Ionothermal Synthesis of Cadmium Coordination Polymers: Ionic Liquid Effects on the Synthesis, Structural, and Thermal Characterization
Abstract
1. Introduction
2. Results and Discussion
2.1. Ionothermal Synthesis
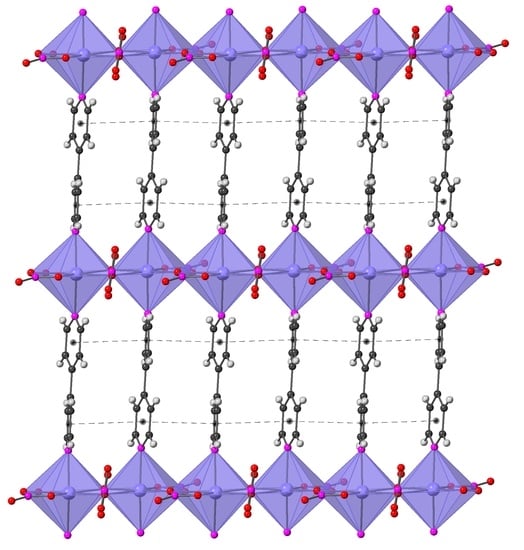
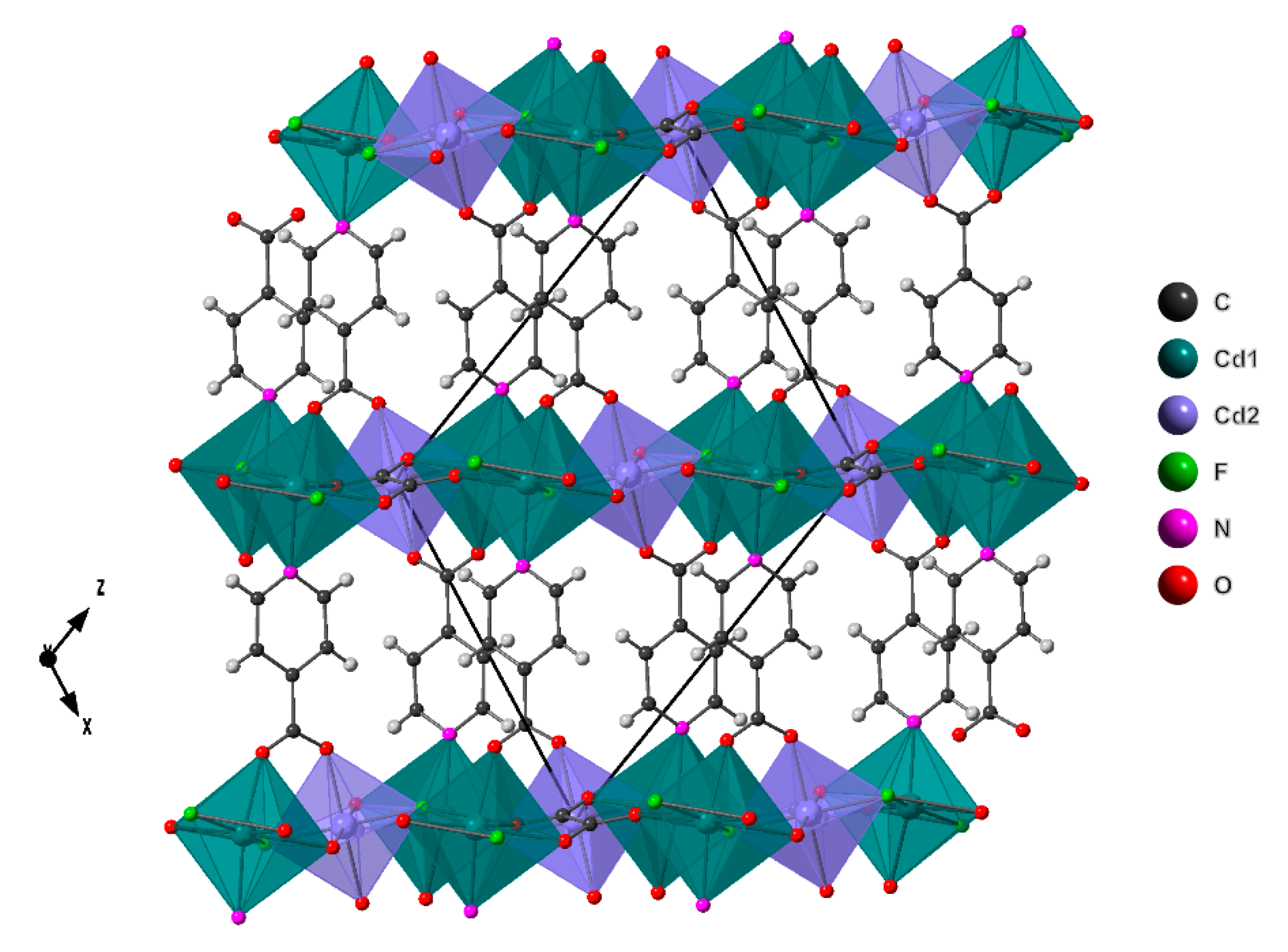
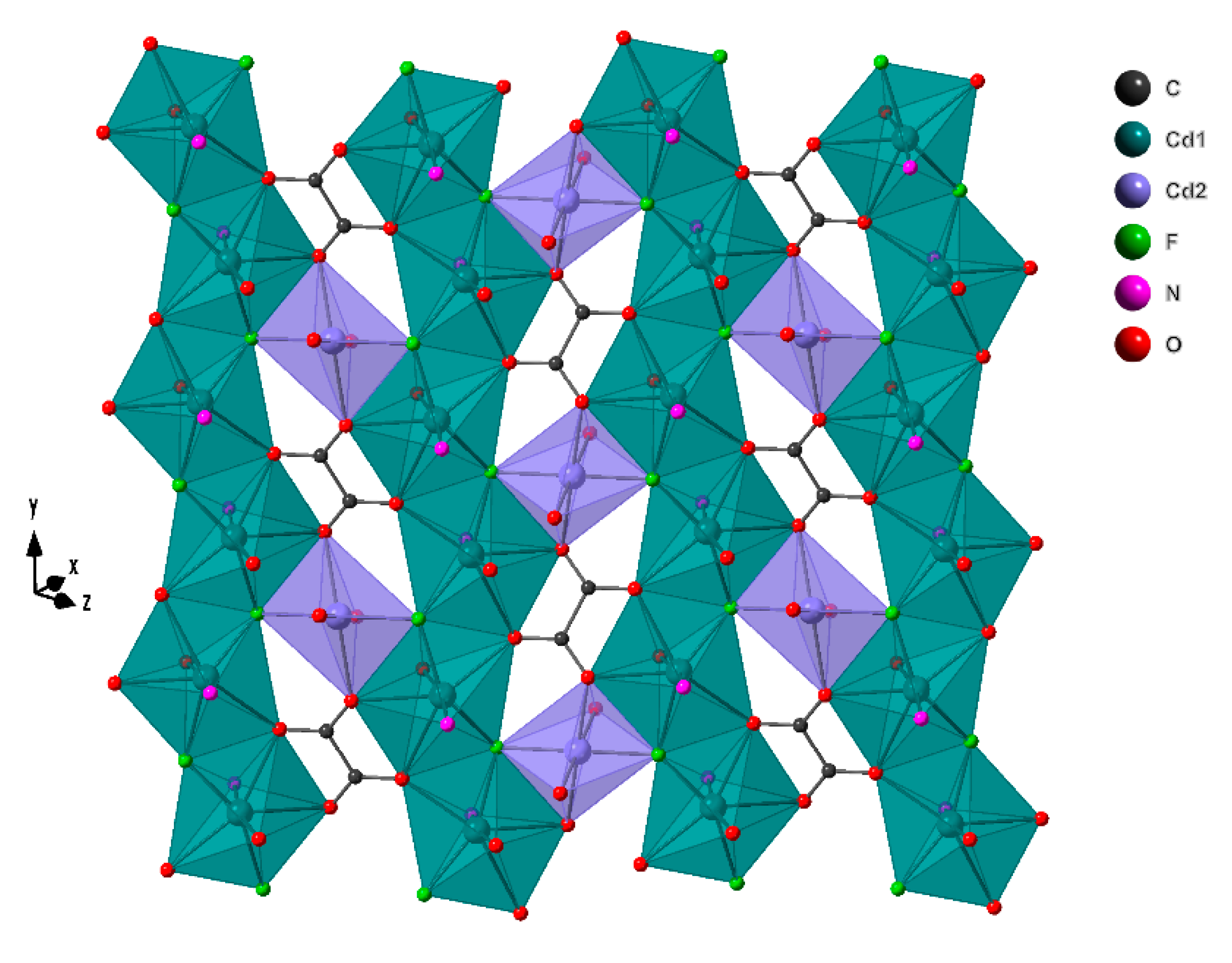
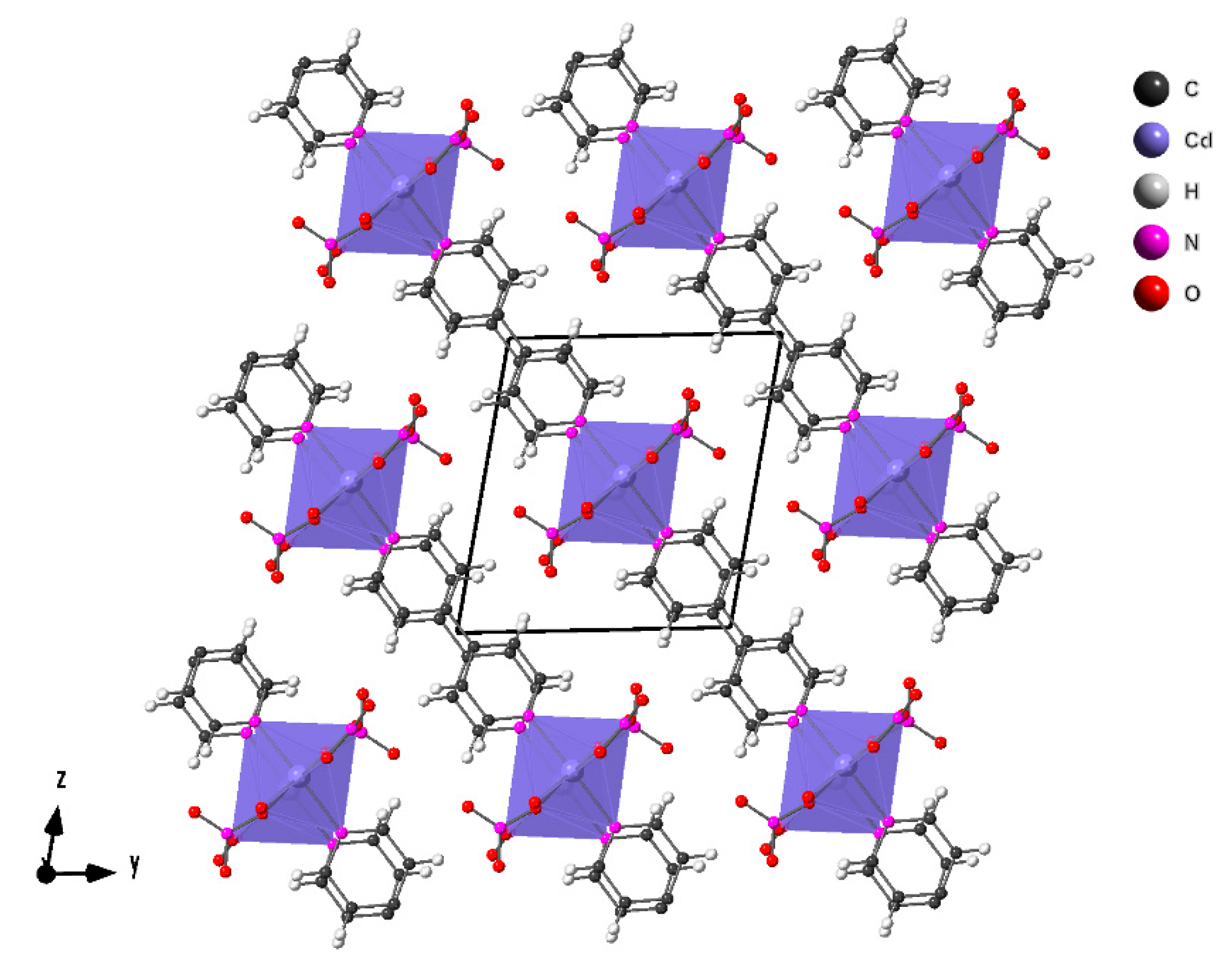
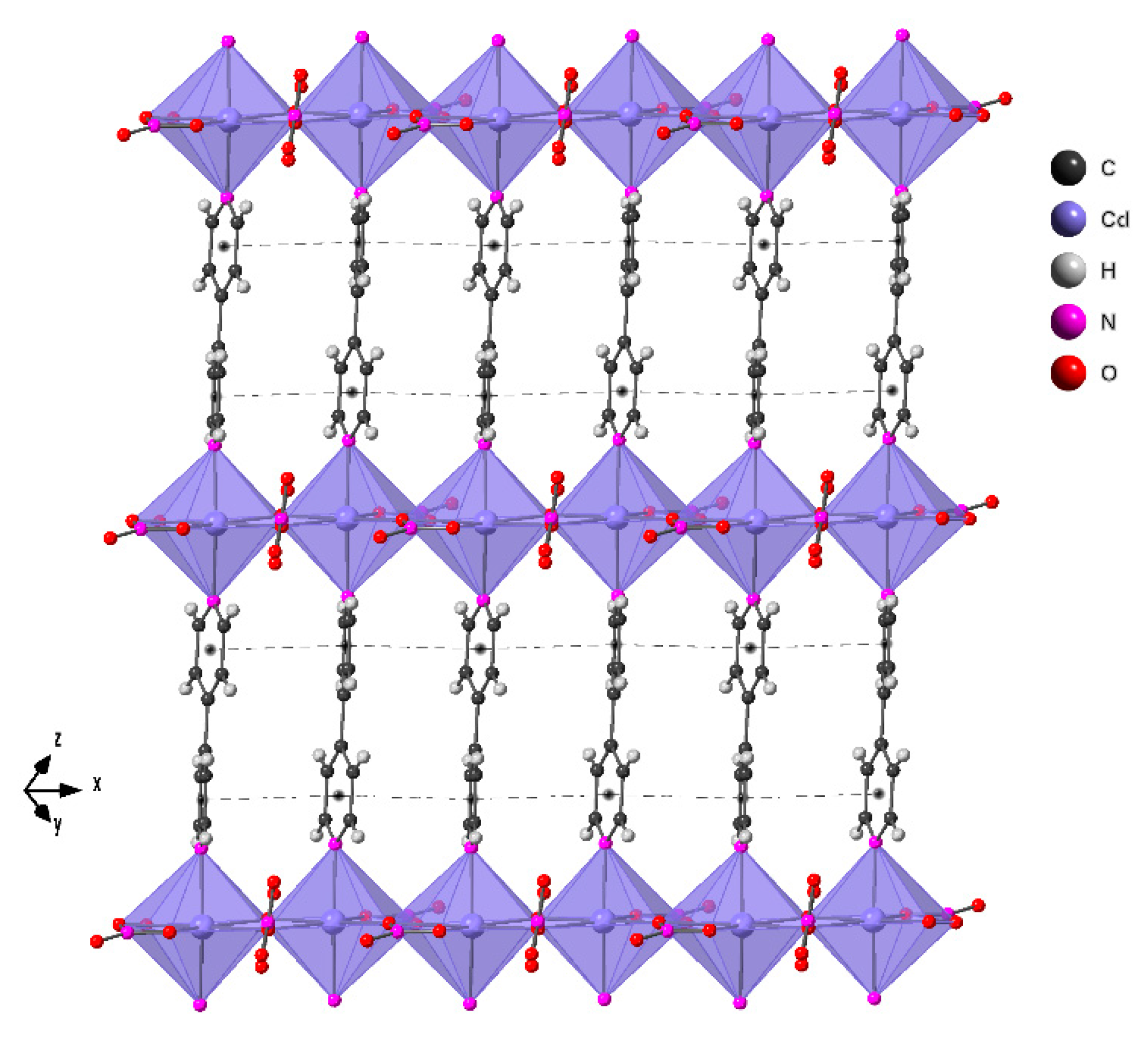
2.2. Crystal Structure
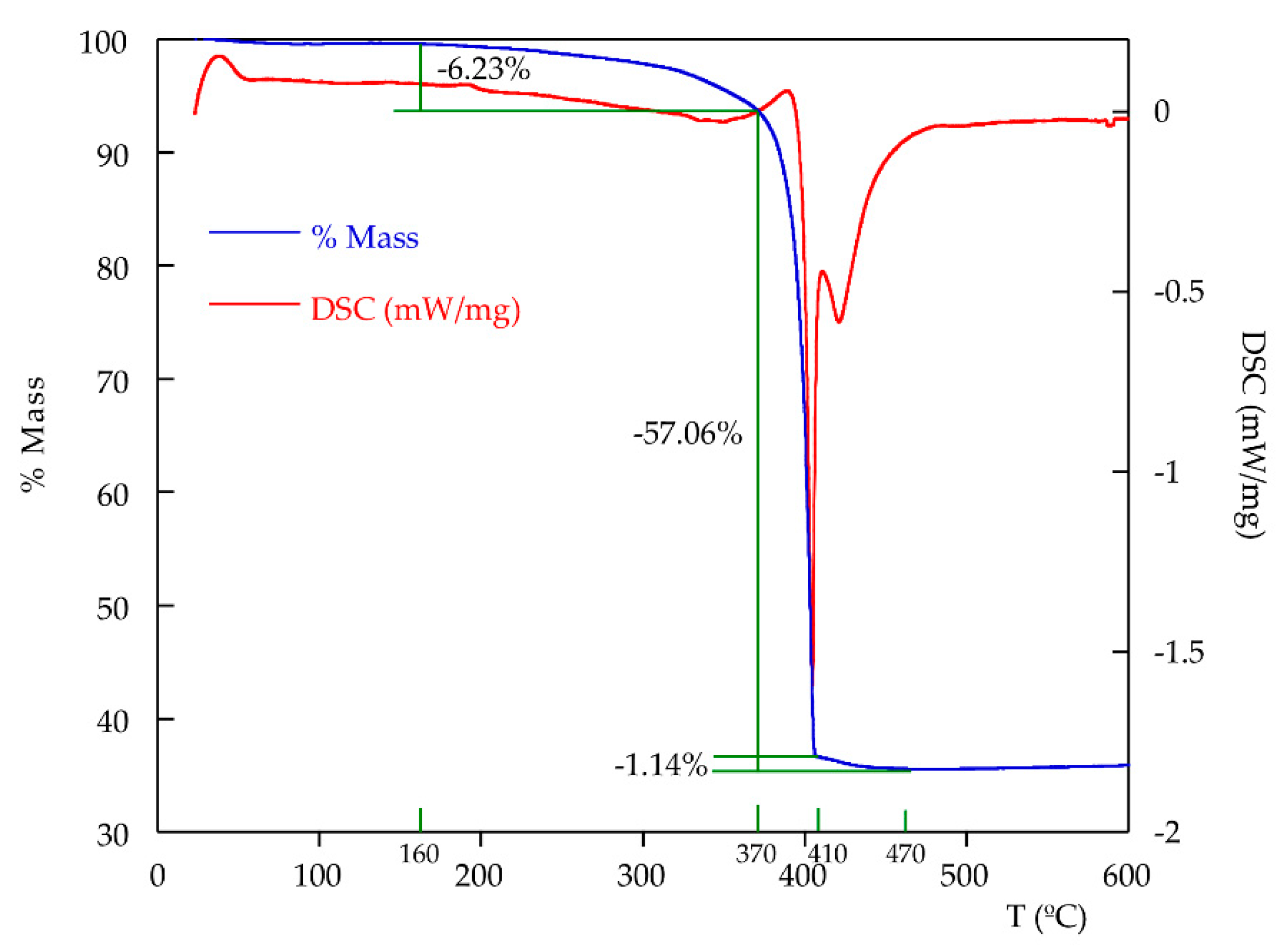
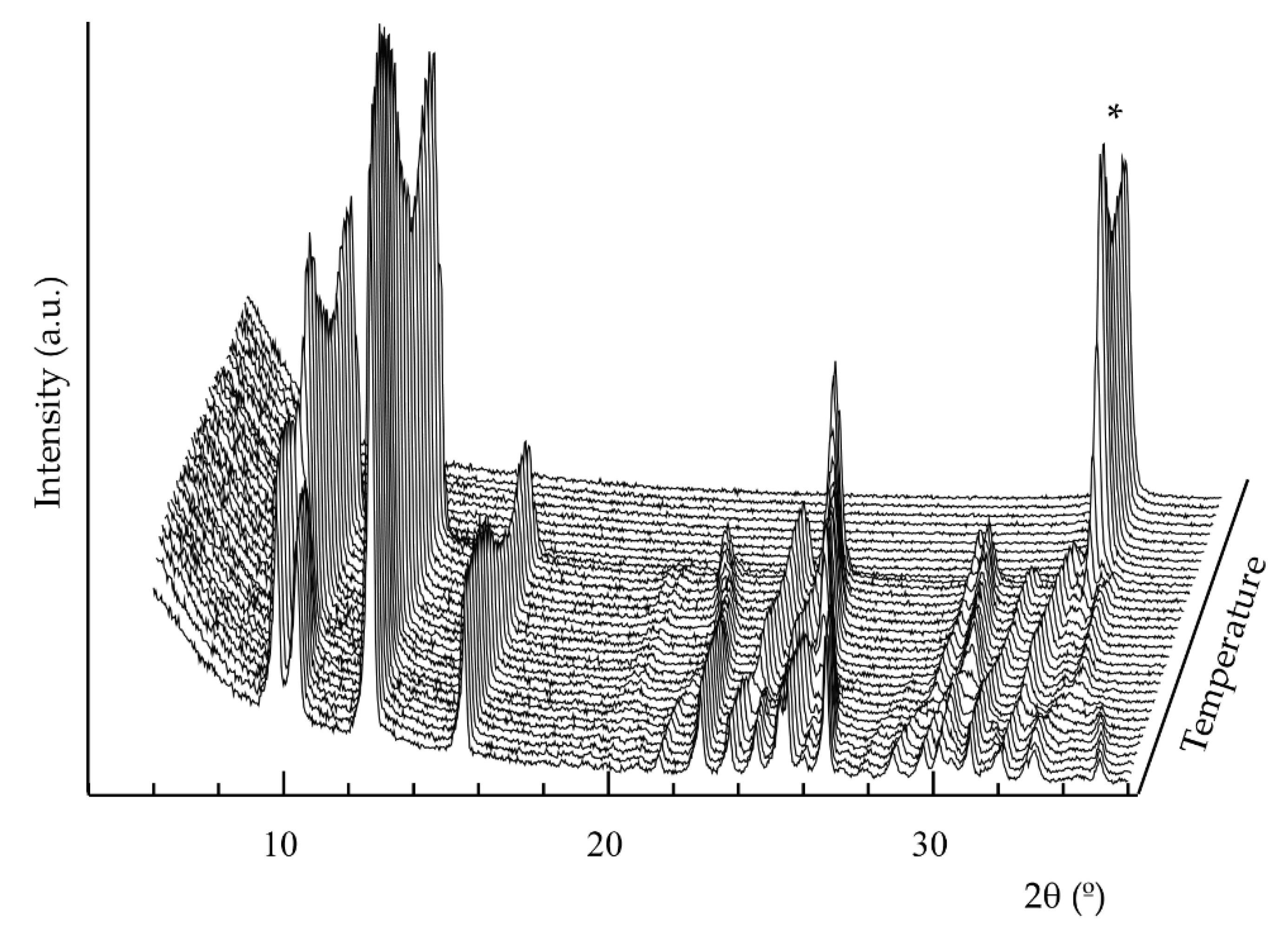
2.3. Thermal Characterization
3. Materials and Methods
3.1. Synthesis of Cd3(Ox)F2(Ina)2 (1)
3.2. Synthesis of Cd(4,4′-Bpy)(NO3)2 (2)
3.3. Single-crystal X-ray Diffraction Characterization
3.4. Physico-Chemical Characterization Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Cota, I.; Fernandez Martinez, F. Recent advances in the synthesis and applications of metal organic frameworks doped with ionic liquids for CO2 adsorption. Coord. Chem. Rev. 2017, 351, 189–204. [Google Scholar] [CrossRef]
- Lanchas, M.; Arcediano, S.; Beobide, G.; Castillo, O.; Luque, A.; Pérez-Yáñez, S. Towards multicomponent MOFs via solvent-free synthesis under conventional oven and microwave assisted heating. Inorg. Chem. Front. 2015, 2, 425–433. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, L.-L.; Pu, W.; Liu, P.; Liu, S.-X.; Li, Y.; Liu, X.-L.; Lu, Z.-X.; Zheng, L.-Y.; Cao, Q.-E. A hydrogel directly assembled from a copper metal–organic polyhedron for antimicrobial application. Chem. Commun. 2019, 55, 2206–2209. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef]
- Li, P.; Cheng, F.-F.; Xiong, W.-W.; Zhang, Q. New synthetic strategies to prepare metal—organic frameworks. Inorg. Chem. Front. 2018, 5, 2693–2708. [Google Scholar] [CrossRef]
- Liu, L.; Wei, H.; Zhang, L.; Li, J.; Dong, J. Ionothermal synthesis of the Metal-Organic Framework compound Cu3(BTC)2. In Studies in Surface Science and Catalysis; Gédéon, A., Massiani, P., Babonneau, F., Eds.; Elsevier Science: Paris, France, 2008; Volume 174, pp. 459–462. [Google Scholar]
- Martins, G.A.V.; Byrne, P.J.; Allan, P.; Teat, S.J.; Slawin, A.M.Z.; Li, Y.; Morris, R.E. The use of ionic liquids in the synthesis of zinc imidazolate frameworks. Dalton Trans. 2010, 39, 1758–1762. [Google Scholar] [CrossRef]
- Fischer, M.; Schwegler, J.; Paula, C.; Schulz, P.S.; Hartmann, M. Direct synthesis of non-breathing MIL-53(Al)(ht) from a terephthalate-based ionic liquid as linker precursor. Dalton Trans. 2016, 45, 18443–18446. [Google Scholar] [CrossRef]
- Tan, B.; Xie, Z.-L.; Huang, X.-Y.; Xiao, X.-R. Ionothermal synthesis, crystal structure, and properties of an anionic two-dimensional cadmium metal organic framework based on paddle wheel-like cluster. Inorg. Chem. Commun. 2011, 14, 1001–1003. [Google Scholar] [CrossRef]
- Xie, Z.-L.; Feng, M.-L.; Tan, B.; Huang, X.-Y. The multifunctional roles of the ionic liquid [Bmim] [BF4] in the creation of cadmium metal–organic frameworks. CrystEngComm 2012, 14, 4894–4901. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Xu, L.; Jiao, H. Ionothermal synthesis, structures, properties of cobalt-1, 4-benzenedicarboxylate metal–organic frameworks. J. Solid State Chem. 2016, 238, 217–222. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Z.-H.; Wang, M.-M.; Xu, L.; Liu, B.; Jiao, H. Combination effect of ligands and ionic liquid components on the structure and properties of manganese metal–organic frameworks. CrystEngComm 2017, 19, 5402–5411. [Google Scholar] [CrossRef]
- Vaid, T.P.; Kelley, S.P.; Rogers, R.D. Structure-directing effects of ionic liquids in the ionothermal synthesis of metal–organic frameworks. IUCrJ 2017, 4, 380–392. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Wasserscheid, P.; Welton, T. Introduction. In Ionic Liquids in Synthesis; Wasserscheid, P., Welton, T., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 1–6. ISBN 978-3-527-62119-4. [Google Scholar]
- Holbrey, J.D.; Rogers, R.D.; Mantz, R.A.; Trulove, P.C.; Cocalia, V.A.; Visser, A.E.; Anderson, J.L.; Anthony, J.L.; Brennecke, J.F.; Maginn, E.J.; et al. Physicochemical Properties. In Ionic Liquids in Synthesis; Wasserscheid, P., Welton, T., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 57–174. ISBN 978-3-527-62119-4. [Google Scholar]
- Pena-Pereira, F.; Namieśnik, J. Ionic liquids and deep eutectic mixtures: Sustainable solvents for extraction processes. Chem. Sus. Chem. 2014, 7, 1784–1800. [Google Scholar] [CrossRef]
- Xu, L.; Choi, E.-Y.; Kwon, Y.-U. Ionothermal Syntheses of Six Three-Dimensional Zinc Metal−Organic Frameworks with 1-Alkyl-3-methylimidazolium Bromide Ionic Liquids as Solvents. Inorg. Chem. 2007, 46, 10670–10680. [Google Scholar] [CrossRef]
- Xu, L.; Choi, E.-Y.; Kwon, Y.-U. Combination Effects of Cation and Anion of Ionic Liquids on the Cadmium Metal−Organic Frameworks in Ionothermal Systems. Inorg. Chem. 2008, 47, 1907–1909. [Google Scholar] [CrossRef]
- Mondal, S.S.; Bhunia, A.; Demeshko, S.; Kelling, A.; Schilde, U.; Janiak, C.; Holdt, H.-J. Synthesis of a Co (II)–imidazolate framework from an anionic linker precursor: Gas-sorption and magnetic properties. CrystEngComm 2014, 16, 39–42. [Google Scholar] [CrossRef]
- Zhou, W.-W.; Zhao, W.; Wei, B.; Du, J.-M.; Chen, Y.-H.; Tong, Y. Crystal structure of bis(µ3-hydroxy)bis(isonicotinato-κO:O’)(oxalato)- biscadmium(II)zinc(II), Cd2Zn(C2O4)(OH)2(C6NO2H4)2. Z. Kristall. 2014, 226, 611–612. [Google Scholar]
- Saha, R.; Sekar, G. Selective oxidation of alkylarenes to aromatic acids/ketone in water by using reusable binaphthyl stabilized Pt nanoparticles (Pt-BNP) as catalyst. Appl. Catal. B Environ. 2019, 250, 325–336. [Google Scholar] [CrossRef]
- Khan, I.U.; Sharif, S.; Sahin, O. Seven-, eight-, and ten-coordinated cerium(III) with highly connective pyridine-2,4,6-tricarboxylate, oxalate, and glycine ligands. J. Coord. Chem. 2013, 66, 3113–3125. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. Shape: Program. for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; Departament de Química Física, Departament de Química Inorgànica, and Institut de Química Teòrica i Computacional—Universitat de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Casanova, D.; Alemany, P.; Bofill, J.M.; Alvarez, S. Shape and Symmetry of Heptacoordinate Transition-Metal Complexes: Structural Trends. Chem. Eur. J. 2003, 9, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Ok, K.M.; Halasyamani, P.S.; Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. Distortions in Octahedrally Coordinated d0 Transition Metal Oxides: A Continuous Symmetry Measures Approach. Chem. Mater. 2006, 18, 3176–3183. [Google Scholar] [CrossRef]
- Alvarez, S.; Avnir, D.; Llunel, M.; Pinsky, M. Continuous symmetry maps and shape classification. The case of six-coordinated metal compounds. New J. Chem. 2002, 26, 996–1009. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Liu, C.-M.; Xiong, R.-G.; You, X.-Z.; Chen, W.; Aleksa, V. A Two-Dimensional Square Network Inclusion Compound Incorporating Guest Molecules Through Both Hydrogen Bonding and Nonionic Electrostatic Attraction. Crystal Structure of [Cd(4,4′-bpy)2(H2O)2]-(ClO4)2.1.5(4,4′-bpy).(C6H4NO3Cl).H2O. Acta Chem. Scand. 1998, 52, 1353–1358. [Google Scholar] [CrossRef]
- Jiang, J.-J.; Liu, Y.-R.; Yang, R.; Pan, M.; Cao, R.; Su, C.-Y. The interplay of coordinative and hydrogen-bonding in directing the [M(4,4′-bpy)2(H2O)2] square-grid networks: Formation of 3D porous framework [Cd(4,4′-bpy)2(H2O)2](ClO4)2(4,4′-bpy)(CH3OH)2. CrystEngComm 2008, 10, 1147–1153. [Google Scholar] [CrossRef]
- Huang, S.D.; Lewandowski, B.J.; Liu, C.; Shan, Y. [Cd(4,4′-bipy)2(NO3)2](2-nitroaniline)2, a novel two-dimensional lattice inclusion compound. Acta Crystallogr. Sect. C 1999, 55, 2016–2018. [Google Scholar] [CrossRef]
- Xiong, R.-G.; Liu, C.-M.; Zuo, J.-L.; You, X.-Z. Guest-induced dimension change. A novel network intercalation complex: {[Cd(4,4′-bipy)2(H2O)2](CF3SO3)2(4,4′-bipy)(H2O)2(C7H8N2O3)2}∞. Inorg. Chem. Commun. 1999, 2, 292–297. [Google Scholar] [CrossRef]
- Huang, S.D.; Xiong, R.-G. Molecular recognition of organic chromosphores by coordination polymers: Design and construction of nonlinear optical supramolecular assemblies. Polyhedron 1997, 16, 3929–3939. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.-Q. A new supramolecular coordination polymer constructed by flexible and rigid organic coligands. Synth. React. Inorg. Met.-Org. Nano-Metal. 2013, 43, 861–863. [Google Scholar] [CrossRef]
- Liu, C.-M.; Xiong, R.-G.; You, X.-Z.; Chen, W.; Lo, K.-M. Molecular recognition of an organic molecule through a two dimensional square network inclusion complex. Synthesis and crystal structure of [Cd(4,4′-Bpy)2(H2O)2] (BF4)2 · 2(4,4′-Bpy) · (C6H6N2O2) · 2H2O. J. Coord. Chem. 1998, 46, 211–220. [Google Scholar] [CrossRef]
- Fujita, M.; Kwon, Y.J.; Washizu, S.; Ogura, K. Preparation, clathration ability, and catalysis of a two-dimensional square network material composed of Cadmium(II) and 4,4′-Bipyridine. J. Am. Chem. Soc. 1994, 116, 1151–1152. [Google Scholar] [CrossRef]
- Hu, C.; Li, Q.; Englert, U. Structural trends in one and two dimensional coordination polymers of cadmium(II) with halide bridges and pyridine-type ligands. CrystEngComm 2003, 5, 519–529. [Google Scholar] [CrossRef]
- Zhang, J. Room-temperature compressibilities of MnO and CdO: Further examination of the role of cation type in bulk modulus systematics. Phys. Chem. Min. 1999, 26, 644–648. [Google Scholar] [CrossRef]
- Yinghua, W. Lorentz–polarization factor for correction of diffraction-line profiles. J. Appl. Crystallogr. 1987, 20, 258–259. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Han, T. International Tables for X-ray Crystallography; Kynoch Press: Birmingham, UK, 1973; Volume 4. [Google Scholar]
Sample Availability: Samples of the compound 2 are available from the authors. |
| 1 | 2 | |
|---|---|---|
| Crystal data | ||
| Chemical formula | C14H8Cd3F2N2O8 | C10H8CdN4O6 |
| Mr | 707.42 | 392.6 |
| Crystal system, space group | Monoclinic, P21/n | Triclinic, P-1 |
| a, b, c (Å) | 10.4405 (4),7.6000(2), 12.2919(5) | 7.842(1), 9.314(1), 10.183(2) |
| α, β, γ (°) | 90, 113.019 (5), 90 | 72.72(1), 68.97(1), 69.54(1) |
| V (Å3) | 897.67 (6) | 637.8 (2) |
| Z | 2 | 2 |
| Dx (g cm−3) | 2.617 | 2.047 |
| F(000) | 664 | 384 |
| μ (mm−1) | 3.59 | 14.10 |
| Crystal size (mm) | 0.33 × 0.10 × 0.05 | 0.18 × 0.07 × 0.06 |
| Data collection | ||
| Radiation type (λ) | Mo Kα (0.71073 Å) | Cu Kα (1.54184 Å) |
| Temperature (K) | 100 | 150 |
| θ range (˚) | 3.2–27.2 | 4.7–74.3 |
| h, k, l ranges | −13 ≤ h ≤ 12, −9 ≤ k ≤ 9, −15 ≤ l ≤ 15 | −9 ≤ h ≤ 9, −11 ≤ k ≤ 10, −12 ≤ l ≤ 12 |
| Tmin, Tmax | 0.542, 0.854 | 0.397, 1 |
| No. of meas. refl. (Rint) | 5712 (0.052) | 2547 (0.057) |
| No. of independent and observed [I > 2σ(I)] refl. | 1846, 1546 | 2547, 2200 |
| (sin θ/λ)max (Å−1) | 0.643 | 0.626 |
| Refinement | ||
| R[F2 > 2σ(F2)], wR(F2), S | 0.034, 0.062, 1.05 | 0.091, 0.265, 1.12 |
| No. of reflections | 1846 | 2547 |
| No. of parameters | 145 | 191 |
| Largest diff. peak and hole (e Å−3) | 0.76 and -0.82 | 4.80and -2.12 |
| BASF | - | 0.493 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
PerezF, I.; S. Larrea, E.; Bazán, B.; Barandika, G.; Urtiaga, M.K.; Arriortua, M.I. Ionothermal Synthesis of Cadmium Coordination Polymers: Ionic Liquid Effects on the Synthesis, Structural, and Thermal Characterization. Molecules 2019, 24, 4059. https://doi.org/10.3390/molecules24224059
PerezF I, S. Larrea E, Bazán B, Barandika G, Urtiaga MK, Arriortua MI. Ionothermal Synthesis of Cadmium Coordination Polymers: Ionic Liquid Effects on the Synthesis, Structural, and Thermal Characterization. Molecules. 2019; 24(22):4059. https://doi.org/10.3390/molecules24224059
Chicago/Turabian StylePerezF, Iñigo, Edurne S. Larrea, Begoña Bazán, Gotzone Barandika, M. Karmele Urtiaga, and Maria I. Arriortua. 2019. "Ionothermal Synthesis of Cadmium Coordination Polymers: Ionic Liquid Effects on the Synthesis, Structural, and Thermal Characterization" Molecules 24, no. 22: 4059. https://doi.org/10.3390/molecules24224059
APA StylePerezF, I., S. Larrea, E., Bazán, B., Barandika, G., Urtiaga, M. K., & Arriortua, M. I. (2019). Ionothermal Synthesis of Cadmium Coordination Polymers: Ionic Liquid Effects on the Synthesis, Structural, and Thermal Characterization. Molecules, 24(22), 4059. https://doi.org/10.3390/molecules24224059