easmin, M.S.; Bt Shamsudin, S.H.; Yunus, K.B. Phytosterols and their extraction from various plant matrices using supercritical carbon dioxide: A review. J. Sci. Food Agric. 2015, 95, 1385–1394. [Google Scholar] [CrossRef]
- Rój, E.; Dobrzyńska-Inger, A.; Grzęda, K.; Kostrzewa, D. Ekstrakcja nadkrytyczna surowców roślinnych. Przem. Chem. 2013, 92, 1358–1363. [Google Scholar]
- King, M.B.; Bott, T.R. Extraction of Natural Products Using Near-Critical Solvents; Chapman and Hall: London, UK, 1993; ISBN 978-94-010-4947-4. [Google Scholar]
- Yu, Z.R.; Singh, B.; Rizvi, S.S.H.; Zollweg, J.A. Solubilities of fatty acids, fatty acid esters, triglycerides, and fats and oils in supercritical carbon dioxide. J. Supercrit. Fluids 1994, 7, 51–59. [Google Scholar] [CrossRef]
- Güçlü-Üstündaǧ; Temelli, F. Correlating the solubility behavior of fatty acids, mono-, di-, and triglycerides, and fatty acid esters in supercritical carbon dioxide. Ind. Eng. Chem. Res. 2000, 39, 4756–4766. [Google Scholar] [CrossRef]
- Yamini, Y.; Moradi, M.; Hojjati, M.; Nourmohammadian, F.; Saleh, A. Solubilities of Some Disperse Yellow Dyes in Supercritical CO2. J. Chem. Eng. Data 2010, 55, 3896–3900. [Google Scholar] [CrossRef]
- Cabral, V.F.; Santos, W.L.F.; Muniz, E.C.; Rubira, A.F.; Cardozo-Filho, L. Correlation of dye solubility in supercritical carbon dioxide. J. Supercrit. Fluids 2007, 40, 163–169. [Google Scholar] [CrossRef]
- Araus, K.A.; Del Valle, J.M.; Robert, P.S.; Fuente, J.C. de la Effect of triolein addition on the solubility of capsanthin in supercritical carbon dioxide. J. Chem. Thermodyn. 2012, 51, 190–194. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Fuente, J.C.; Uquiche, E. A refined equation for predicting the solubility of vegetable oils in high-pressure CO2. J. Supercrit. Fluids 2012, 67, 60–70. [Google Scholar] [CrossRef]
- Khamda, M.; Hosseini, M.H.; Rezaee, M. Measurement and correlation solubility of cefixime trihydrate and oxymetholone in supercritical carbon dioxide (CO2). J. Supercrit. Fluids 2013, 73, 130–137. [Google Scholar] [CrossRef]
- Kostrzewa, D.; Dobrzyńska-Inger, A.; Rój, E. Experimental data on xanthohumol solubility in supercritical carbon dioxide. Fluid Phase Equilibria 2013, 360, 445–450. [Google Scholar] [CrossRef]
- Arenas-Quevedo, M.G.; Elizalde-Solis, O.; Zúñiga-Moreno, A.; Macías-Salinas, R.; García-Sánchez, F. Solubilities of Palmitic Acid + Capsaicin in Supercritical Carbon Dioxide. J. Chem. Eng. Data 2017, 62, 3861. [Google Scholar] [CrossRef]
- Kurnik, R.T.; Reid, R.C. Solubility of solid mixtures in supercritical fluids. Fluid Phase Equilibria 1982, 8, 93–105. [Google Scholar] [CrossRef]
- Fernández-Ronco, M.P.; Ortega-Noblejas, C.; Gracia, I.; de Lucas, A.; García, M.T.; Rodríguez, J.F. Supercritical fluid fractionation of liquid oleoresin capsicum: Statistical analysis and solubility parameters. J. Supercrit. Fluids 2010, 54, 22–29. [Google Scholar] [CrossRef]
- Uquiche, E.; del Valle, J.M.; Ortiz, J. Supercritical carbon dioxide extraction of red pepper (Capsicum annuum L.) oleoresin. J. Food Eng. 2004, 65, 55–66. [Google Scholar] [CrossRef]
- Illés, V.; Daood, H.G.; Biacs, P.A.; Gnayfeed, M.H.; Meszaros, B. Supercritical CO2 and subcritical propane extraction of spice red pepper oil with special regard to carotenoid and tocopherol content. J. Chromatogr. Sci. 1999, 37, 345–352. [Google Scholar] [CrossRef]
- Ambrogi, A.; Cardarelli, D.A.; Eggers, R. Fractional extraction of paprika using supercritical carbon dioxide and on-line determination of carotenoids. J. Food Sci. 2002, 67, 3236–3241. [Google Scholar] [CrossRef]
- Özkal, S.G.; Yener, M.E.; Bayındırlı, L. Mass transfer modeling of apricot kernel oil extraction with supercritical carbon dioxide. J. Supercrit. Fluids 2005, 35, 119–127. [Google Scholar] [CrossRef]
- Özkal, S.G.; Salgın, U.; Yener, M.E. Supercritical carbon dioxide extraction of hazelnut oil. J. Food Eng. 2005, 69, 217–223. [Google Scholar] [CrossRef]
- Goodrum, J.W.; Kilgo, M.B. Peanut oil extraction with SC-CO2: Solubility and kinetic functions. Trans. ASAE 1987, 30, 1865–1868. [Google Scholar] [CrossRef]
- Palazoglu, T.K.; Balaban, M.O.; Walker, L.P.; LanghansCarey, R.W.; Evangelou Davis, V.P.; Sumner, H.R.; Chandler, L.D. Supercritical CO2 extraction of lipids from roasted pistachio nuts. Trans. ASAE 1998, 41, 679–684. [Google Scholar] [CrossRef]
- Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 1982, 86, 3016–3021. [Google Scholar] [CrossRef]
- Garlapati, C.; Madras, G. Solubilities of palmitic and stearic fatty acids in supercritical carbon dioxide. J. Chem. Thermodyn. 2010, 42, 193–197. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Aguilera, J.M. An improved equation for predicting the solubility of vegetable oils in supercritical carbon dioxide. Ind. Eng. Chem. Res. 1988, 27, 1551–1553. [Google Scholar] [CrossRef]
- Adachi, Y.; Lu, B.C.-Y. Supercritical fluid extraction with carbon dioxide and ethylene. Fluid Phase Equilibria 1983, 14, 147–156. [Google Scholar] [CrossRef]
- Sparks, D.L.; Hernandez, R.; Estévez, L.A. Evaluation of density-based models for the solubility of solids in supercritical carbon dioxide and formulation of a new model. Chem. Eng. Sci. 2008, 63, 4292–4301. [Google Scholar] [CrossRef]
- Bian, X.; Du, Z.; Tang, Y. An improved density-based model for the solubility of some compounds in supercritical carbon dioxide. Thermochim. Acta 2011, 519, 16–21. [Google Scholar] [CrossRef]
- Daood, H.G.; Illés, V.; Gnayfeed, M.H.; Mészáros, B.; Horváth, G.; Biacs, P.A. Extraction of pungent spice paprika by supercritical carbon dioxide and subcritical propane. J. Supercrit. Fluids 2002, 23, 143–152. [Google Scholar] [CrossRef]
- FAO Compendium of food Additive Specifications, 69rd Meeting FAO JECFA Monographs 5. 2008. Available online: http://www.fao.org/3/i0345e/i0345e00.htm (accessed on 13 November 2019).
- Rój, E.; Kostrzewa, D.; Dobrzyńska-Inger, A. Ekstrakcja barwników karotenoidowych z papryki ditlenkiem węgla o parametrach nadkrytycznych. Przem. Chem. 2013, 92, 966–969. [Google Scholar]
- Datta, P.R. Official Analytical Methods of the American Spice Trade Association, 3rd ed.; ASTA: Englewood Cliffs, NJ, USA, 1985; pp. 41–42. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |


| Components/Parameters | Value |
|---|---|
| Extract color value (ASTA) | 2500.00 |
| Carotenoids content (%) | 7.33 |
| Violaxanthin (%) | 0.22 |
| Capsorubin (%) | 0.70 |
| Capsanthin (%) | 5.04 |
| Zeaxanthin (%) | 0.42 |
| β-cryptoxanthin (%) | 0.20 |
| β-carotene (%) | 0.64 |
| Palmitic acid (C16:0) (%) | 11.55 |
| Stearic acid (C18:0) (%) | 2.11 |
| Oleic acid (C18:1) (%) | 9.08 |
| Linoleic acid (C18:2) (%) | 48.53 |
| Behenic acid (C22:0) (%) | 0.21 |
| Arachidic acid (C20:0) (%) | 0.27 |
| α-Linolenic acid (C18:3) (%) | 3.16 |
| Lauric acid (C12:0) (%) | 0.40 |
| T (K) | P (MPa) | ρ b (kg m−3) | S (g kg−1) |
|---|---|---|---|
| 313.15 | 20 | 839.81 | 4.00 c ± 0.28 d |
| 30 | 909.89 | 4.80 ± 0.31 | |
| 40 | 956.07 | 5.50 ± 0.25 | |
| 50 | 991.30 | 6.10 ± 0.38 | |
| 323.15 | 20 | 784.29 | 3.90 ± 0.26 |
| 30 | 870.43 | 5.50 ± 0.29 | |
| 40 | 923.32 | 6.40 ± 0.35 | |
| 50 | 962.45 | 7.20 ± 0.41 | |
| 333.15 | 20 | 723.68 | 3.80 ± 0.27 |
| 30 | 829.71 | 6.00 ± 0.25 | |
| 40 | 890.14 | 7.50 ± 0.32 | |
| 50 | 933.50 | 8.60 ± 0.45 |
| Parameters | Equation | ||||
|---|---|---|---|---|---|
| Chrastil | del Valle and Aguilera | Adachi and Lu | Sparks et al. | Bian et al. | |
| k | 4.01 | 4.01 | - | - | - |
| A | −2538.33 | −2537.98 | −2527.27 | −2529.23 | −2529.92 |
| B | −17.74 | −17.74 | −66.20 | −65.38 | −36.82 |
| C | - | −1.23 × 10−4 | - | −6.52 × 10−3 | −1.08 |
| e0 | - | - | 12.62 | 12.47 | 4.78 |
| e1 | - | - | −2.11 × 10−3 | −2.06 × 10−3 | −7.34 × 10−5 |
| e2 | - | - | 5.02 × 10−7 | 4.85 × 10−7 | 14.69 |
| AARD (%) | 1.99 | 1.99 | 1.20 | 1.20 | 1.15 |
| R2 | 0.9954 | 0.9954 | 0.9982 | 0.9982 | 0.9983 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostrzewa, D.; Dobrzyńska-Inger, A.; Turczyn, A. Experimental Data and Modelling of the Solubility of High-Carotenoid Paprika Extract in Supercritical Carbon Dioxide. Molecules 2019, 24, 4174. https://doi.org/10.3390/molecules24224174
Kostrzewa D, Dobrzyńska-Inger A, Turczyn A. Experimental Data and Modelling of the Solubility of High-Carotenoid Paprika Extract in Supercritical Carbon Dioxide. Molecules. 2019; 24(22):4174. https://doi.org/10.3390/molecules24224174
Chicago/Turabian StyleKostrzewa, Dorota, Agnieszka Dobrzyńska-Inger, and August Turczyn. 2019. "Experimental Data and Modelling of the Solubility of High-Carotenoid Paprika Extract in Supercritical Carbon Dioxide" Molecules 24, no. 22: 4174. https://doi.org/10.3390/molecules24224174
APA StyleKostrzewa, D., Dobrzyńska-Inger, A., & Turczyn, A. (2019). Experimental Data and Modelling of the Solubility of High-Carotenoid Paprika Extract in Supercritical Carbon Dioxide. Molecules, 24(22), 4174. https://doi.org/10.3390/molecules24224174