Abstract
Five new meroterpenoids, clavipols A–B (1–2) with a 12-membered ether ring and clavilactones G–I (3–5) having a 10-membered carbocycle connected to a hydroquinone and an α,β-epoxy/unsaturated lactone, were obtained from the fruiting bodies of the basidiomycete Clitocybe clavipes. Their structures were determined by comprehensive analysis of their spectroscopic data, and the absolute configuration of 1 was established by quantum chemical calculations of electronic circular dichroism (ECD). All the isolated compounds (1–5) were tested for their cytotoxic activity against three human tumor cell lines (Hela, SGC-7901, and SHG-44) in vitro after treatment for 48 h. Compound 4 exhibited moderate cytotoxic activity against Hela and SGC-7901 tumor cell lines, with IC50 values of 23.5 and 14.5 µM, respectively.
1. Introduction
Secondary metabolites from fungi have attracted the attention of chemists, pharmacologists, and biologists because of their unique chemical structures and potential biological activities [1,2,3]. Meroterpenoids are defined as compounds partially derived from terpenoids [4]. In the past decades, fungal meroterpenoids, including pyripyropene A [5], arisugacins [6], and territrems [6], have been reported to have novel and fascinating chemical structures. They not only have diverse structural skeletons but also show biological activity, such as antitumor [7], anti-inflammatory [8], antioxidant [9], antibacterial [10], and antifungal [10] activities. Considering their unique structure and significant biological activities, total synthesis of fungal meroterpenoids has been achieved by synthetic chemists [11,12].
The fungus Clitocybe clavipes has rarely been chemically investigated. To the best of our knowledge, only 10 of its chemical constituents, 5 meroterpenoids, clavilactones A–E, and 5 fatty acid derivatives, have been isolated [13,14,15]. Moreover, the meroterpenoids clavilactones A–E have been reported to have potent pharmacological activity, such as antifungal activity and inhibition of protein tyrosine kinases [13,14]. Especially, clavilactone D was shown to inhibit epidermal growth factor receptor tyrosine kinase, with an IC50 value of 5.5 μM [16]. Recently, we have reported the isolation of three novel meroterpenoids from the basidiomycete C. clavipes, clavipines A–C, possessing a benzoquinone fused to an azepine ring and a 10-membered carbocycle with α,β-epoxy/unsaturated-ɤ-lactone. [17]. In our ongoing search for structurally unique and biologically valuable metabolites, an investigation of the extracts of the fruiting bodies of the basidiomycete C. clavipes led to the isolation of five new meroterpenoids, clavipols A–B (1–2) and clavilactones G–I (3–5) (Figure 1). This paper reports the isolation and structural elucidation of the five isolated new meroterpenoids, as well as their cytotoxic activities.
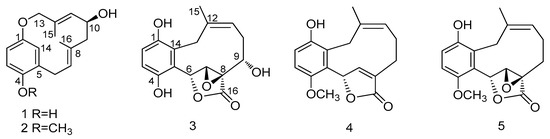
Figure 1.
Structures of compounds 1–5.
2. Results
Compound 1 was isolated as a colorless powder. Its molecular formula was established to be C16H20O3 on the basis of high-resolution electrospray ionization mass spectroscopy (HRESIMS) at m/z 259.1329 [M − H]− (calcd for 259.1340), indicating seven degrees of unsaturation. It had an IR absorption band at 3417 cm−1, which suggested the presence of hydroxyl groups. The 1H-NMR spectrum (Table 1, supplementary Figure S1) of 1 revealed the presence of one ABX aromatic system at [δH 6.60(1H, d, J = 8.4 Hz, H-3), 6.56(1H, dd, J = 3.0, 8.4 Hz, H-2), 7.23(1H, d, J = 3.0 Hz, H-14)], three aliphatic methylenes [δH 3.04(1H, dd, J = 7.2, 16.8 Hz, H-6b), 3.34 (1H, dd, J = 8.4, 16.8 Hz, H-6a), 2.36 (1H, t, J = 12Hz, H-9b), 2.71(1H, dd, J = 4.2, 12 Hz, H-9a), 4.46(1H, d, J = 12.6Hz, H-13b), 4.64(1H, d, J = 12.6 Hz, H-13a)], two olefinic methines [δH 5.74(1H, t, J = 7.2Hz, H-7), 5.56(1H, d, J = 10.2Hz, H-11)], one oxygenated methine [δH 4.68(1H, m, H-10)], and two methyl groups [δH 1.56(3H, s, H3-15), 1.24(3H, s, H3-16)]. 13C-NMR analysis with the aid of the HSQC spectra of 1 revealed 20 carbon signals composed of two methyls, three methylenes, five olefinic methines, five olefinic quaternary carbons, and one oxygenated methine. The proton signal at δH 4.68 (1H, m, H-10), together with the downfield methine carbon signal at δC 66.0, suggested the existence of a hydroxy group. Comprehensive analysis of 1D-NMR data indicated the existence of a 1,2,4-substituted hydrobenzene unit and the monoterpene moiety.

Table 1.
NMR spectral data of 1 and 2 (600 MHz for 1H-NMR and 150 MHz for 13C-NMR).
Spectra Data were Recorded in CDCl3
The 1H-1H COSY spectrum (Figure 2) indicated the presence of two coupling fragments H-6/H-7 and H-9/H-10/H-11. A hydroxy group located at C-10 in 1 was ascertained by the COSY correlations of H-10/H-11 and H-10/H-9, as well as by the HMBC correlations from H-10 to C-9/C-11. The HMBC correlations (Figure 2) from H-6 to C-4/C-5/C-7/C-14 established that the monoterpene was attached to C-5. Meanwhile, the HMBC correlations from H-9 to C-8/C-15 and from H-13 to C-11/C-12/C-13/C-15 indicated compound 1 was an ansa-type monoterpenylbenzenoid with a 12-membered ether ring [18]. The NMR data and biogenetical considerations indicated that the configuration of the two double bonds should be E. Finally, the planar structure of 1 was established, as shown in Figure 1, and the compound was given the name clavipol A.
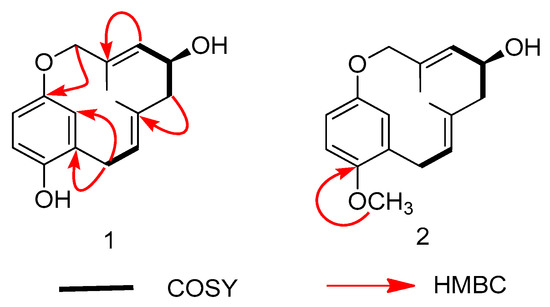
Figure 2.
Key 1H-1H COSY and HMBC correlations for compounds 1–2.
In the ROESY spectrum, correlations from H-10, H-9a, and H3-15 to H3-16 were observed (Figure 3), which allowed H-10 to be placed on the same side of H3-15 and H3-16. In order to establish the absolute configuration of compound 1, density functional theory (DFT) calculations at the APFD/6-311+g (2d, p) level of the ECD spectra were carried out and compared with the experimental ones (Figure 4); their identical spectral profiles supported the S configuration of C-10.
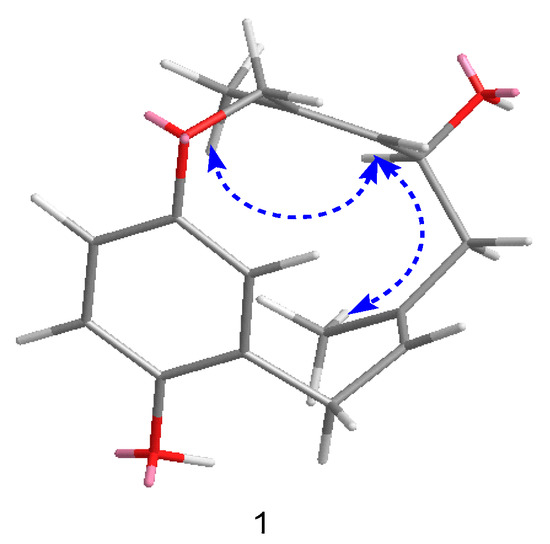
Figure 3.
Key rotating overhauser effect correlations for compound 1.
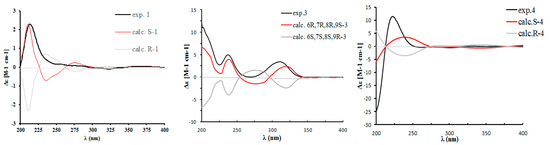
Figure 4.
Calculated and experimental electronic circular dichroism (ECD) spectra of 1, 3, and 4 in methanol.
Compound 2 was obtained as a colorless powder with the molecular formula of C17H22O3 according to its negative ion HRESIMS peak at m/z 273.1487 [M − H]− (calcd 273.1496), indicative of 7 degrees of unsaturation and 14 more mass units with respect to 1. The 1H- and 13C-NMR spectroscopic data of compound 2 were quite similar to those of compound 1, except for an additional methoxy group [δH 3.83 (3H, s); δC 56.1]. In the HMBC spectrum, the methoxy signals had correlations with C-4 (δC 151.4), indicating its connection to C-4 (Figure 2). Finally, the entire structure of compound 2 was elucidated as 4-methylated clavipol A. The absolute configuration of 2 was determined to be identical with that of 1 by comparing their ECD spectra (supplementary Figures S7 and S14), and the compound was given the name clavipol B.
Compound 3 was isolated as a yellow powder, having the formula of C16H16O6 with nine degrees of unsaturation based on the HRESIMS at m/z 303.0885 [M − H]− (cal. 303.0874). Overall consideration of 1D-NMR data (Table 2) suggested that compound 3 was a meroterpenoid similar to clavilactone A [13]. In detail, two aromatic protons at δH 6.77(1H, d, J = 8.4 Hz, H-2), 6.66(1H, d, J = 8.4 Hz, H-3), together with six olefinic carbons at δC 151.0 (C-1), 118.9 (C-2), 115.7 (C-3), 151.0 (C-4), 120.8 (C-5), 128.1 (C-14), indicated the presence of one four-substituted benzene ring. 13C-APT NMR signals of δC 77.1 (C-6), 64.7 (C-7), 62.8 (C-8), and 172.8 (C-16) indicated the presence of an α,β-epoxy ɤ-lactone moiety, which was further confirmed by HMBC correlations from H-7(4.02, s) to C-5, C-6, C-8, and C-16 (Figure 5). The methine signal at δH 3.47(1H, dd, J = 3.0, 12.0 Hz) and δC 71.3(C-3) indicated that the hydroxyl group was substituted at C-9, which was further supported by the HMBC correlations from δH 3.47 to C-8 (62.8), C-10 (33.4), and C-16 (172.8). By analyzing the COSY spectrum, one proton-bearing structure fragment [=CH–CH2–CH–] was readily established (indicated by bold bonds in Figure 2). In the HMBC spectrum, the correlations from H-13b to C-11, C-12, C-14, and C-15 and from H-9 to C-8, C-10, and C-16, indicated that compound 3 was a clavilactone homologue containing hydrobenzene fused to a 10-member carbocycle with α,β-epoxy ɤ-lactone. As a result, compound 3 was established as 9-hydroxyl-substituted clavilactone A. In the 1H-NMR spectrum, no vicinal coupling between the two adjacent protons H-6 and H-7 suggested these protons form a dihedral angle of approximately 90°, which means that the relative configurations of C-6, C-7, and C-8 are 6R, 7R, 8R or 6S, 7S, 8S. In the NOESY spectrum, the correlations of H-7a/H-9, H-11, H-13, H3-15; H-9/H-11, H-7a, and H-10a/H-13b revealed the close proximity of these protons, and the observed correlation of H-9 to H-7a and H-11 (Figure 6) allowed H-9 to be placed on the side H-9b due to the reported correlations between H-9b to H-7a in clavilactone A [13,17]. The positive cotton effects at 237 and 308 nm showed in the ECD spectrum of 3 (supplementary Figure S21) were in good agreement with the calculated CD spectrum of 6R, 7R, 8R, 9S configuration for 3, as shown in Figure 4. Thus, the absolute configuration of 3 was determined to be 6R, 7R, 8R, 9S, and the compound was given the name clavilactone G.

Table 2.
NMR spectral data of 3–5 (600 MHz for 1H-NMR and 150 MHz for 13C-NMR).
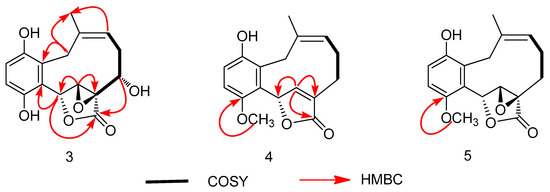
Figure 5.
Key 1H-1H COSY and HMBC correlations for compounds 3–5.
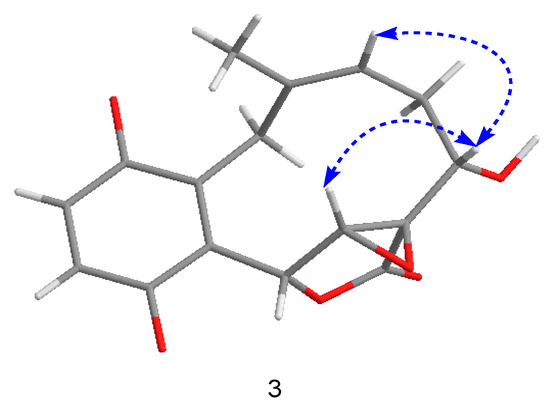
Figure 6.
Key nuclear overhauser effect (NOE) correlations for compound 3.
Compound 4 was isolated as a yellow powder. The HRESIMS displayed an [M − H]− ion peak at m/z 285.1132 (calcd 285.1132), which showed the molecular formula C17H18O4. The IR spectrum suggested the presence of hydroxyl (3370 cm−1) and carbonyl groups (1714 cm−1). By intensive comparison of the 1H and 13C-NMR data (Table 2) with those of clavilactone A, significant differences were the absence of two oxygenated carbon signals and the presence of a double bond [δH 6.80 (1H, s); δC 149.7, 129.2] as well as of a methoxy group [δH 3.83(3H, s); δC 53.1] in 4. In the HMBC spectrum, the correlations from δH 6.80 to C-6, C-8, and C-16 established that the α,β-epoxy-ɤ-lactone moiety in clavilactone A had been cracked and dehydrated to form an α,β-unsaturated-ɤ-lactone unit in 4. The methoxy group was attached to C-4 on the basis of the HMBC correlation from protons (δH 3.83) to C-4 (δC 153.1). The experimental ECD spectrum of 4 exhibited the positive cotton effect at 222 nm (supplementary Figure S28), in agreement with the calculated CD spectrum of 6S configuration for 4 (Figure 4). Thus, the absolute configuration of 4 was determined as S, and the compound was given the name clavilactone H.
Compound 5 was isolated as a yellow amorphous powder. The HRESIMS displayed an [M − H]− ion peak at m/z 301.1070 [M − H]− (calcd 301.1081), which showed the molecular formula C17H18O5 and 14 more mass units than clavilactone A [13]. Its IR bands at 3436 cm−1 and 1749cm−1 suggested the existence of hydroxyl and carbonyl groups. Overall consideration of 1H and 13C data of 5 indicated compound 5 could be a methylation derivative of clavilactone A, which was further supported by its HMBC spectrum. In the HMBC spectrum, the correlation between δH (3.83, s) and C-4 (δC 153.1) established the presence of a methoxy group (δC 57.1) attaching to C-4 in compound 5 (Figure 5). Considering the identical CD spectra of compound 5 and clavilactone A (supplementary Figures S34 and S35), compound 5 should have the same absolute configuration of calvilactone A, and was given the name clavilactone I.
Furthermore, all isolated compounds (1–5) were evaluated for their cytotoxicity against three human tumor cell lines (Hela, SGC-7901, and SHG-44) in vitro by treatment for 48 h, using the MTT assay [19]; cisplatin was used as the positive control drug. The results of cytotoxicity are displayed in Table 3. Compound 4 exhibited moderate cytotoxic activity against Hela and SGC-7901 tumor cell lines, with IC50 values of 23.5 and 14.5 µM, respectively.

Table 3.
In vitro cytotoxic activity of compounds 1–5.
3. Discussion
The chemical investigation of the fungus C. clavipes led to the isolation of five new meroterpenoids, clavipols A–B (1–2) with a 12-membered ether ring and clavilactones G–I (3–5) having a 10-membered carbocycle connected to a hydroquinone and an α,β-epoxy/unsaturated lactone. This study contributes to broadening the list of known chemically diverse meroterpenoids from the fungus C. clavipes.
Until now, only about 20 naturally occurring meroterpenoids with a benzo-fused 10-membered carbocycle unit have been isolated from plants and fungi [17]. These meroterpenoids have been shown to display potent cytotoxic activities [20]. For example, terreumols A, isolated from the mushroom Tricholoma terreum, displayed potent cytotoxic activity against A-549 cancer cell line, with an IC50 value of 4.2 μM [20]. Compared with the cytotoxic activities of compounds 3 and 5, compound 4 exhibited moderate cytotoxic activity against Hela and SGC-7901 cancer cell lines, with IC50 values of 23.5 and 14.5 µM, respectively. Therefore, we speculate that different degrees of oxidation of such compounds may affect their cytotoxic activities.
4. Materials and Methods
4.1. General Experimental Procedures
1D and 2D-NMR spectra were obtained with a Bruker AV 600 NMR spectrometer (chemical shift are presented as δ values with TMS as the internal standard) (Bruker, Billerica, Germany). HRESIMS was performed on a Q-tof spectrometer (Waters, Milford, MA, USA). UV and IR data were obtained using a Shimadzu UV2550 spectrophotometer and a FTIR-8400S spectrometer (Shimadzu, Kyoto, Japan), respectively. CD spectra were obtained using a JASCO J-815 spectropolarimeter (Tokyo, Japan). Thin-layer chromatography (TLC) was performed on pre-coated silica gel GF254 (Zhi Fu Huang Wu Pilot Plant of Silica Gel Development, Yantai, China). Semi-preparative HPLC was conducted on an analytic LC equipped with a pump of P230 and a DAD detector of 230+ (Ellte, Dalian, China) with a C18 ODS-A (5 µm, YMC, Kyoto, Japan). Column chromatography used silica gel columns (200–300 mesh, Qingdao Marine Chemical plant, Qingdao, China). All solvents used were of analytical grade (Beijing Chemical Plant, China).
4.2. Computational Methods
The ECD calculations were carried out using Gaussian 09 program (Inc., WALLINGFORD, CT, USA). Conformers were generated by MMFF94s force field, each conformer was optimized with the HF/6-31G(d) method, and further optimized with the DFT method at the B3LYP/6-311+g(d, p) level. Frequency calculations were also performed at the same level to confirm that each optimized conformer was true minimum and to estimate their relative thermal free energy (ΔG) at 298.15 K. Conformers with the Boltzmann distribution over 1% were chosen for ECD calculations in methanol at the APFD/6-311+g(2d, p) level. The ECD spectra were simulated by the SpecDis program. To obtain the final conformationally averaged data, the simulated spectra of the predominant conformers were averaged according to the Boltzmann distribution theory.
4.3. Fungal Material
The fruiting bodies of Clitocybe clavipes were collected from Hotan Prefecture, Xinjiang Uygur Autonomous Region, China, in July 2018. The fungus was identified by Prof. Leiling Shi, Xinjiang Institute of Chinese and Ethnic Medicine, where a voucher specimen of C. clavipes (No. 201812) was preserved.
4.4. Extraction and Isolation
The dried fruiting bodies of C. clavipes (0.6 kg) were macerated three times with EtoAc. The solvents were filtrated and evaporated in vacuum to give the total extract (45 g), and this residue was subjected to silica-gel (200–300 mesh) column chromatography (CC) with two gradient systems (ether/EtOAc 30:1, 10:1, 5:1,1:1; CH2Cl2/MeOH 20:1, 10:1, 5:1, 1:1, v/v) to give 8 fractions (F1–F8). F3 was purified by semi-preparative HPLC (CH3CN/H2O 60:49, v/v) to yield 1 (3 mg, tR = 15.5 min) and 2 (2 mg, tR = 17.1 min). F4–5 were subjected to C-18 reversed-phase (RP) silica-gel CC using MeOH/H2O in a linear gradient (30:70, 45:55, 60:40, 80:20, 100:0, v/v) to obtain 5 fractions (F4–5.a–F4–5.e). F4–5.d was purified by semi-preparative HPLC with CH3CN-H2O as mobile phase (45:55, v/v), to give 3 (2 mg, tR = 12.4 min), 4 (2 mg, tR = 20.0 min), and 5 (3 mg, tR = 22.1 min).
The structures of compounds 1–5 were determined by HRESIMS, UV, IR, 1D and 2D-NMR spectra.
Clavipol A (1), colorless powder; [α]25D +18.6 (c 0.11, MeOH); UV(MeOH) λmax (log ε) 204(3.54), 291(3.2) nm; IR(KBr) vmax 3417, 2917, 2851,1596, 1385, 1118, 768, 544 cm−1; 1H and 13C-NMR data see Table 1; (-)HRESIMS m/z 259.1329 [M − H]− (calcd for 259.1340).
Clavipol B (2), colorless powder; [α]25D +18.0 (c 0.10, MeOH); UV(MeOH) λmax (log ε) 203(3.3), 290(3.1) nm; IR(KBr) vmax 3418, 2914, 2849,1593, 1383, 1115, 763, 546 cm−1; 1H and 13C NMR data see Table 1; (-)HRESIMS m/z 273.1487 [M − H]− (calcd 273.1496).
Clavilactone G (3), yellow powder; [α]25D +50.8 (c 0.10, MeOH); UV(MeOH) λmax (log ε) 235(3.91), 310(3.52) nm; IR(KBr) vmax 3440, 2944, 1776, 1487, 1419, 1233, 1210, 802 cm−1; 1H and 13C-NMR data see Table 2; (-)HRESIMS m/z 303.0885 [M − H]− (cal. 303.0874).
Clavilactone H (4), yellow powder; [α]25D −21.8 (c 0.06, MeOH); UV(MeOH) λmax (log ε) 209(3.91), 281(3.52) nm; IR(KBr) vmax 3370, 2925, 2853, 1743, 1599, 1453, 1267, 1076, 807cm−1; 1H and 13C NMR data see Table 2; (+)HRESIMS m/z 285.1132(calcd 285.1132).
Clavilactone I (5), yellow powder; [α]25D +90.5 (c 0.12, MeOH); UV(MeOH) λmax (log ε) 236(3.70), 310(3.56) nm; IR(KBr) vmax 3435, 2930, 1749, 1487, 1260, 1152, 1021, 800 cm−1; 1H and 13C NMR data see Table 2; (-)HRESIMS m/z 301.1070 [M − H]− (calcd 301.1081).
4.5. Cytotoxicity Assays
Compounds 1–5 were evaluated for their cytotoxic activity by the MTT method using Hela, SGC-7901, and SHG-44 cancer cell lines. Cells were grown in DMEM medium and cultured at a density of 6 × 104 cells/mL per well in a 96-well microtiter plate. Then, different concentrations of the isolated compounds dissolved in dimethyl sulfoxide (DMSO) were added to each well. Each concentration was tested in triplicate. After incubation at 37 °C in 5% CO2 for 48 h, 10 μL of MTT was added to each well, and incubation was continued for additional 4 h. Then, the liquid was removed from the wells and DMSO was added to each well. The absorbance was recorded on a microplate reader at a wavelength of 570 nm.
Supplementary Materials
Supplementary Materials are available online.
Author Contributions
G.M. and L.S. conceived and designed the experiments; Z.S. wrote the paper and performed the experiments; X.X. (Xudong Xu) helped during the structural elucidation; H.L. and X.X. (Xinyi Xia) assisted in the collating of NMR data.
Funding
The work was financially supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2017-I2M-1-013) and the National Natural Sciences Foundation of China (No. 81502945).
Conflicts of Interest
The authors have declared no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| IR | Infrared |
| NMR | Nuclear magnetic resonance |
| HR-ESI-MS | High resolution electrospray ionization mass spectroscopy |
| HMBC | Heteronuclear multiple bond correlation |
| HSQC | Heteronuclear single quantum correlation |
| COSY | Homonuclear chemical shift Correlation Spectroscopy |
| NOESY | Nuclear overhauser effect spectroscopy |
| ROESY | Rotating frame overhauser effect spectroscopy |
| APFD | Austin-Frisch-Petersson functional with dispersion |
| TMS | Tetramethylsilane |
| ODS | Octadecyl silane |
| HPLC | High performance liquid chromatography |
| CH2Cl2 | Dichloromethane |
| EtOAc | Ethyl acetate |
| MeOH | Methanol |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
References
- Niu, Z.; Chen, Y.; Guo, H.; Li, S.N.; Li, H.H.; Liu, H.X.; Liu, Z.; Zhang, W. Cytotoxic Polyketides from a Deep-Sea Sediment Derived Fungus Diaporthe phaseolorum FS431. Molecules 2019, 24, 3062. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.C.; Kharwar, R.N.; Strobel, G.A. Chemical and functional diversity of natural products from plant associated endophytic fungi. Nat. Prod. Commun. 2009, 4, 1511–1532. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xue, Y.; Du, G.; Wang, J.; Liu, J.; Sun, B.; Li, X.N.; Yao, G.; Luo, Z.; Zhang, Y. Structural revisions of a class of natural products: Scaffolds of aglycon analogues of fusicoccins and cotylenins isolated from fungi. Angew. Chem. Int. Ed. 2016, 128, 4137–4141. [Google Scholar] [CrossRef]
- Geris, R.; Simpson, T.J. Meroterpenoids produced by fungi. Nat. Prod. Rep. 2009, 26, 1063. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, H.; Nishida, H.; Kim, Y.K.; Obata, R.; Sunazuka, T.; Omura, S. Relative and absolute stereochemistry of pyripyropene A, a potent, bioavailable inhibitor of Acyl-CoA:Cholesterol Acyltransferase (ACAT). J. Am. Chem. Soc. 1994, 116, 12097–12098. [Google Scholar] [CrossRef]
- Kuno, F.; Shiomi, K.; Otoguro, K.; Sunazuka, T.; Omura, S. Arisugacins A and B, novel and selective acetylcholinesterase inhibitors from penicillium sp. F0-4259. II. structure elucidation. J. Antibiot. 1996, 49, 748–751. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, Y.; Zheng, M.; Li, Q.; Hu, Z.; Zhu, H.; Liu, J.; Wang, J.; Xue, Y.; Li, H.; Zhang, Y. Asperspiropene A, a novel fungal metabolite as an inhibitor of cancer-associated mutant isocitrate dehydrogenase 1. Org. Chem. Front. 2017. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, H.; Chen, Y.; She, Z. A new anti-inflammatory meroterpenoid from the fungus Aspergillus terreus H010. Nat. Prod. Res. 2017, 32, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Li, L.; Wang, X.; Zhu, G.; Li, Z.; Qiu, M. Antioxidant farnesylated hydroquinones from Ganoderma capense. Fitoterapia 2016, 111, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Meng, W.; Cao, C.; Wang, J.; Shan, W.; Wang, Q. Antibacterial and antifungal compounds from marine fungi. Mari. Drugs 2015, 13, 3479–3513. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, I.; Da Silva, M.I.; Gómez, P.M.; Hannen, P.; Ko, E.; Lenger, S.R.; White, A.J.P.; Wilton, D.; Barrett, A.G.M. Highly convergent three component benzyne coupling: The total synthesis of ent-clavilactone B. J. Am. Chem. Soc. 2006, 128, 14042–14043. [Google Scholar] [CrossRef] [PubMed]
- Takao, K.; Mori, K.; Kasuga, K.; Nanamiya, R.; Namba, A.; Fukushima, Y.; Nemoto, R.; Mogi, T.; Yasui, H.; Ogura, A.; et al. Total Synthesis of Clavilactones. J. Org. Chem. 2018, 83, 7060–7075. [Google Scholar] [CrossRef] [PubMed]
- Arnone, A.; Cardillo, R.; Meille, S.V.; Nasini, G.; Tolazz, M. Secondary mould metabolites. Part 47. isolation and structure elucidation of clavilactones A-C, new metabolites from the fungus Clitocybe clavipes. J. Chem. Soc. Perkin Trans. 1 1994, 2165–2168. [Google Scholar] [CrossRef]
- Merlini, L.; Nasini, G.; Scaglioni, L.; Cassinelli, G.; Lanzi, C. Structure elucidation of clavilactone D: An inhibitor of protein tyrosine kinases. Phytochemistry 2000, 53, 1039–1041. [Google Scholar] [CrossRef]
- Kawagishi, H.; Miyazawa, T.; Kume, H.; Arimoto, Y.; Inakuma, T. Aldehyde dehydrogenase inhibitors from the mushroom Clitocybe clavipes. J. Nat. Prod. 2002, 65, 1712–1714. [Google Scholar] [CrossRef] [PubMed]
- Cassinelli, G.; Lanzi, C.; Pensa, T.; Gambetta, R.A.; Nasini, G.; Cuccuru, G.; Cassinis, M.; Pratesi, G.; Polizzi, D.; Tortoreto, M.; et al. Clavilactones, a novel class of tyrosine kinase inhibitors of fungal origin. Biochem. Pharmacol. 2000, 59, 1539–1547. [Google Scholar] [CrossRef]
- Sun, Z.; Zhu, N.; Zhou, M.; Huo, X.; Wu, H.; Tian, Y.; Yang, J.; Ma, G.; Yang, Y.L.; Xu, X. Clavipines A–C, antiproliferative meroterpenoids with a fused azepine skeleton from the basidiomycete Clitocybe clavipes. Org. Chem. Front. 2019. [Google Scholar] [CrossRef]
- Yao, X.S.; Ebizuka, Y.; Noguchi, H.; Kiuchi, F.; Shibuya, M.; Iitaka, Y.; Seto, H.; Ushio, S. Biologically active constituents of arnebia euchroma: Structure of arnebinol, an ansa-type monoterpenylbenzenoid with inhibitory activity on prostaglandin biosynthesis. Chem. Pharm. Bull. 1991, 39, 2956–2961. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Hung, H.C.; Sung, P.J.; Chen, I.S.; Kuo, W.L. Aporphine alkaloids and cytotoxic lignans from the roots of Illigera luzonensis. Phytochemistry 2011, 72, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Feng, T.; Li, Z.H.; Dong, Z.J.; Li, Y.; Liu, J. Highly oxygenated meroterpenoids from fruiting bodies of the mushroom Tricholoma terreum. J. Nat. Prod. 2013, 76, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds (1–5) are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).