The Early Years of 2,2′-Bipyridine—A Ligand in Its Own Lifetime
Abstract
1. Introduction
2. Discovery and the Discoverer
3. Fundamentals
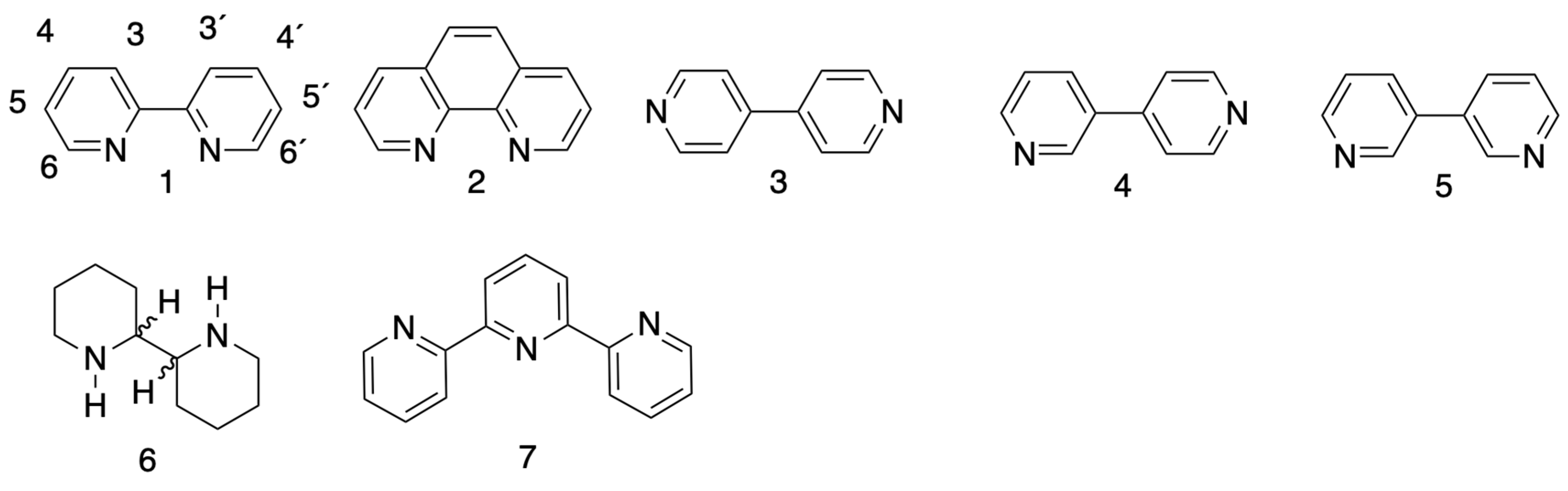
3.1. Nomenclature
3.2. Structure
3.3. Protonation
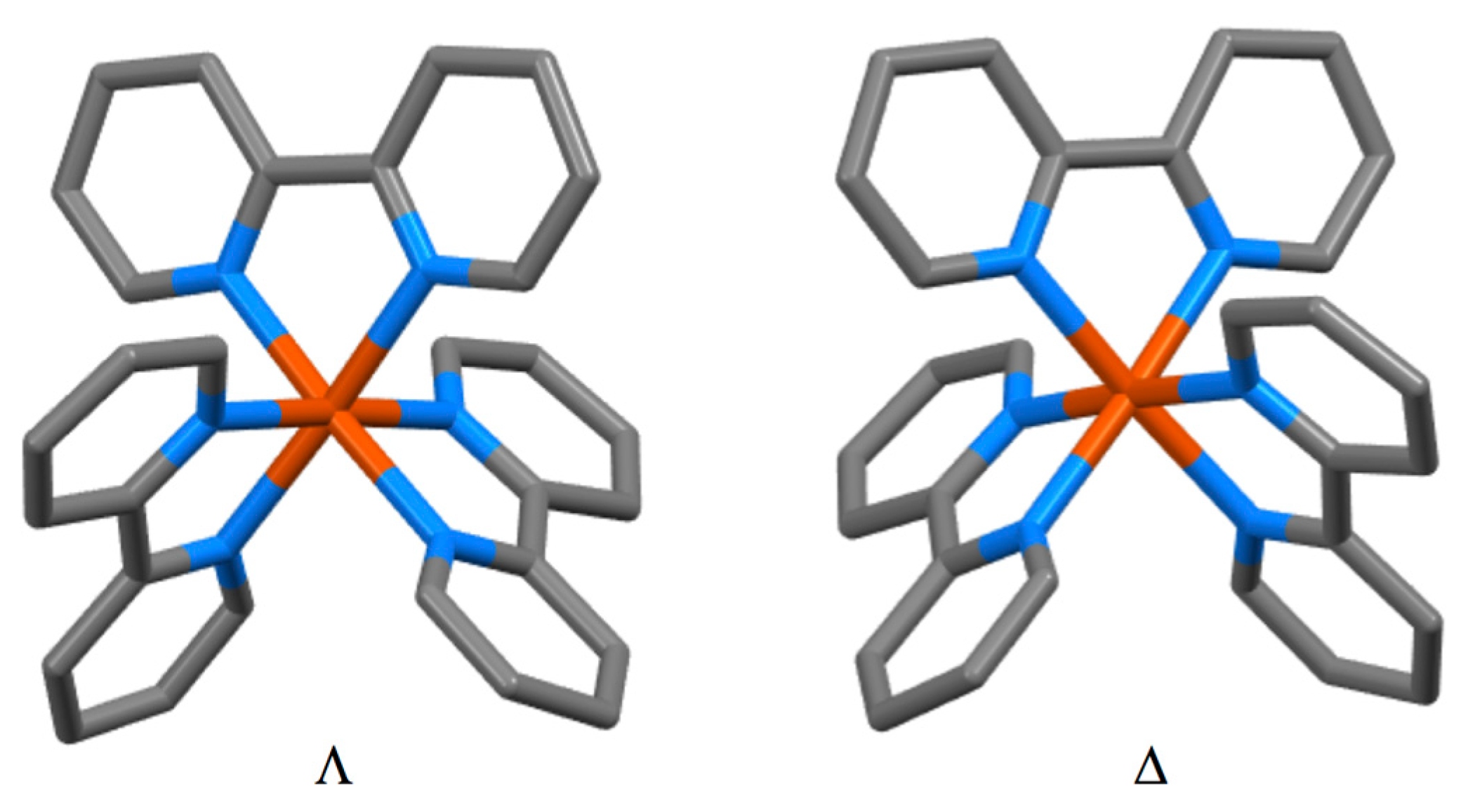
3.4. Coordination Behaviour
4. The First 30 Years—from 1899 to 1929
4.1. Physicochemical Properties
4.2. Biological Activity
4.3. Synthetic Developments
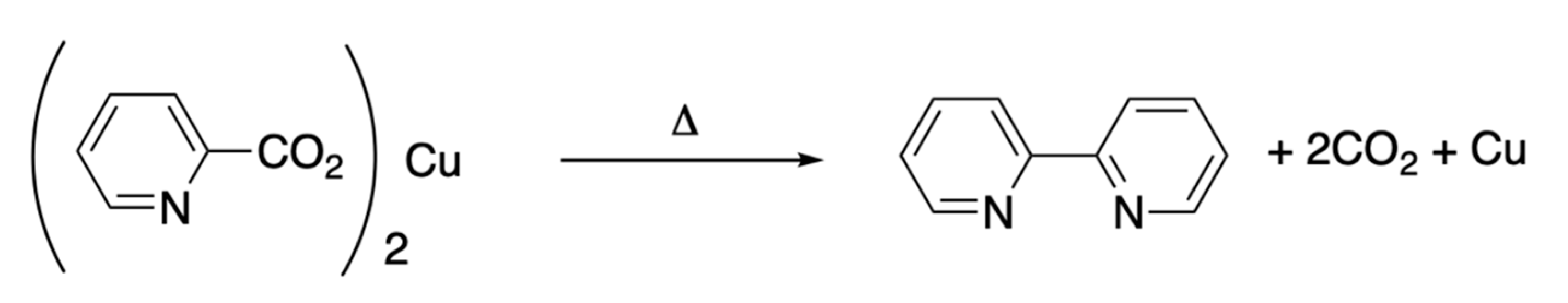
4.3.1. Dimerization with Electropositive Metals
4.3.2. Reductive Coupling of a 2-Functionalized Pyridine
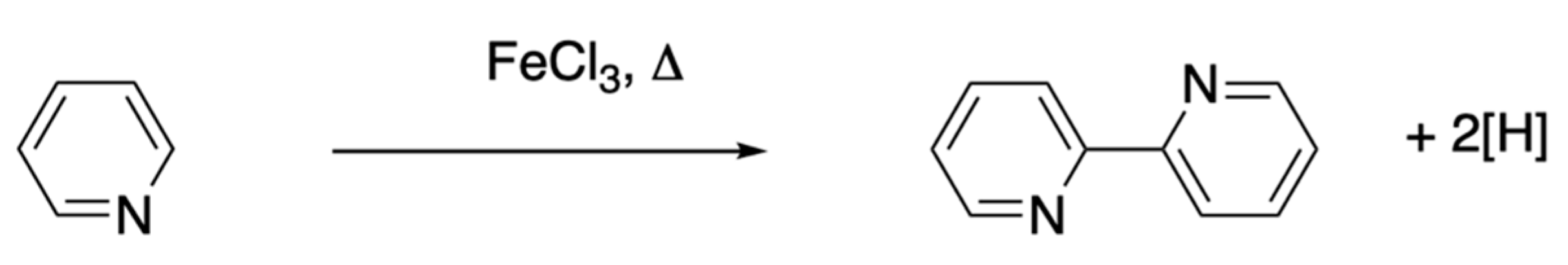
4.3.3. Oxidation and Dehydrogenation of Pyridines and Piperidines
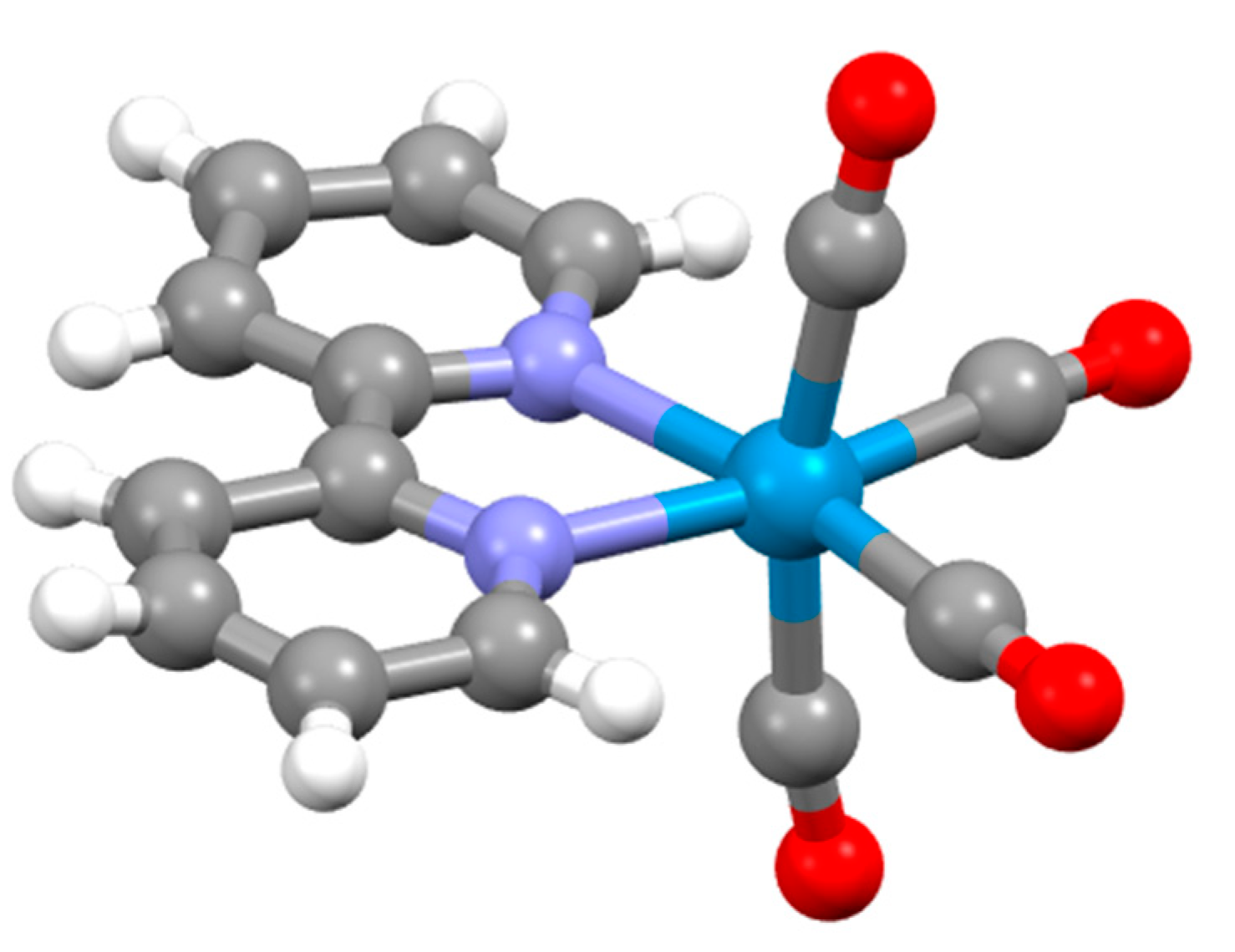
4.4. Coordination Chemistry
5. 1930–1939—Golden Years and then Back into the Abyss
5.1. Synthetic Developments
5.1.1. Oxidation and Dehydrogenation of Pyridines
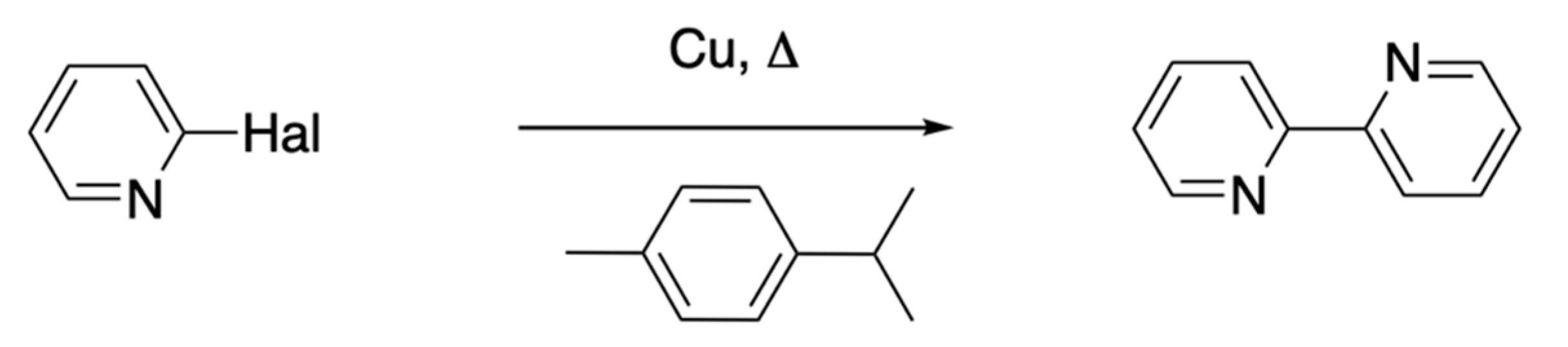
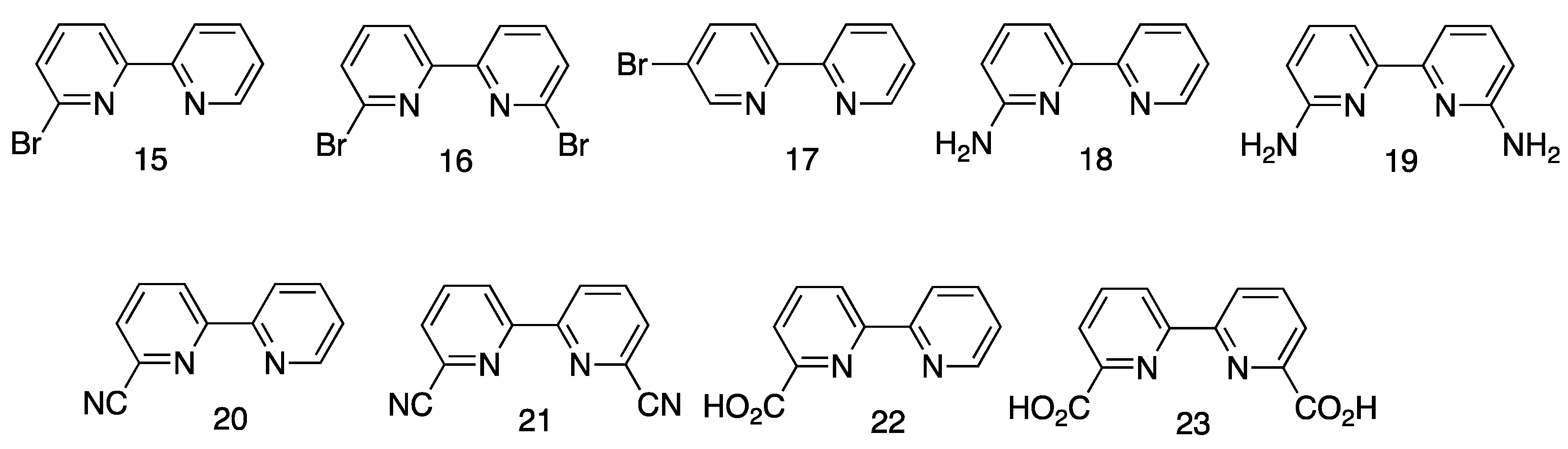
5.1.2. Other Routes to 2,2′-Bipyridines
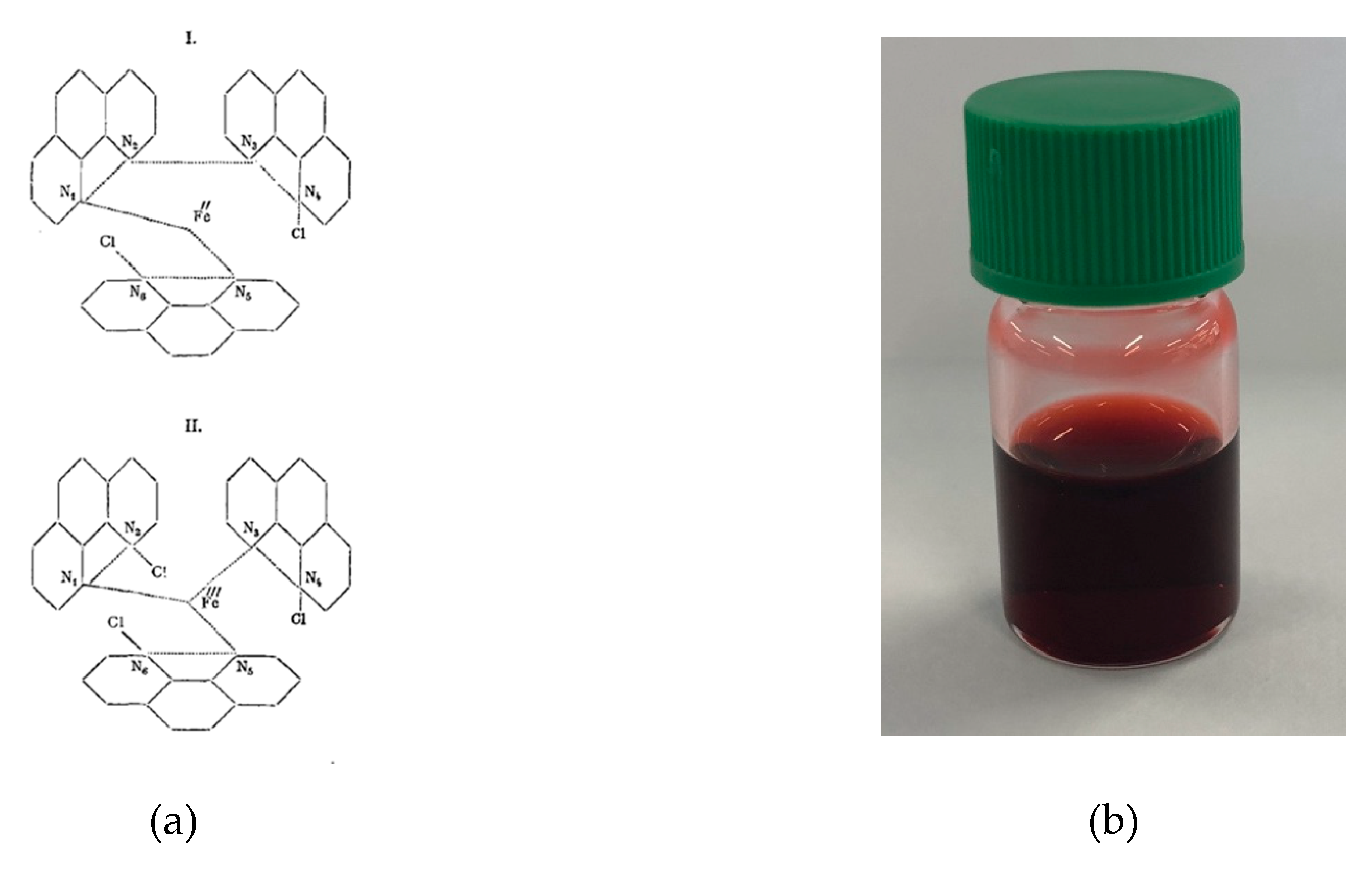
5.2. Coordination Chemistry
5.2.1. Complexes of Elements in Groups 1 and 2
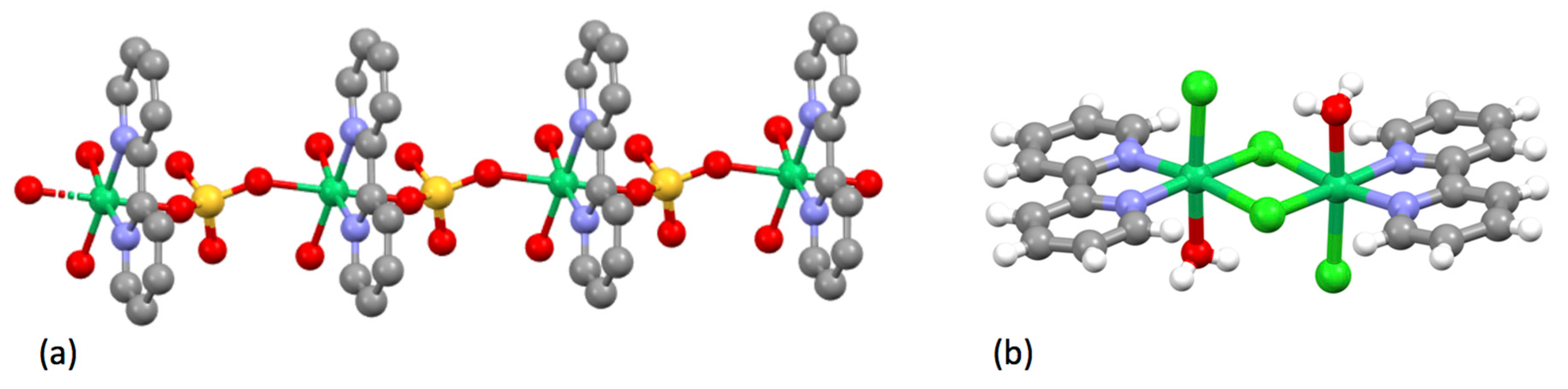
5.2.2. Complexes of Elements in Groups 3 to 12
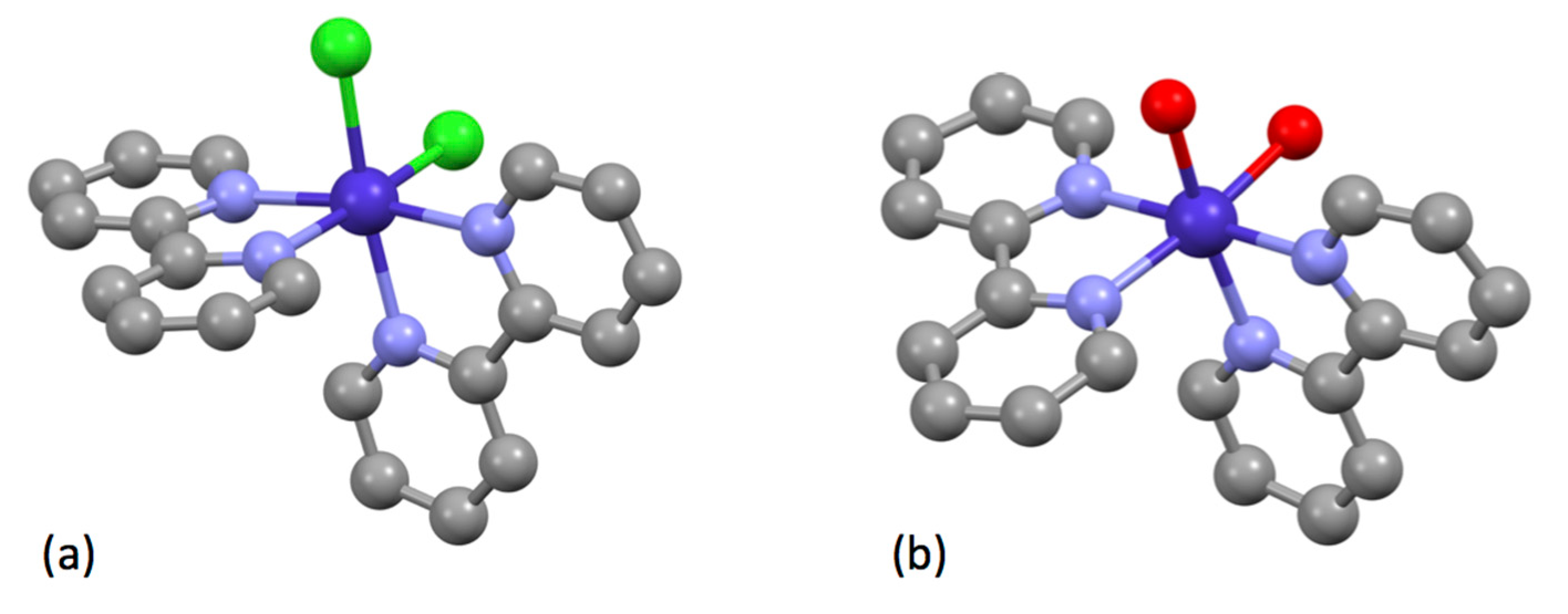
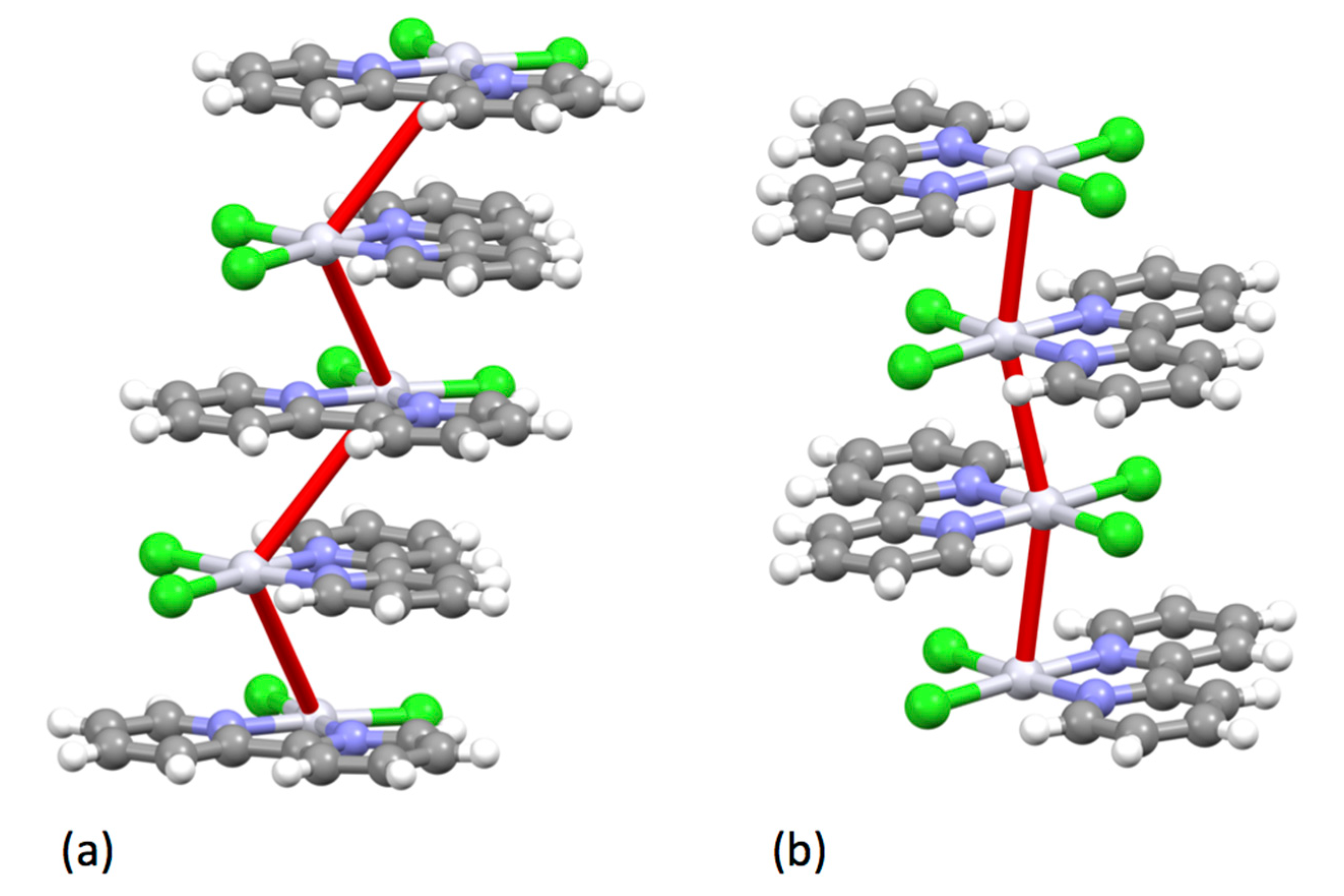
5.2.3. Complexes of Elements in Groups 13 to 18
5.3. Analytical Applications
5.4. Biological Studies
5.5. Physicochemical Properties
5.6. Other Applications
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaes, C.; Katz, A.; Hosseini, M.W. Bipyridine: The most widely used ligand. A review of molecules comprising at least two 2,2′-bipyridine units. Chem. Rev. 2000, 100, 3553–3590. [Google Scholar] [CrossRef] [PubMed]
- Summers, L.A. The bipyridines. Adv. Heterocycl. Chem. 1984, 35, 281–374. [Google Scholar] [CrossRef]
- Summers, L.A. The phenanthrolines. Adv. Heterocycl. Chem. 1978, 24, 1–69. [Google Scholar] [CrossRef]
- Constable, E.C. Homoleptic complexes of 2,2′-bipyridine. Adv. Inorg. Chem. 1989, 34, 1–63. [Google Scholar] [CrossRef]
- Accorsi, G.; Listorti, A.; Yoosaf, K.; Armaroli, N. 1,10-Phenanthrolines: Versatile building blocks for luminescent molecules, materials and metal complexes. Chem. Soc. Rev. 2009, 38, 1690–1700. [Google Scholar] [CrossRef]
- Bencini, A.; Lippolis, V. 1,10-Phenanthroline: A versatile building block for the construction of ligands for various purposes. Coord. Chem. Rev. 2010, 254, 2096–2180. [Google Scholar] [CrossRef]
- Lindoy, L.F.; Livingstone, S.E. Complexes of iron(II),cobalt(II) and nickel(II) with α-diimines and related bidentate ligands. Coord. Chem. Rev. 1967, 2, 173–193. [Google Scholar] [CrossRef]
- McWhinnie, W.R.; Miller, J.D. The chemistry of complexes containing 2,2′-bipyridyl, 1, 10-phenanthroline, or 2,2′,6′,2”-terpyridyl as ligands. Adv. Inorg. Chem. Radiochem. 1970, 12, 135–215. [Google Scholar] [CrossRef]
- Brandt, W.W.; Dwyer, F.P.; Gyarfas, E.D. Chelate complexes of 1,10-phenanthroline and related compounds. Chem. Rev. 1954, 54, 959–1017. [Google Scholar] [CrossRef]
- Constable, E.C. The Coordination Chemistry of 2,2′:6′,2″-Terpyridine and Higher Oligopyridines. Adv. Inorg. Chem. 1986, 30, 69–121. [Google Scholar] [CrossRef]
- Fallahpour, R.-A. The Higher Oligopyridines and their Metal Complexes. Curr. Org. Synth. 2006, 3, 19–39. [Google Scholar] [CrossRef]
- Constable, E.C. Higher Oligopyridines as a Structural Motif in Metallosupramolecular Chemistry. Prog. Inorg. Chem. 1994, 42, 67–138. [Google Scholar] [CrossRef]
- Schubert, U.S.; Winter, A.; Newkome, G.R. Terpyridine-Based Materials: For Catalytic, Optoelectronic and Life Science Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar] [CrossRef]
- Constable, E.C. 2,2′:6′,2″-Terpyridines: From chemical obscurity to common supramolecular motifs. Chem. Soc. Rev. 2007, 36, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar] [CrossRef]
- Blau, F. Die Destillation pyridinmonocarbonsaurer Salze. Ber. Dtsch. Chem. Ges. 1888, 21, 1077–1078. [Google Scholar] [CrossRef]
- Weidel, H. Studien über Verbindungen aus dem animalischen Theer. Ber. Dtsch. Chem. Ges. 1879, 12, 1077–1078. [Google Scholar] [CrossRef]
- Anderson, T. Ueber die Producte der trockenen Destillation thierischer Materien. Ann. Chem. Pharm. 1870, 154, 270–286. [Google Scholar] [CrossRef]
- Anderson, T. On the products of the destructive distillation of animal substances. J. Chem. Soc. 1869, 22, 406–417. [Google Scholar] [CrossRef]
- Weidel, H.; Russo, M. Studien über das Pyridin. Monatsh. Chem. 1882, 3, 850–885. [Google Scholar] [CrossRef]
- Emmert, B. Über Verbindungen des Pyridins mit den Alkalimetallen. III. Mitteilung. Ber. Dtsch. Chem. Ges. 1917, 50, 31–35. [Google Scholar] [CrossRef]
- Smith, C.R. Dipyridyls from pyridine. J. Am. Chem. Soc. 1924, 46, 414–419. [Google Scholar] [CrossRef]
- Hartley, W.N. Researches on the relation between the molecular structure of carbon compounds and their absorption spectra. (Part VII). J. Chem. Soc. Trans. 1885, 47, 685–757. [Google Scholar] [CrossRef]
- Heuser, A.; Stoehr, C. 4. Ueber methylirte Dipyridyle; I. Abhandlung. J. Prakt. Chem. 1890, 42, 429–440. [Google Scholar] [CrossRef]
- Heuser, A.; Stoehr, C. Ueber methylirte Dipyridyle; II. Abhandlung. J. Prakt. Chem. 1891, 44, 404–410. [Google Scholar] [CrossRef]
- Stoehr, C.; Wagner, M. Ueber methylirte Dipyridyle. J. Prakt. Chem. 1893, 48, 1–16. [Google Scholar] [CrossRef]
- Blau, F. Über die trockene Destillation von pyridincarbonsauren Salzen. Monatsh. Chem. 1889, 10, 375–388. [Google Scholar] [CrossRef]
- Blau, F. Neuerungen beim gebräuchlichen Verbrennungsverfahren. Monatsh. Chem. 1889, 10, 357–371. [Google Scholar] [CrossRef]
- Blau, F. Über neue organische Metallverbindungen. Monatsh. Chem. 1898, 19, 647–689. [Google Scholar] [CrossRef]
- Werner, A. Beitrag zur Konstitution anorganische Verbindungen. Z. Anorg. Chem. 1893, 3, 267–330. [Google Scholar] [CrossRef]
- Tobies, R. Iris Runge: A Life at the Crossroads of Mathematics, Science, and Industry; Springer: Basel, Switzerland, 2012. [Google Scholar]
- Tobies, R. Morgen möchte ich wieder 100 herrliche Sachen ausrechnen. In Iris Runge bei Osram und Telefunken; Franz Steiner: Stuttgart, Germany, 2010. [Google Scholar]
- Pirani, M. Fritz Blau. Naturwissenschaften 1930, 18, 97–101. [Google Scholar] [CrossRef]
- Favre, H.A.; Powell, W.H. Nomenclature of Organic Chemistry—IUPAC Recommendations and Preferred Names 2013; Rule P-13.5.1; Royal Society of Chemistry: Cambridge, UK, 2013; p. 19. [Google Scholar]
- Connelly, N.G.; Damhus, T.; Hartshorn, R.M.; Hutton, A.T. Nomenclature of Inorganic Chemistry: IUPAC Recommendations; Issued by the Division of Chemical Nomenclature and Structure Representation in collaboration with the Division of Inorganic Chemistry; RSC Publishing: Cambridge, UK, 2005; pp. 261–268. [Google Scholar]
- Favre, H.A.; Powell, W.H. Nomenclature of Organic Chemistry—IUPAC Recommendations and Preferred Names 2013; Rule P-25.2.1; Royal Society of Chemistry: Cambridge, UK, 2013; p. 211. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Merritt jr, L.L.; Schroeder, E.D. The crystal structure of 2,2′-bipyridine. Acta Crystallogr. 1956, 9, 801–803. [Google Scholar] [CrossRef]
- Nakatsu, K.; Yoshioka, H.; Matsui, M.; Koda, S.; Ooi, S. Structural studies on 2,2′-bipyridinium and 1,10-phenanthrolinium cations. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1972, 28, S24. [Google Scholar]
- Cagle, F.W. A preliminary examination of the crystal structure of 2,2′-bipyridyl and its relation to biphenyl. Acta Crystallogr. 1948, 1, 158–159. [Google Scholar] [CrossRef]
- Chisholm, M.H.; Huffman, J.C.; Rothwell, I.P.; Bradley, P.G.; Kress, N.; Woodruff, W.H. Bis(2,2′-bipyridyl)diisopropoxymolybdenum(II). Structural and spectroscopic evidence for molybdenum-to-bipyridyl π* bonding. J. Am. Chem. Soc. 1981, 103, 4945–4947. [Google Scholar] [CrossRef]
- Kuhn, F.E.; Groarke, M.; Bencze, E.; Herdtweck, E.; Prazeres, A.; Santos, A.M.; Calhorda, M.J.; Romao, C.C.; Goncalves, I.S.; Lopes, A.D.; et al. Octahedral bipyridine and bipyrimidine dioxomolybdenum(VI) complexes: Characterization, application in catalytic epoxidation, and density functional mechanistic study. Chem. Eur. J. 2002, 8, 2370–2383. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Roussel, C. A theoretical study of the conformation, basicity and NMR properties of 2,2′-, 3,3′- and 4,4′-bipyridines and their conjugated acids. Comput. Theor. Chem. 2011, 966, 334–339. [Google Scholar] [CrossRef]
- Krishnan, C.V.; Creutz, C.; Schwarz, H.A.; Sutin, N. Reduction potentials for 2,2′-bipyridine and 1,10-phenanthroline couples in aqueous solutions. J. Am. Chem. Soc. 1983, 105, 5617–5623. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. More Hydra than Janus—Non-classical coordination modes in complexes of oligopyridine ligands. Coord. Chem. Rev. 2017, 350, 84–104. [Google Scholar] [CrossRef]
- Purvis, J.E. The absorption spectra of various derivatives of pyridine, piperidine, and piperazine in solution and as vapours. J. Chem. Soc. Trans. 1913, 103, 2283–2296. [Google Scholar] [CrossRef]
- IUPAC Gold Book. Available online: https://goldbook.iupac.org/terms/view/A00511 (accessed on 9 August 2019).
- Mascarelli, L. Diphenyl and its derivatives (and dinaphthyls). Cleavage into optical antipodes of compounds which do not contain asymmetric atoms. II. Gazz. Chim. Ital. 1928, 58, 627–630. [Google Scholar]
- Summers, L.A. The Bipyridinium Herbicides; Academic Press: London, UK, 1980. [Google Scholar]
- Richardson, C.H.; Smith, C.R. Studies on Contact Insecticides; United States Department of Agriculture: Washington, DC, USA, 1923. [Google Scholar]
- Richardson, C.H.; Smith, C.R. Toxicity of dipyridyls and certain other organic compounds as contact insecticides. J. Agric. Res. 1923, 33, 597–609. [Google Scholar] [CrossRef]
- Smith, C.R.; Richardson, C.H.; Shepard, H.H. Neonicotine and certain other derivatives of the dipyridyls as insecticides. J. Econ. Entomol. 1930, 23, 863–867. [Google Scholar] [CrossRef]
- Smith, C.R. Neonicotine and isomeric pyridylpiperidines. J. Am. Chem. Soc. 1931, 53, 277–283. [Google Scholar] [CrossRef]
- Emmert, B. Über die Verbindungen des Pyridins und Picolins mit metallischem Natrium. Ber. Dtsch. Chem. Ges. 1914, 47, 2598–2601. [Google Scholar] [CrossRef]
- Emmert, B. Über Verbindungen des Pyridins mit den Alkalimetallen. Ber. Dtsch. Chem. Ges. 1916, 49, 1060–1062. [Google Scholar] [CrossRef]
- Emmert, B.; Buchert, R. Über Verbindungen des Pyridins mit den Alkalimetallen (IV). Ber. dtsch. Chem. Ges. A/B 1921, 54, 204–209. [Google Scholar] [CrossRef][Green Version]
- Weitz, E.; Nelken, A.; Ludwig, R. Das Benzylpyridinium. Justus Liebigs Ann. Chem. 1921, 425, 187–207. [Google Scholar] [CrossRef]
- Hofmann, A.W. Zur Geschichte der Pyridinbasen. Ber. Dtsch. Chem. Ges. 1881, 14, 1497–1506. [Google Scholar] [CrossRef]
- Emmert, B. Über die Konstitution der von A. W. Hofmann aufgefundenen Dialkyl-tetrahydro-dipyridyle. Ber. Dtsch. Chem. Ges. A/B 1919, 52, 1351–1353. [Google Scholar] [CrossRef]
- Weitz, E.; Ludwig, R. Über freie Ammonium Radikale, III. Mitteilung: Die Existenz des N-Benzyl-pyridiniums. Ber. Dtsch. Chem. Ges. A/B 1922, 55, 395–413. [Google Scholar] [CrossRef]
- Weitz, E.; König, T.; von Wistinghausen, L. Über freie Ammonium-Radikale, V.: Vergleich von N,N′-Dibenzyl- und N,N′-Diphenyl-γ,γ′-dipyridinium. Ber. Dtsch. Chem. Ges. A/B 1924, 57, 153–175. [Google Scholar] [CrossRef]
- Weitz, E.; König, T. Über freie Ammoniumradikale, IV. Mitteilung: Weitere Untersuchungen über das NN′-Dibenzyl-γ,γ′-dipyridinium und seine Homologen, sowie die sog. NN′-disubstituierten Tetrahydro-γ,γ′-dipyridyle. Ber. Dtsch. Chem. Ges. A/B 1922, 55, 2864–2889. [Google Scholar] [CrossRef]
- Emmert, B.; Varenkamp, O. Über N,N′-Dialkyl-[tetrahydro-γ,γ′-dipyridyle]. Ber. Dtsch. Chem. Ges. A/B 1922, 55, 2322–2326. [Google Scholar] [CrossRef]
- Emmert, B.; Jungck, G.; Häffner, H. Über chinhydron-artige Verbindungen des Dihydro-γ,γ-dipyridyls. Ber. Dtsch. Chem. Ges. A/B 1924, 57, 1792–1797. [Google Scholar] [CrossRef]
- Mumm, O.; Ludwig, H. Zur Kenntnis der N,N′-Dialkyl-[tetrahydro-dipyridyle]. Ber. Dtsch. Chem. Ges. A/B 1926, 59, 1605–1616. [Google Scholar] [CrossRef]
- Mumm, O.; Roder, O.; Ludwig, H. Zur Kenntnis der N,N′-Dialkyl-[tetrahydro-dipyridyle]. Ber. Dtsch. Chem. Ges. A/B 1924, 57, 865–880. [Google Scholar] [CrossRef]
- Emmert, B.; Werb, O. Über N,N′-Dimethyl-[tetrahydro-γ,γ′-dikollidyl]. Ber. Dtsch. Chem. Ges. A/B 1922, 55, 1352–1358. [Google Scholar] [CrossRef]
- Emmert, B.; Varenkamp, O. Über chinhydronartige Verbindungen der N,N′-Dialkyl-[dihydro-γ,γ′-dipyridyle]. Ber. Dtsch. Chem. Ges. A/B 1923, 56, 491–501. [Google Scholar] [CrossRef]
- Dimroth, O.; Heene, R. Reduktion von Pyridin mit Zinkstaub und Essigsäure-anhydrid. Ber. Dtsch. Chem. Ges. A/B 1921, 54, 2934–2942. [Google Scholar] [CrossRef][Green Version]
- Dimroth, O.; Frister, F. Reduktion von Pyridin mit Zinkstaub und Essigsäure-anhydrid (2. Mitteilung). Ber. Dtsch. Chem. Ges. A/B 1922, 55, 1223–1232. [Google Scholar] [CrossRef]
- Fanta, P.E. The Ullmann Synthesis of Biaryls. Synthesis 1974, 1947, 9–21. [Google Scholar] [CrossRef]
- Wibaut, J.P.; Overhoff, J. Über eine neue Darstellungsmethode von 2,2′-Dipyridyl. Recl. Trav. Chim. Pays Bas 1928, 47, 761–763. [Google Scholar] [CrossRef]
- Meyer, H.; Hofmann-Meyer, A. Über Pyrokondensationen in der Pyridinreihe. J. Prakt. Chem. 1921, 102, 287–294. [Google Scholar] [CrossRef]
- Wibaut, J.P.; van de Lande, L.M.F. Sur la Formation de L’aminopyridine par L’action du gaz Ammoniac sur la Pyridine en Présence de Catalyseurs. Recl. Trav. Chim. Pays-Bas 1929, 48, 1005–1009. [Google Scholar] [CrossRef]
- Wibaut, J.P.; Dingemanse, E. Über eine Pyrogene Umlagerung von n-Methyl-(α-Pyridyl)-α-Pyrrol. Recl. Trav. Chim. Pays-Bas 1926, 45, 671–673. [Google Scholar] [CrossRef]
- Sabatier, P.; Fernandez, A. Déshydrogénations et hydrogénations catalysées par des oxydes métalliques. C. R. Hebd. Seances Acad. Sci. 1927, 185, 241–244. [Google Scholar]
- Hein, F.; Retter, W. Eine neue Methode zur Darstellung von α,α′-Dipyridyl. Ber. Dtsch. Chem. Ges. A/B 1928, 61, 1790–1791. [Google Scholar] [CrossRef]
- Werner, A. Über Spiegelbild-Isomerie bei Eisenverbindungen. Ber. Dtsch. Chem. Ges. 1912, 45, 433–436. [Google Scholar] [CrossRef]
- Freudlich, H.; Birstein, V. Über einige Eigenschaften der Blauschen Komplexsalze. Kolloidchem. Beih. 1926, 23, 27–40. [Google Scholar] [CrossRef]
- Morgan, G.T.; Drew, H.D.K. Researches on residual affinity and co-ordination. Part II. Acetylacetones of selenium and tellurium. J. Chem. Soc. Trans. 1920, 117, 1456–1465. [Google Scholar] [CrossRef]
- Lowry, T.M. Stability of co-ordination compounds. J. Soc. Chem. Ind. London Rev. Sect. 1923, 42, 711–715. Available online: https://archive.org/details/ost-chemistry-chemistryindustr01soci/page/n735 (accessed on 12 August 2019). [CrossRef]
- Smith, J.D.M. Chelate co-ordination. J. Soc. Chem. Ind. Lond. Rev. Sect. 1923, 42, 847–850. [Google Scholar] [CrossRef]
- Lowry, T.M. Stability of co-ordination compounds. J. Soc. Chem. Ind. London Rev. Sect. 1923, 42, 1004–1007. Available online: https://archive.org/details/ost-chemistry-chemistryindustr01soci/page/n1029 (accessed on 12 August 2019). [CrossRef]
- Biltz, W. Über Molekular- und Atomvolumina. XVII. Zur Raumchemie und Magnetochemie fester Cyanide. Z. Anorg. Allg. Chem. 1928, 170, 161–183. [Google Scholar] [CrossRef]
- Hein, F.; Schwedler, H. Zur α, α′-Dipyridyl-Synthese aus Pyridin und Eisen(III)-chlorid, sowie über einige Dipyridyl-Komplexsalze. Ber. Dtsch. Chem. Ges. B 1935, 68, 681–684. [Google Scholar] [CrossRef]
- Jaeger, F.M.; van Dijk, J.A. Complex salts with α,α′-dipyridyl. Complex salts of bivalent nickel. Proc. K. Ned. Akad. Wet. 1934, 37, 10–15. [Google Scholar]
- Morgan, G.T.; Burstall, F.H. Dehydrogenation of pyridine by anhydrous ferric chloride. J. Chem. Soc. 1932, 20–30. [Google Scholar] [CrossRef]
- Morgan, G.T.; Burstall, F.H. Dehydrogenation of pyridine by anhydrous metallic chlorides. J. Indian Chem. Soc. Prafulla Chandra Ray Commemoration Vol. 1933, 1–16. [Google Scholar]
- Willink, H.D.T., Jr.; Wibaut, J.P. Preparation of 2,2′-bipyridyl and some of its derivatives. Recl. Trav. Chim. Pays-Bas 1935, 54, 275–283. [Google Scholar] [CrossRef]
- Thate, H. The hydrogenation of pyridine with hydrogen under pressure by the Bergius process. Recl. Trav. Chim. Pays-Bas 1931, 50, 77–90. [Google Scholar] [CrossRef]
- Burstall, F.H. Researches on the Polypyridyls. J. Chem. Soc. 1938, 1662–1672. [Google Scholar] [CrossRef]
- Wibaut, J.P.; Willink, H.D.T. Eine Darstellungsmethode von 22″-Dipyridyl durch Katalytische Dehydrierung von Pyridin unter Druck. Recl. Trav. Chim. Pays-Bas 1931, 50, 287–290. [Google Scholar] [CrossRef]
- Wibaut, J.P.; Van De Lande, L.M.F. Über die Einwirkung von Ammoniakgas auf Pyridin und 2-Methylpyridin in Gegenwart von Dehydrogenations-Katalysatoren. (Zweite Mitteilung). Recl. Trav. Chim. Pays-Bas 1931, 50, 1056–1059. [Google Scholar] [CrossRef]
- Kabachnik, M.I. The mechanism of the reaction of pyridine and its derivatives with the alkali metal amides. Bull. Acad. Sci. U. R. S. S. Classe Sci. Math. Nat. 1935, 971–976. [Google Scholar]
- Smith, C.R. Skraup’s reaction applied to the phenylenediamines. Preparation of the phenanthrolines and related bipyridyls. J. Am. Chem. Soc. 1930, 52, 397–403. [Google Scholar] [CrossRef]
- Barbieri, G.A.; Tettamanzi, A. Compounds of bivalent chromium. ATTI Accad. Naz. Lincei Cl. Sci. Fis. Mat. Nat. Rend. 1932, 15, 877–882. [Google Scholar]
- Balthis, J.H., Jr.; Bailar, J.C., Jr. Some chromous and chromic ammines. J. Am. Chem. Soc. 1936, 58, 1474–1476. [Google Scholar] [CrossRef]
- Komarovskii, A.S.; Poluektov, N.S. Color reaction of molybdenum with α,α‘-bipyridyl. Zh. Prikl. Khim. 1937, 10, 565–566. [Google Scholar]
- Komarovskii, A.S.; Poluektov, N.S. Über eine Farbreaktion auf Molybdän mit α-α′-Dipyridyl. Microchim. Acta 1937, 1, 264–266. [Google Scholar]
- Hieber, W.; Romberg, E. Über Metallcarbonyle. XXII. Derivate des Wolframhexacarbonyls. Z. Anorg. Allg. Chem. 1935, 221, 349–353. [Google Scholar] [CrossRef]
- Ye, Q.; Wu, Q.; Zhao, H.; Song, Y.-M.; Xue, X.; Xiong, R.-G.; Pang, S.-M.; Lee, G.-H. Two noncentrosymmetric complexes: [W(CO)4(bipy)] and [W(CO)4(phen)] (bipy=2,2′-bipyridine, phen=1,10-phenanthroline) obtained through solvothermal synthesis and their optical properties. J. Organomet. Chem. 2005, 690, 286–290. [Google Scholar] [CrossRef]
- Chapman, J.; Kolawole, G.; Long, N.; White, A.J.P.; Williams, D.J.; O′Brien, P. Syntheses and X-ray structural analyses of the mononuclear tungsten hexacarbonyl complexes of 2,2′-bipyridine and 2,2′-bipyrimidine. S. Afr. J. Sci. 2005, 101, 454. [Google Scholar]
- Morgan, G.T.; Davies, G.R. Residual affinity and co-ordination. XL. Some complex compounds of rhenium. J. Chem. Soc. 1938, 1858–1861. [Google Scholar] [CrossRef]
- Akimov, V.M.; Romero de Endara, M.; Babeshkina, G.K.; Stepanovich, V.M.; Dolganev, V.P. Mono- and bis(α,α’-dipyridylium) hexachlororhenates. Izv. Vyssh. Uchebn. Zaved., Khim. Khim. Tek. 1971, 14, 1835–1840. [Google Scholar]
- Englert, U.; Koelle, U.; Nageswara, R.N. Crystal structure of bis(2,2′-bipyridinium-(1-H))-hexachlororhenate, (C10H9N2)2ReCl6. Z. Krist. Cryst. Mater. 1994, 209, 780. [Google Scholar] [CrossRef]
- Ferrari, C. Analytical applications of α,α’-bipyridine. Ann. Chim. Appl. 1937, 27, 479–482. [Google Scholar]
- Ferrari, C. Researches on the ferrotribipyridylic complex. Gazz. Chim. Ital. 1937, 67, 604–608. [Google Scholar]
- Barbieri, G.A.; Ferrari, C. A reversible reaction between complex metalammine ions and hydrogen ions. Ric. Sci. 1936, 7, 390–391. [Google Scholar]
- Jaeger, F.M.; van Dijk, J.A. Complex salts α,α’-bipyridyl with bivalent iron. Proc. K. Ned. Akad. Wet. 1934, 37, 333–336. [Google Scholar]
- Cambi, L.; Cagnasso, A. The structure and magnetic susceptibility of complex ferrous compounds. Gazz. Chim. Ital. 1933, 63, 767–778. [Google Scholar]
- Simon, A.; Knauer, H. Active iron. XII. Magnetic character of a few complex iron salts. Z. Elektrochem. 1939, 45, 678–685. [Google Scholar]
- Klemm, W. Über physikalische Methoden im chemischen Laboratorium. XVIII. Die Bedeutung magnetischer Messungen für chemische Fragen. Angew. Chem. 1931, 44, 250–259. [Google Scholar] [CrossRef]
- Klemm, W. Die Bedeutung magnetischer Messungen für chemische Fragen. II. Angew. Chem. 1935, 48, 617–624. [Google Scholar] [CrossRef]
- Cambi, L.; Cagnasso, A. New types of complex paramagnetic salts of the iron series. Rend. Lombardo Sci. 1934, 67, 741–747. [Google Scholar]
- Barbieri, G.A. Compounds intermediate between ferrocyanides and ferroamines. ATTI Accad. Naz. Lincei Cl. Sci. Fis. Mat. Nat. Rend. 1934, 20, 273–278. [Google Scholar]
- Jaeger, F.M.; van Dijk, J.A. Die verschiedenen Typen von Komplexsalzen des α-α′-Dipyridyls mit Kupfer, Zink, Cadmium, Eisen, Nickel, Kobalt und Rhodium. Z. Anorg. Allg. Chem. 1936, 227, 273–327. [Google Scholar] [CrossRef]
- Belamri, Y.; Setifi, F.; Francuski, B.M.; Novaković, S.; Zouaoui, S. Crystal structure of tetraaqua(5,5′-dimethyl-2,2′-bipyridyl-κ2N,N′)iron(II) sulfate. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, 544–546. [Google Scholar] [CrossRef]
- Kuhn, R.; Wassermann, A. Komplexbildung und Katalyse, hochaktive Zwischenstufen. Justus Liebigs Ann. Chem. 1933, 503, 203–232. [Google Scholar] [CrossRef]
- Simon, A.; Haufe, W. Über aktives Eisen. IX. Mitteilung. Über die αα′-Dipyridyl- und die o-Phenantrolinreaktion. Z. Anorg. Allg. Chem. 1936, 230, 160–175. [Google Scholar] [CrossRef]
- Simon, A.; Reetz, T. Über aktives Eisen XI. Mitteilung Die Ferrisalzkatalyse im System Oxalsäure-Hydroperoxyd und Oxalsäure-Sublimat-Hydroperoxyd. Z. Anorg. Allg. Chem. 1937, 231, 217–237. [Google Scholar] [CrossRef]
- Manchot, W.; Pflaum, W. Über den Mechanismus der Oxydationsvorgänge und die Oxydation von Eisen(II)salzen durch Wasserstoffsuperoxyd. Z. Anorg. Allg. Chem. 1933, 211, 1–15. [Google Scholar] [CrossRef]
- Simon, A.; Morgenstern, G.; Arbrecht, W.H. Über aktives Eisen X. Mitteilung. Über die magnetische Charakterisierung des Ferrimonodipyridylkomplexes und den Magnetismus einiger komplexer Ferropentacyanide. Z. Anorg. Allg. Chem. 1937, 230, 225–238. [Google Scholar] [CrossRef]
- Shahbazi-Raz, F.; Notash, B.; Amani, V.; Safari, N. 4,4′-Dimethyl-2,2′-bithiazole: Potent co-former in coordination compounds. Polyhedron 2016, 119, 227–237. [Google Scholar] [CrossRef]
- Eckenhoff, W.T.; Biernesser, A.B.; Pintauer, T. Structural characterization and investigation of iron(III) complexes with nitrogen and phosphorus based ligands in atom transfer radical addition (ATRA). Inorg. Chim. Acta 2012, 382, 84–95. [Google Scholar] [CrossRef]
- Driessen, W.L.; de Graaff, R.A.G.; Vos, J.G. Structure of fac-(2,2′-bipyridyl)trichloro(1H-1,2,4-triazole-N4)iron(III), [Fe(C2H3N3)(C10H8N2)Cl3]. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1983, 39, 1635–1637. [Google Scholar] [CrossRef]
- Amani, V.; Safari, N.; Khavasi, H.R.; Mirzaei, P. Iron(III) mixed-ligand complexes: Synthesis, characterization and crystal structure determination of iron(III) hetero-ligand complexes containing 1,10-phenanthroline, 2,2′-bipyridine, chloride and dimethyl sulfoxide, [Fe(phen)Cl3(DMSO)] and [Fe(bipy)Cl3(DMSO)]. Polyhedron 2007, 26, 4908–4914. [Google Scholar] [CrossRef]
- Figgis, B.N.; Reynolds, P.A.; Lehner, N. cis-Bis(bipyridyl)dichloroiron(III) tetrachloroferrate(III), [Fe(bpy)2Cl2][FeCl4]; structure at 4.2 and at 115 K by neutron diffraction. Acta Crystallogr. Sect. B Struct. Sci. 1983, 39, 711–717. [Google Scholar] [CrossRef]
- Figgis, B.N.; Patrick, J.M.; Reynolds, P.A.; Skelton, B.W.; White, A.H.; Healy, P.C. Structural studies in the iron(III)/chloride/α,α’-diimine system. II. Ionic derivatives of stoichiometry FeCl3(phen,bpy)1,1.5. Aust. J. Chem. 1983, 36, 2043–2055. [Google Scholar] [CrossRef]
- Figgis, B.N.; Reynolds, P.A.; White, A.H. Covalent bonding in cis-[Fe(bpy)2Cl2][FeCl4] studied by x-ray diffraction at 120 K. Inorg. Chem. 1985, 24, 3762–3770. [Google Scholar] [CrossRef]
- Witten, E.H.; Reiff, W.M.; Lazar, K.; Sullivan, B.W.; Foxman, B.M. The ferric chloride-.alpha.-diamine system. 3. X-ray crystallographic, magnetic susceptibility, and zero- and high-field Moessbauer spectroscopy investigation of bis(2,2′-bipyridine)dichloroiron(2+) tetrachloroferrate(2–): Slow paramagnetic relaxation and magnetic ordering of complex bimetallic salts. Inorg. Chem. 1985, 24, 4585–4591. [Google Scholar] [CrossRef]
- Morgan, G.T. Recent researches on certain of the rarer elements. J. Chem. Soc. 1935, 554–570. [Google Scholar] [CrossRef]
- Burstall, F.H. Optical activity dependent on co-ordinated bivalent ruthenium. J. Chem. Soc. 1936, 173–175. [Google Scholar] [CrossRef]
- Morgan, G.T.; Burstall, F.H. Residual affinity and co-ordination. XXXIX. Complex ruthenium derivatives containing nitric oxide and polypyridyls. J. Chem. Soc. 1938, 1675–1678. [Google Scholar] [CrossRef]
- Haukka, M.; Venalainen, T.; Ahlgren, M.; Pakkanen, T.A. Reactions of [Ru(bpy)(CO)2Cl2] in Acidic Media: Formation and Structural Characterization of [Ru(bpy)Cl3(NO)], [Ru2N(bpy)2Cl5(H2O)], and (H5O2)[Ru2N(bpy)2Cl6]. Inorg. Chem. 1995, 34, 2931–2936. [Google Scholar] [CrossRef]
- Nagao, H.; Ooyama, D.; Howell, F.S.; Mukaida, M.; Mizumachi, K. Crystal Structure of Bis-(2,2′- bipyridine)chloronitrosylruthenium(II) Perchlorate. Anal. Sci. 1998, 14, 645–646. [Google Scholar] [CrossRef][Green Version]
- Nagao, H.; Nishimura, H.; Funato, H.; Ichikawa, Y.; Howell, F.S.; Mukaida, M.; Kakihana, H. Synthesis, properties, and molecular structure of trans-chloro(nitrosyl)bis(2,2′-bipyridine)ruthenium(2+): Trans and cis isomer characteristics compared. Inorg. Chem. 1989, 28, 3955–3959. [Google Scholar] [CrossRef]
- Godward, L.N.W.; Wardlaw, W. The Valency and Covalency of Ruthenium in the Blue Chloride Solution. J. Chem. Soc. 1938, 1422–1424. [Google Scholar] [CrossRef]
- Durham, B.; Cox, D.I.; Cordes, A.W.; Barsoum, S. Structure of potassium (2,2′-bipyridine)tetrachlororuthenate(III). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1990, 46, 321–322. [Google Scholar] [CrossRef]
- Eskelinen, E.; Da Costa, P.; Haukka, M. The synthesis and electrochemical behavior of ruthenium(III) bipyridine complexes: [Ru(dcbpy)Cl4]− (dcbpy=4,4′-dicarboxylic acid-2,2′-bipyridine) and [Ru(bpy)Cl3L] (L=CH3OH, PPh3, 4,4′-bpy, CH3CN). J. Electroanal. Chem. 2005, 579, 257–265. [Google Scholar] [CrossRef]
- Seddon, K.R.; Seddon, E.A. The Chemistry of Ruthenium; Elsevier Science Publishers, B.V.: Amsterdam, The Netherlands, 2008; pp. 312–314, 342–348. [Google Scholar]
- Jaeger, F.M.; van Dijk, J.A. Complex salts of bipyridyl with bivalent and trivalent cobalt. Proc. K. Ned. Akad. Wet. 1936, 39, 164–175. [Google Scholar]
- Arun Kumar, K.; Amuthaselvi, M.; Dayalan, A. cis-Bis(2,2′-bipyridine-κN,N’)dichloridocobalt(II) trihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, m468. [Google Scholar] [CrossRef] [PubMed]
- Ciornea, V.; Mingalieva, L.; Costes, J.-P.; Novitchi, G.; Filippova, I.; Galeev, R.T.; Shova, S.; Voronkova, V.K.; Gulea, A. Structural determinations, magnetic and EPR studies of complexes involving the Cr(OH)2Cr unit. Inorg. Chim. Acta 2008, 361, 1947–1957. [Google Scholar] [CrossRef]
- Andersen, P.; Josephsen, J.; Lindberg, B.; Svensson, S.; Koskikallio, J.; Kachi, S. Configurational Correlations of cis-Bis(2,2′-bipyridine) and of cis-Bis(1,10-phenanthroline) Complexes of Trivalent Metals by Means of X-Ray Powder Photographs. Acta Chem. Scand. 1971, 25, 3255–3260. [Google Scholar] [CrossRef]
- Strenger, I.; Rosu, T.; Negoiu, M. Refinement of the crystal structure of cw-bis(2,2′-bipyridyl)- dichlorocobalt(III) chloride dihydrate, [C20H16N4CoCl2]Cl·2H2O. Z. Kristallogr. New Cryst. Struct. 2000, 215, 489–490. [Google Scholar] [CrossRef][Green Version]
- Niederhoffer, E.C.; Martell, A.E.; Rudolf, P.; Clearfield, A. Structures of (carbonato)bis(2,2′-bipyridine)cobalt(III) and (carbonato)bis(1,10-phenanthroline)cobalt(III) complexes. Inorg. Chem. 1982, 21, 3734–3741. [Google Scholar] [CrossRef]
- Li, X.-X.; Cao, Z.-S.; Jiang, W.-J.; Cai, T.-J. Synthesis and determination of structure of [Co(2)CO3]NO3·5H2O. Hunan Keji Daxue Xuebao Ziran Kexueban 2007, 22, 98–101. [Google Scholar]
- Jaeger, F.M.; van Dijk, J.A. Complex salts of trivalent rhodium with 2,2′-bipyridyl. Proc. K. Ned. Akad. Wet. 1934, 37, 284–290. [Google Scholar]
- Lahuerta, P.; Latorre, J.; Martinez-Manez, R.; Garcia-Granda, S.; Gomez-Beltran, F. Structure of bis(2,2′-bipyridine)dichlororhodium(III) chloride dihydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1991, 47, 519–522. [Google Scholar] [CrossRef]
- Morgan, G.T.; Burstall, F.H. Optical activity dependent on co-ordinated nickel. Nature 1931, 127, 854. [Google Scholar] [CrossRef]
- Morgan, G.T.; Burstall, F.H. Residual affinity and co-ordination. Part XXXIII. Optical activity dependent on coordinated nickel. J. Chem. Soc. 1931, 2213–2218. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Tappermann, F. Dipyridyl- und phenanthrolinhaltige Komplexsalze zweiwertiger Metalle. Z. Anorg. Allg. Chem. 1933, 215, 273–287. [Google Scholar] [CrossRef]
- Xia, L.; Zhu, Y.; Wang, Y.-M.; Liu, P.; Xie, J.-M. Syntheses, Structures, Fluorescence and Heterogeneous Catalysis of Coordination Polymers with 4-Benzoimidazol-1-yl-methyl Benzoic Acid and 2,2′-Dipyridine. Jiegou Huaxue 2016, 35, 577–585. [Google Scholar]
- Boonlue, S.; Theppitak, C.; Chainok, K. Tetra-aqua-(2,2′-bipyridine-κ2N,N’)nickel(II) sulfate. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, m908. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, P.; Nakatsuka, Y. Aktivierung von Komplexsalzen in wäßriger Lösung (II. Mitteil.). Ber. Dtsch. Chem. Ges. B 1933, 66, 415–418. [Google Scholar] [CrossRef]
- Jaeger, F.M.; van Dijk, J.A. On some complex dipyridyl-salts of nickel and copper. Proc. K. Akad. Wet. Amst. 1935, 38, 972–977. [Google Scholar]
- Pedireddi, V.R.; Shimpi, M.R.; Yakhmi, J.V. Room-Temperature Ionic Liquids: For a Difference in the Supramolecular Synthesis. Macromol. Symp. 2006, 241, 83–87. [Google Scholar] [CrossRef]
- Charron, G.; Malkin, E.; Rogez, G.; Batchelor, L.J.; Mazerat, S.; Guillot, R.; Guihéry, N.; Barra, A.L.; Mallah, T.; Bolvin, H. Unraveling σ and π Effects on Magnetic Anisotropy in cis-NiA4B2 Complexes: Magnetization, HF-HFEPR Studies, First-Principles Calculations, and Orbital Modeling. Chem. Eur. J. 2016, 22, 16850–16862. [Google Scholar] [CrossRef] [PubMed]
- Garai, M.; Dey, D.; Yadav, H.R.; Choudhury, A.R.; Kole, N.; Biswas, B. Catalytic aspects of a nickel(II)–bipyridine complex towards phosphatase and catechol dioxygenase activity. Polyhedron 2017, 129, 114–122. [Google Scholar] [CrossRef]
- Ikotun, O.F.; Ouellette, W.; Lloret, F.; Julve, M.; Doyle, R.P. Synthesis, X-ray Structure, Thermal and Magnetic Behavior of [(bipy)2Ni2(μ-Cl)2Cl2(H2O)2]: The First Neutral Ferromagnetically Coupled Six-Coordinate Dichlorido-Bridged Nickel(II) Dimer. Eur. J. Inorg. Chem. 2007, 2083–2088. [Google Scholar] [CrossRef]
- Vasileiadou, E.; Angaridis, P.A.; Raptis, R.G.; Mathivathanan, L. Aquabis(2,2′-bipyridine-κ2N,N′)chloridonickel(II) chloride chloroform monosolvate hemihydrate. IUCrData 2016, 1, x161834. [Google Scholar] [CrossRef]
- Boutebdja, M.; Beghidja, A.; Beghidja, C.; Setifi, Z.; Merazig, H. Bis(2,2′-bipyridyl-κ(2) N,N’)chloridonickel(II) nitrate trihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, m190-1. [Google Scholar] [CrossRef]
- Cambi, L.; Cagnasso, A. Complexes of metals of the first transition series with dipyridyl and phenanthroline. ATTI Accad. Naz. Lincei Cl. Sci. Fis. Mat. Nat. Rend. 1934, 19, 458–461. [Google Scholar]
- Mann, F.G.; Purdie, D. The constitution of complex metallic salts. IV. The constitution of certain bridged dipalladium derivatives. A novel type of tautomerism. J. Chem. Soc. 1936, 873–890. [Google Scholar] [CrossRef]
- Chatt, J.; Mann, F.G. The constitution of complex metallic salts. Part VIII. The bridged thio-derivatives of palladous halides with tertiary phosphines. J. Chem. Soc. 1938. [Google Scholar] [CrossRef]
- Maekawa, M.; Munakata, M.; Kitagawa, S.; Nakamura, M. Crystal Structure of (2,2′-Bipyridine)dichloropalladium(II). Anal. Sci. 1991, 7, 521–522. [Google Scholar] [CrossRef]
- Canty, A.J.; Skelton, B.W.; Traill, P.R.; White, A.H. Structural Chemistry of the Platinum Group-Metals: MCl2(bpy) (M = Pd, Pt, bpy = 2,2′-Bipyridine). Aust. J. Chem. 1992, 45, 417–422. [Google Scholar] [CrossRef]
- Kim, N.-H.; Hwang, I.-C.; Ha, K. Redetermination of (2,2′-bipyridine-κ2N,N′)dichloridopalladium(II) dichloromethane solvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, m615–m616. [Google Scholar] [CrossRef]
- Gutiérrez Márquez, R.A.; Crisóstomo-Lucas, C.; Morales-Morales, D.; Hernández-Ortega, S. (2,2′-Bi-pyridine-κ(2) N,N’)di-chloridopalladium(II) 1,4-dioxane hemisolvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2014, 70, m218. [Google Scholar] [CrossRef]
- Rosenblatt, F.; Schleede, A. Der räumliche Bau der Platin-tetrammin-salze. Justus Liebigs Ann. Chem. 1933, 505, 51–58. [Google Scholar] [CrossRef]
- Hazell, A.C. Bis 2,2′-bipyridine platinum(II) dinitrate monohydrate. Acta Crystallogr. Sect. A Found. Crystallogr. 1984, 40, C310. [Google Scholar] [CrossRef]
- Hazell, A.; Simonsen, O.; Wernberg, O. Complexes of 2,2′-bipyridine (bpy) and 1,10-phenanthroline (phen) with platinum(II). Structures of [PtII(bpy)1.3(phen)0.7](NO3)2.0.3H2O and [PtII(bpy)2](NO3)2.H2OActa Crystallogr. Sect. C Cryst. Struct. Commun. 1986, 42, 1707–1711. [Google Scholar] [CrossRef]
- Palmans, R.; MacQueen, D.B.; Pierpont, C.G.; Frank, A.J. Synthesis and Characterization of Bis(2,2′-bipyridyl)platinum(I): A Novel Microtubular Linear-Chain Complex. J. Am. Chem. Soc. 1996, 118, 12647–12653. [Google Scholar] [CrossRef]
- Fedotova, T.N.; Minacheva, L.K.; Kuznetsova, G.N. Formation of new platinum blues by the reaction of platinum(III) acetamidate chloride [Pt2(μ-NHCOCH3)4Cl2] with 2,2′-bipyridine: The crystal structure of [Pt(Bipy)2](CF3SO3)2. Zh. Neorg. Khim. 2003, 48, 417–425. [Google Scholar]
- Clare, B.R.; McInnes, C.S.; Blackman, A.G. Bis(2,2′-bi-pyridine-κ2N,N′)platinum(II) bis--(perchlorate). Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, m2042. [Google Scholar] [CrossRef]
- Hudson, T.A.; Robson, R. A New Class of TCNQ Derivatives Easily Generated from TCNQH2 Containing Discrete TCNQ2−Anions and Noncoordinating Cations. Cryst. Growth Des. 2009, 9, 1658–1662. [Google Scholar] [CrossRef]
- Stork, J.R.; Rios, D.; Pham, D.; Bicocca, V.; Olmstead, M.M.; Balch, A.L. Metal−Metal Interactions in Platinum(II)/Gold(I) or Platinum(II)/Silver(I) Salts Containing Planar Cations and Linear Anions. Inorg. Chem. 2005, 44, 3466–3472. [Google Scholar] [CrossRef]
- Endres, H.; Keller, H.J.; Moroni, W.; Nöthe, D.; Dong, V. The 1:3 radical salt bis(2,2′-dipyridyl)platinum(II)–7,7,8,8-tetracyanoquinodimethane, [Pt(dipy)2]2+[TCNQ]32−. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1978, 34, 1823–1827. [Google Scholar] [CrossRef]
- Dong, V.; Endres, H.; Keller, H.J.; Moroni, W.; Nöthe, D. Dimerization of 7,7,8,8-tetracyanoquinodimethane (TCNQ) radical anions via σ-bond formation: Crystal structure and EPR properties of bis(dipyridyl)platinum(II)–TCNQ, [Pt(2,2′-dipy)2 2+(TCNQ)2 2−]. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1977, 33, 2428–2431. [Google Scholar] [CrossRef]
- Chieh, P.C. Crystal and molecular structure of aquobis-(2,2′-bipyridyl)palladium dinitrate. J. Chem. Soc. Dalton Trans. 1972, 15, 1643–1646. [Google Scholar] [CrossRef]
- Hinamoto, M.; Ooi, S.; Kuroya, H. Crystal structure of bis-(2,2′-bipyridyl)palladium(II) nitrate monohydrate and the stereochemistry of palladium(II). J. Chem. Soc. Chem. Commun. 1972, 356–357. [Google Scholar] [CrossRef]
- Gao, E.J.; Zhang, Y.X.; Zhu, M.C.; Liu, H.Y.; Huang, Y.; Zhang, M.; Su, M.; Guo, M.J.; Guan, F.; Gao, X.N.; et al. Synthesis and characterization of a novel complex [Pd(Bipy)2](Bpcc)·11H2O. Russ. J. Coord. Chem. 2010, 36, 853–858. [Google Scholar] [CrossRef]
- Henling, L.M.; Marsh, R.E. Some more space-group corrections. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2014, 70, 834–836. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Nishida, Y.; Okawa, H.; Kida, S. The Crystal Structure of Bis(2,2′-bipyridyl)palladium(II) Picrate [Pd(bpy)2](pic)2. A Novel “Bow” Distortion. Bull. Chem. Soc. Jpn. 1986, 59, 2013–2014. [Google Scholar] [CrossRef]
- Geremia, S.; Randaccio, L.; Mestroni, G.; Milani, B. Bow–step and twist conformations and stacking interactions in palladium bipyridine and phenanthroline complexes. J. Chem. Soc. Dalton Trans. 1992, 2117–2118. [Google Scholar] [CrossRef]
- Stoccoro, S.; Alesso, G.; Cinellu, M.A.; Minghetti, G.; Zucca, A.; Bastero, A.; Claver, C.; Manassero, M. New complexes of palladium(II) with chelating heterocyclic nitrogen ligands. J. Organomet. Chem. 2002, 664, 77–84. [Google Scholar] [CrossRef]
- Milani, B.; Anzilutti, A.; Vicentini, L.; Sessanta o Santi, A.; Zangrando, E.; Geremia, S.; Mestroni, G. Bis-Chelated Palladium(II) Complexes with Nitrogen-Donor Chelating Ligands Are Efficient Catalyst Precursors for the CO/Styrene Copolymerization Reaction. Organometallics 1997, 16, 5064–5075. [Google Scholar] [CrossRef]
- Wehman, P.; Dol, G.C.; Moorman, E.R.; Kamer, P.C.J.; van Leeuwen, P.W.N.M.; Fraanje, J.; Goubitz, K. Ligand Effects in the Palladium-Catalyzed Reductive Carbonylation of Nitrobenzene. Organometallics 1994, 13, 4856–4869. [Google Scholar] [CrossRef]
- Yue, C.-Y.; Jiang, F.-L.; Yuan, D.-Q.; Chen, L.; Wu, M.-Y.; Hong, M.-C. Synthesis, Crystal Structure and Photoluminescent Property of a 3D Hydrogen-bonded Supramolecular Compound with Large Channels. Chin. J. Struct. Chem. 2008, 27, 467–470. [Google Scholar]
- Morgan, G.T.; Burstall, F.H. Residual affinity and co-ordination. XXXIV. 2,2′-Bipyridyl platinum salts. J. Chem. Soc. 1934, 965–971. [Google Scholar] [CrossRef]
- Randall, J.T. Luminescence of solids at low temperatures. Nature 1938, 142, 113–114. [Google Scholar] [CrossRef]
- Osborn, R.S.; Rogers, D. Crystal structure of the red form of 2,2′-bipyridyldichloroplatinum(II). J. Chem. Soc. Dalton Trans. 1974, 1002–1004. [Google Scholar] [CrossRef]
- Connick, W.B.; Henling, L.M.; Marsh, R.E.; Gray, H.B. Emission Spectroscopic Properties of the Red Form of Dichloro(2,2′-bipyridine)platinum(II). Role of Intermolecular Stacking Interactions. Inorg. Chem. 1996, 35, 6261–6265. [Google Scholar] [CrossRef]
- Falvello, L.R.; Garde, R.; Miqueleiz, E.M.; Tomás, M.; Urriolabeitia, E.P. Evidence of C-H activation of acetone by a platinum(II) complex. Synthesis and structural characterization of [Pt(CH2COCH3)Cl(bipy)] (bipy = 2,2′-bipyridyl). Inorg. Chim. Acta 1997, 264, 297–303. [Google Scholar] [CrossRef]
- Textor, M.; Oswald, H.R. Röntgenographische und spektroskopische Untersuchungen an zwei Modifikationen von Dichloro-2,2′-dipyridyl-platin(II). Zeit. Anorg. Allg. Chem. 1974, 407, 244–256. [Google Scholar] [CrossRef]
- Canty, A.J.; Gardiner, M.G.; Jones, R.C.; Sharma, M. Structural Chemistry of [MX2(bipy)] (M = Pd, Pt; X = Cl, Br, I): The Yellow Polymorph of Dichlorido(2,2′-bipyridine)platinum(II) and Diiodido(2,2′-bipyridine)palladium(II), and Overview of this System. Aust. J. Chem. 2011, 64, 1355–1359. [Google Scholar] [CrossRef]
- Kato, M.; Kosuge, C.; Yano, S.; Kimura, M. π-Stacking of [Pt(2,2′-bipyridine)(ethylenediamine)]2+as its Hexafluorophosphate Salt. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1997, 53, 838–840. [Google Scholar] [CrossRef]
- Kato, M.; Takahashi, J.; Sugimoto, Y.; Kosuge, C.; Kishi, S.; Yano, S. Selective formation of integrated stacks of (α-diimine)(ethylenediamine)platinum(II) and neutral π systems of the phenanthrene type. J. Chem. Soc. Dalton Trans. 2001, 747–752. [Google Scholar] [CrossRef]
- Masuda, H.; Yamauchi, O. A structural basis for nucleic base‚Äîmetallointercalator lnteractions: Crystal structure of [Pt(2,2′-bipyridine)(ethylenediamine)].(AMP).10H2O (AMP = adenosine 5′-monophosphate). Inorg. Chim. Acta 1987, 136, L29–L31. [Google Scholar] [CrossRef]
- Cavigliasso, G.; Stranger, R.; Lo, W.K.C.; Crowley, J.D.; Blackman, A.G. The nature of species derived from [Pt(bipy)2]2+ in aqueous solution: X-ray structural, mass spectral, NMR, and computational studies. Polyhedron 2013, 64, 238–246. [Google Scholar] [CrossRef]
- Rotondo, E.; Bruschetta, G.; Bruno, G.; Rotondo, A.; Di Pietro, M.L.; Cusumano, M. NMR Investigation and Dynamic Behaviour of [2,2′-Bipyridylbis(pyridine)platinum(II)]2+ and Related Cationic Complexes—Crystal Structure of [Pt(bipy)(py)2](PF6)2. Eur. J. Inorg. Chem. 2003, 2003, 2612–2618. [Google Scholar] [CrossRef]
- Corbo, R.; Georgiou, D.C.; Wilson, D.J.; Dutton, J.L. Reactions of [PhI(pyridine)2]2+ with model Pd and Pt II/IV redox couples. Inorg. Chem. 2014, 53, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Reihlen, H.; Seipel, G.; Weinbrenner, E. Über das asymmetrische Platinatom. VII. Eine neue Art optisch aktiver Verbindungen. Justus Liebigs Ann. Chem. 1935, 520, 256–269. [Google Scholar] [CrossRef]
- Reihlen, H.; Hühn, W. Über das asymmetrische Platinatom. VI. Justus Liebigs Ann. Chem. 1935, 519, 80–96. [Google Scholar] [CrossRef]
- Tartarini, G. New color reactions of cuprous salts. Gazz. Chim. Ital. 1933, 63, 597–600. [Google Scholar]
- Mann, F.G.; Purdie, D.; Wells, A.F. The constitution of complex metallic salts. Part V. The constitution of the phosphine and arsine derivatives of cuprous iodide. The configuration of the co-ordinated cuprous complex. J. Chem. Soc. 1936, 1503. [Google Scholar] [CrossRef]
- Skelton, B.W.; Waters, A.F.; White, A.H. Lewis-Base Adducts of Group 11 Metal(I) Compounds. LXIII. Stereochemistries and Structures of the 1:1 Adducts of Cu1X (X = Cl, Br, I) With 2,2′-Bipyridine. Aust. J. Chem. 1991, 44, 1207–1215. [Google Scholar] [CrossRef]
- Huang, P.C.; Parthasarathy, K.; Cheng, C.H. Copper-catalyzed intramolecular oxidative C-H functionalization and C-N formation of 2-aminobenzophenones: Unusual pseudo-1,2-shift of the substituent on the aryl ring. Chem. Eur. J. 2013, 19, 460–464. [Google Scholar] [CrossRef]
- Jaeger, F.M.; van Dijk, J.A. Complex salts of α,α’-dipyridyl with bivalent copper. Proc. K. Ned. Akad. Wet. 1934, 37, 395–400. [Google Scholar]
- Tedenac, J.C.; Phung, N.D.; Avinens, C.; Maurin, M. Stereochimie du motif de coordination dans les complexes a ligands mixtes du type MII(bipy)(H2O)2AB4 (MII = NiII ou CuII.; AB42- = SO42– ou BeF42–) bipy: 2,2′-bipyridine. J. Inorg. Nucl. Chem. 1976, 38, 85–89. [Google Scholar] [CrossRef]
- Chattopadhyay, S.K.; Mak, T.C.W. Study of Cu2+ mediated oxidation of thiosemicarbazide, thiocarbohydrazide and thiourea. Inorg. Chem. Commun. 2000, 3, 111–113. [Google Scholar] [CrossRef]
- Zheng, Y.-Q.; Lin, J.-L. Crystal Structures of [Cu2(bpy)2(H2O)(OH)2(SO4)].4H2O and [Cu(bpy)(H2O)2]SO4 with bpy = 2,2′-Bipyridine. Z. Anorg. Allg. Chem. 2003, 629, 1622–1626. [Google Scholar] [CrossRef]
- Chattopadhyay, S.K.; Seth, S.; Mak, T.C.W. Studies of Copper(II) Thiosemicarbazide Complexes and their Reactivities. X-Ray Structure of Two Unusual Reaction Products, [Cu(bpy)(H2O)2SO4] and [(bpy)2Cu2(C2O4)Cl2].H2O. J. Coord. Chem. 2002, 55, 259–270. [Google Scholar] [CrossRef]
- Clemente, D.A.; Marzotto, A. 30 Space-group corrections: Two examples of false polymorphism and one of incorrect interpretation of the fine details of an IR spectrum. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Kuppukannu, R.; Gabriele, B.; Andrea, C. Synthesis, spectral and single crystal X-ray structural studies on bis(2,2′-bipyridine)sulphido M(II) (M=Cu, Zn) and diaquo 2,2′-bipyridine zinc(II)sulphate dihydrate. J. Serb. Chem. Soc. 2010, 75, 1085–1092. [Google Scholar]
- Tedenac, J.-C.; Philippot, E. Structure du sulfate de bisaquomonobipyridyl-cuivre Cu(C10H8N2)H2O)2SO4. J. Inorg. Nucl. Chem. 1975, 37, 846–848. [Google Scholar] [CrossRef]
- Hernández-Molina, M.; González-Platas, J.; Ruiz-Pérez, C.; Lloret, F.; Julve, M. Crystal structure and magnetic properties of the single-μ-chloro copper(II) chain [Cu(bipy)Cl2] (bipy = 2,2′-bipyridine. Inorg. Chim. Acta 1999, 284, 258–265. [Google Scholar] [CrossRef]
- Garland, M.T.; Grandjean, D.; Spodine, E.; Atria, A.M.; Manzur, J. Structures of catena-di-[mu]-chloro- and catena-di-[mu]-bromo-(2,2-bipyridine)copper(II). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1988, 44, 1209–1212. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Bi, W.-H.; Li, X.; Cao, R. (2,2′-Bi-pyridine-κ2N,N′)-di-chloro-copper(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2004, 60, m876–m877. [Google Scholar] [CrossRef]
- Garai, M.; Dey, D.; Yadav, H.R.; Choudhury, A.R.; Maji, M.; Biswas, B. Catalytic Fate of Two Copper Complexes towards Phenoxazinone Synthase and Catechol Dioxygenase Activity. Chem. Sel. 2017, 2, 11040–11047. [Google Scholar] [CrossRef]
- Liu, E.; Zhang, Y.; Li, L.; Yang, C.; Fettinger, J.C.; Zhang, G. New copper(II) species from the copper/2,2′-bypyridine and copper/4-dimethylaminopyridine catalyzed aerobic alcohol oxidations. Polyhedron 2015, 99, 223. [Google Scholar] [CrossRef]
- Kostakis, G.E.; Nordlander, E.; Haukka, M.; Plakatouras, J.C. Di-μ-chloro-bis-[(2,2-bi-pyridine)chloro-copper(II)]. Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, m77–m79. [Google Scholar] [CrossRef]
- Jaeger, F.M.; van Dijk, J.A. Complex salts of α,α’-dipyridyl with bivalent copper. II. Proc. K. Ned. Akad. Wet. 1934, 37, 618–623. [Google Scholar]
- Kumar, D.; Kapoor, I.P.S.; Singh, G.; Fröhlich, R. Preparation, characterization, and kinetics of thermolysis of nickel and copper nitrate complexes with 2,2′-bipyridine ligand. Thermochim. Acta 2012, 545, 67–74. [Google Scholar] [CrossRef]
- Nakai, H.; Ooi, S.; Kuroya, H. The Crystal Structure of Nitratotriaquo(2,2′-bipyridine)copper(II) Nitrate [Cu(NO3)(H2O)3(bipy)]NO3. Bull. Chem. Soc. Jpn. 1977, 50, 531–532. [Google Scholar] [CrossRef]
- Mathews, I.I.; Manohar, H. Redetermination of the structure of triaqua(2,2′-bipyridine)nitratocopper(II) nitrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1991, 47, 2213–2214. [Google Scholar] [CrossRef]
- Jianmin, L.; Jianbin, Z.; Yanxiong, K.; Xintao, W. A Novel Structure of Bipyridine Coordinated with Copper(II): [Cu(bipy)(H2O)3]·(NO3)2. Cryst. Res. Technol. 1996, 31, 589–593. [Google Scholar] [CrossRef]
- Potapov, A.S.; Domina, G.A.; Petrenko, T.V.; Khlebnikov, A.I. Synthesis and crystal structure of discrete complexes and coordination polymers containing 1,3-bis(pyrazol-1-yl)propane ligands. Polyhedron 2012, 33, 150–157. [Google Scholar] [CrossRef]
- Marjani, K.; Davies, S.C.; Durrant, M.C.; Hughes, D.L.; Khodamorad, N.; Samodi, A. Bis(2,2′-bi-pyridine)-nitratocopper(II) nitrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, m11–m14. [Google Scholar] [CrossRef]
- Lobana, T.S.; Rani, A.; Jassal, A.K.; Jasinski, J.P. Reactivity of thiazolidine-2-thione towards CuI/CuII: Synthesis and structures of [3-(2-thiazolin-2-yl)thiazolidine-2-thione]copper(I) bromide and [bis(2,2′-bipyridine)nitratocopper(II)] nitrate. J. Chem. Sci. 2015, 127, 149–153. [Google Scholar] [CrossRef]
- Proctor, I.M.; Stephens, F.S. An example of a cis-distorted octahedral copper(II) complex: The crystal structure of nitritobis-2,2′-bipyridylcopper(II) nitrate. J. Chem. Soc. A 1969, 1248–1255. [Google Scholar] [CrossRef]
- Simmons, C.; Clearfield, A.; Fitzgerald, W.; Tyagi, S.; Hathaway, B. The X-ray crystal structure and electronic properties of [Cu(bipy)2(ONO)][NO3](bipy = 2,2′-bipyridyl) at 298 and 165 K, a fluxional cis-distorted octahedral CuN4O2 chromophore. J. Chem. Soc. Chem. Commun. 1983, 189–190. [Google Scholar] [CrossRef]
- Simmons, C.J.; Hathaway, B.J.; Amornjarusiri, K.; Santarsiero, B.D.; Clearfield, A. The first determination of the energy difference between solid-state conformers by x-ray diffraction. 1. The crystal structure of the pseudo-Jahn-Teller complex (nitrito)bis(2,2′-bipyridyl)copper(II) nitrate at 20, 100, 165 and 296 K and of its isostructural zinc(II) analog at 295 K. 2. The possibility of using x-ray diffraction to characterize adiabatic potential energy surfaces and relative ligand strengths. J. Am. Chem. Soc. 1987, 109, 1947–1958. [Google Scholar] [CrossRef]
- Simmons, C.J.; Clearfield, A.; Fitzgerald, W.; Tyagi, S.; Hathaway, B.J. Fluxional behavior of a pseudo-Jahn-Teller complex: X-ray crystal structure of [Cu(bppy)2(ONO)][NO3] at 165 and 296 K. Inorg. Chem. 1983, 22, 2463–2466. [Google Scholar] [CrossRef]
- Fereday, R.J.; Hodgson, P.; Tyagi, S.; Hathaway, B.J. Crystal structure and electronic properties of bis(2,2′-bipyridyl)-nitratocopper(II) nitrate monohydrate. J. Chem. Soc. Dalton Trans. 1981, 2070–2077. [Google Scholar] [CrossRef]
- Nakai, N. The Crystal Structure of Bis(2,2′-bipyridine)nitratocopper(II) Nitrate Monohydrate [Cu(NO3)(bpy)2]NO3·H2O. Bull. Chem. Soc. Jpn. 1980, 53, 1321–1326. [Google Scholar] [CrossRef]
- Catalan, K.J.; Jackson, S.; Zubkowski, J.D.; Perry, D.L.; Valente, E.J.; Feliu, L.A.; Polanco, A. Copper(II) nitrate compounds with heterocyclic ligands: Structures of [Cu(NO3)(2,2′-dipyridyl)2][NO3]·H2O and [Cu(H2O)(1,10-phenanthroline)2][NO3]2. Polyhedron 1995, 14, 2165–2171. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Christeleit, W. Complex copper salts of alpha-amino acids. Stiasny Festschr. 1937, 332–338. [Google Scholar]
- do Nascimento Neto, J.A.; da Silva, C.C.; Ribeiro, L.; Valdo, A.K.S.M. Competition between coordination bonds and hydrogen bonding interactions in solvatomorphs of copper(II), cadmium(II) and cobalt(II) complexes with 2,2′-bipyridyl and acetate. Z. Kristallogr. Cryst. Mater. 2018, 234, 119–128. [Google Scholar] [CrossRef]
- Perlepes, S.P.; Libby, E.; Streib, W.E.; Folting, K.; Christou, G. The reactions of Cu2(O2CMe)4(H2O)2 with 2,2′-bipyridine (bpy): Influence of the Cu: Bpy ratio, and the structure of a linear polymer comprising two alternating types of Cu2 units. Polyhedron 1992, 11, 923–936. [Google Scholar] [CrossRef]
- Koo, B.K. Synthesis, Crystal Structure, and Characterization of Copper(II) Acetate Complex. Bull. Kor. Chem. Soc. 2001, 22, 113–117. [Google Scholar]
- Morgan, G.T.; Burstall, F.H. Researches on residual affinity and co-ordination. Part XXXII. Complex salts of bivalent silver. J. Chem. Soc. 1930, 2594–2598. [Google Scholar] [CrossRef]
- Sugden, S. 20. Magnetism and valency. Part I. Copper and silver compounds. J. Chem. Soc. 1932, 161–170. [Google Scholar] [CrossRef]
- Barbieri, G.A. The electrolytic preparation of several complex salts of bivalent silver. ATTI Accad. Naz. Lincei Cl. Sci. Fis. Mat. Nat. Rend. 1932, 16, 44–47. [Google Scholar]
- Kandaiah, S.; Huebner, R.; Jansen, M. Electrocrystallisation and single crystal structure determination of Bis(2,2′-bipyridyl)silver(II) perchlorate [Ag(bipy)2](ClO4)2. Polyhedron 2012, 48, 68–71. [Google Scholar] [CrossRef]
- Atwood, J.L.; Simms, M.L.; Zatko, D.A. Bis(2,2′-bipyridine)silver(II) Nitrate Monohydrate, Ag(N2C10H8)2.(NO3)2.H2O. Cryst. Struct. Commun. 1973, 2, 279–282. [Google Scholar]
- Morgan, G.T.; Sugden, S. Paramagnetism of Bivalent Silver. Nature 1931, 128, 31. [Google Scholar] [CrossRef]
- McMillan, J.A.; Smaller, B. Paramagnetic Resonance of Some Silver (II) Compounds. J. Chem. Phys. 1961, 35, 1698–1701. [Google Scholar] [CrossRef]
- Cervone, E. Electronic spectra of some Ag++ complexes. Annali Chimica 1962, 52, 1167–1173. [Google Scholar]
- Thorpe, W.G.; Kochi, J.K. Silver(II) complexes of dipyridyl. J. Inorg. Nucl. Chem. 1971, 33, 3958–3962. [Google Scholar] [CrossRef]
- Hieber, W.; Schulten, H. Über Metallcarbonyle. XXIV. Bildungsweise und Metallsalzreaktionen des Kobaltcarbonylwasserstoffs. Z. Anorg. Allg. Chem. 1937, 232, 17–28. [Google Scholar] [CrossRef]
- Dothie, H.J.; Llewellyn, F.J.; Wardlaw, W.; Welch, A.J.E. Stereochemistry of quadricovalent atoms: Gold. J. Chem. Soc. 1939, 426–428. [Google Scholar] [CrossRef]
- Jones, P.G.; Clegg, A.; Sheldrick, G.M. Potassium Dicyanoaurate(I)-2,2′-Bipyridyl. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1980, 36, 160–162. [Google Scholar] [CrossRef]
- Döring, C.; Strey, M.; Jones, P.G. The march of progress: Structures of the adducts of potassium dicyanidoaurate(I) with 2,2′-bipyridyl (redetermination) and 1,10-phenanthroline. Acta Crystallogr. Sect. C Struct. Chem. 2017, 73, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Brain, F.H.; Gibson, C.S. Organic compounds of gold. VII. Methyl and ethyl compounds. J. Chem. Soc. 1939, 762–767. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Quehl, K. Aktivierung von Komplexsalzen in wäßriger Lösung (II. Mitteil.). Ber. Dtsch. Chem. Ges. B 1932, 65, 560–565. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Quehl, K. Über einen neuen Effekt in Lösungen optisch-aktiver Substanzen (I. Mitteil.). Ber. Dtsch. Chem. Ges. B 1931, 64, 2667–2671. [Google Scholar] [CrossRef]
- Jaeger, F.M.; van Dijk, J.A. Complex salts of α,α’-bipyridyl with zinc and cadmium. Proc. K. Ned. Akad. Wet. 1934, 37, 753–760. [Google Scholar]
- Harvey, M.; Baggio, S.; Mombrú, A.; Baggio, R. Three new ZnII sulfate complexes. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2000, 56, 771–774. [Google Scholar] [CrossRef]
- Behera, J.N.; Rao, C.N. Organically-Templated Zinc and Thorium Sulfates with Chain and Layered Structures. Z. Anorg. Allg. Chem. 2005, 631, 3030–3036. [Google Scholar] [CrossRef]
- Lan, C.-L.; Zhang, S.-H.; Zhou, Z.-Y. Synthesis and crystal structure of one-dimensional coordination polymers [Zn(bipy)(H2O)2SO4]. Chem. Res. Appln. 2007, 19, 318–322. [Google Scholar]
- Karmakar, A.; Baruah, J.B.; Shankar, R.B. Zinc(II) and cobalt(II) complexes of (3-carboxymethoxy-naphthalen-2-yloxy)-acetic acid: A structural study. CrystEngComm 2009, 11, 832–840. [Google Scholar] [CrossRef]
- Tai, X.-S.; Xu, J.; Feng, Y.-M. Synthesis and crystal structure of 3D network Zn(II) complex. Huaxue Shiji 2009, 31, 691–692. [Google Scholar]
- Wang, L.H. Synthesis and Structural Characterization of Zn(II) Coordination Polymer Material. Adv. Mater. Res. 2011, 282, 100–103. [Google Scholar] [CrossRef]
- Park, H.-M.; Hwang, I.-H.; Bae, J.-M.; Jo, Y.-D.; Kim, C.; Kim, H.-Y.; Kim, Y.-M.; Kim, S.-J. Anion Effects on Crystal Structures of CdII Complexes Containing 2,2′-Bipyridine: Photoluminescence and Catalytic Reactivity. Bull. Korean Chem. Soc. 2012, 33, 1517–1522. [Google Scholar] [CrossRef]
- Rodesiler, P.F.; Turner, R.W.; Charles, N.G.; Griffith, E.A.H.; Amma, E.L. Solution and solid-state cadmium-113 NMR of Cd(alpha.alpha.’-bpy)2X2 (X = Cl−,Br−, NCS−,NO3−,H2O) and crystal structures of the nitrate (monohydrate) and the isothiocyanate derivatives. Inorg. Chem. 1984, 23, 999–1004. [Google Scholar] [CrossRef]
- Turner, R.W.; Rodesiler, P.F.; Amma, E.L. The crystal structure, solid and solution 113Cd NMR of bis(α,α′-dipyridyl)cadmium(II) nitrate hemihydrate and its relationship to Cd containing proteins. Inorg. Chim. Acta 1982, 66, L13–L15. [Google Scholar] [CrossRef]
- Jaeger, F.M.; van Dijk, J.A. Complex salts of alpha-alpha’-bipyridyl with zinc and cadmium. II. Proc. Acad. Sci. Amst. 1935, 38, 235–242. [Google Scholar]
- Cherni, S.N.; Cherni, A.; Driss, A. Crystal Structure of Bicapped Trigonal-Antiprismatic Coordinated Cd(II) Complex [Cd(C10H8N2)(NO3)2(H2O)]. X-Ray Struct. Anal. Online 2012, 28, 13–14. [Google Scholar] [CrossRef]
- Freire, E.; Baggio, S.; Baggio, R.; Suescun, L. Mercury(II) halide complexes with N-donor organic ligands: Crystal and molecular structure of HgI2R, R = 1,10-phenanthroline, 2,9-dimethyl-1,10-phenanthroline, bipyridine. J. Chem. Crystallogr. 1999, 29, 825–830. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Christeleit, W. Komplexsalze der Alkali- und Erdalkalimetalle. Z. Anorg. Allg. Chem. 1938, 239, 133–144. [Google Scholar] [CrossRef]
- Snell, F.D. Colorimetric Analysis; Van Nostrand: New York, NY, USA, 1921. [Google Scholar]
- Rosenfeld, L. Four Centuries of Clinical Chemistry; Gordon and Breach: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Hill, R. Method for the estimation of iron in biological material. Proc. R. Soc. Lond. Ser. B 1930, 107, 205–214. [Google Scholar] [CrossRef]
- Feigl, F.; Hamburg, H. The detection of iron. Z. Anal. Chem. 1931, 86, 1–13. [Google Scholar]
- Feigl, F.; Krumholz, P.; Hamburg, H. Colorimetric determination of iron with α,α’-dipyridyl. Z. Anal. Chem. 1932, 90, 199–202. [Google Scholar] [CrossRef]
- van Nieuwenburg, C.J.; Blumendal, H.B. Cerimetric titration of small amounts of iron by means of α,α’-bipyridyl as indicator. Mikrochemie 1935, 18, 39–42. [Google Scholar] [CrossRef]
- Poluektov, N.S.; Nazarenko, V.A. An application of a ferrous bipyridyl complex in micro-chemical analysis. Zh. Prikl. Khim. (S. Peterburg Russ. Fed.) 1937, 10, 2105–2108. [Google Scholar]
- McFarlane, W.D. Determination of iron by titanium titration and by alpha,alpha-bipyridine colorimetry—Improved analytical procedure. Ind. Eng. Chem. Anal. Ed. 1936, 8, 124–126. [Google Scholar] [CrossRef]
- Kohler, G.O.; Elvehjem, C.A.; Hart, E.B. Modifications of the bipyridine method for available iron. J. Biol. Chem. 1936, 113, 49–53. [Google Scholar]
- Szebelledy, L.; Ajtai, M. Detection of iron. Magy. Gyogyszeresztud. Tarsasag Ert. 1937, 13, 791–794. [Google Scholar]
- Thiel, A.; van Hengel, E. Grundlagen und Anwendungen der Absolutcolorimetrie, XVI. Mitteil.: Über die absolutcolorimetrische Bestimmung des Eisens. Ber. Dtsch. Chem. Ges. B 1937, 70, 2491–2497. [Google Scholar] [CrossRef]
- Szelenyi, T. Methods of quantitative spectral analysis. Magy. Mern. Epitesz-Egylet Kozl. 1938, 12, 20–26. [Google Scholar]
- Nießner, M.; Wien, T.H. Mikroanalytische Forschungen in der Metallkunde. Angew. Chemie 1939, 52, 721–726. [Google Scholar] [CrossRef]
- Klockmann, R. Newer organic reagents in medical-chemical analysis. E. Merck’s Jahresber. 1939, 53, 59–68. [Google Scholar]
- Hill, R.; Keilin, D. Estimation of hematin iron and the oxidation-reduction equivalent of cytochrome c. Proc. R. Soc. Lond. Ser. B 1933, 114, 104–109. [Google Scholar] [CrossRef]
- Muller, H. Die Verwendung von α-α′-Dipyridyl zur Bestimmung von Ferro- und Gesamteisen in Natürlichen Wässern. Mikrochemie 1933, 12, 307–314. [Google Scholar] [CrossRef]
- Cooper, L.H.N. Iron in the sea and in marine plankton. Proc. R. Soc. Lond. Ser. B 1935, 118, 419–438. [Google Scholar] [CrossRef]
- Sirks, H.A. The determination of the iron content of water used in butter making. Versl. Landbouwkd. Onderz. C 1939, 45, 575–585. [Google Scholar]
- Hutchinson, G.E.; Deevey, E.S., Jr.; Wollack, A. The oxidation-reduction potentials of lake waters and their ecological significance. Proc. Natl. Acad. Sci. USA 1939, 25, 87–90. [Google Scholar] [CrossRef][Green Version]
- Pal, J.C. Ionizable iron in cow and human milk. Indian Med. Gaz. 1939, 74, 470–471. [Google Scholar]
- Bode, G. Estimation of iron in beer by means of α,α’-bipyridyl. Wochenschr. Brau. 1933, 50, 321–323. [Google Scholar]
- Gray, P.P.; Stone, I.M. Direct determination of iron in malt beverages. Ind. Eng. Chem. Anal. Ed. 1938, 10, 415–417. [Google Scholar] [CrossRef]
- Kok, J.A.F. Removal of metals from foods. Acta Brevia Neerl. Physiol. Pharmacol. Microbiol. 1933, 3, 109–110. [Google Scholar]
- Elvehjem, C.A.; Hart, E.B.; Sherman, W.C. The availability of iron from different sources for hemoglobin formation. J. Biol. Chem. 1933, 103, 61–70. [Google Scholar]
- Ranganathan, S. The available iron in some common Indian foodstuffs, determined by the α,α’-bipyridine method. Indian J. Med. Res. 1938, 25, 677–684. [Google Scholar]
- Harris, R.S.; Mosher, L.M.; Bunker, J.W.M. The nutritional availability of iron in molasses. Am. J. Dig. Dis. Nutr. 1939, 6, 459–462. [Google Scholar] [CrossRef]
- Yang, E.F.; Dju, M.Y. The total and available iron in vegetable foods. Zhongguo Shenglixue Zazhi 1939, 14, 479–487. [Google Scholar]
- Goswami, H.; Basu, U. “Available” iron in Indian foodstuffs. Indian J. Med. Res. 1938, 25, 893–899. [Google Scholar]
- Smith, M.C.; Otis, L. The banana as a source of iron for hemoglobin formation. J. Home Econ. 1936, 28, 395–398. [Google Scholar]
- Shackleton, L.; McCance, R.A. The ionizable iron in foods. Biochem. J. 1936, 30, 582–591. [Google Scholar] [CrossRef]
- Schlegel, H. A critical investigation on the possibility of the determination of traces of iron in silicic acid, with special consideration of the quantitative spectrum analysis. Beiheft Z. Ver. Deut. Chem. 1936, 411–412. [Google Scholar]
- Hackl, O. Micro tests for ferrous and ferric oxides in silicates. Mikrochemie 1937, 21, 224–230. [Google Scholar] [CrossRef]
- Dyer, W.J.; McFarlane, W.D. A study of the iron in a podzol soil by means of an improved bipyridine method. Can. J. Res. Sect. B 1938, 16, 91–96. [Google Scholar] [CrossRef]
- Kazarinova-Oknina, V.A. Colorimetric determination of ferrous oxide in phosphate rocks. Zavod. Lab. 1938, 7, 1106–1109. [Google Scholar]
- Ignatieff, V. Method for determining ferrous iron in soil solutions and a study of the effect of light on the reduction of iron by citrate and 2,2′-dipyridyl. J. Soc. Chem. Ind. Lond. 1937, 56, 407-10T. [Google Scholar]
- Engel, L.L. Method for the determination of iron in dental enamel. J. Dent. Res. 1934, 14, 273–276. [Google Scholar] [CrossRef]
- Murray, M.M.; Glock, G.E.; Lowater, F. Chemical and spectrographic determination of iron in tooth material. Br. Dent. J. 1939, 66, 345–350. [Google Scholar]
- Shorland, F.B.; Wall, E.M. A rapid method for the estimation of total iron in blood. Biochem. J. 1936, 30, 1047–1048. [Google Scholar] [CrossRef] [PubMed]
- Coombs, H.I. The hemoglobin and iron in the blood. I. The determination of the total iron. Biochem. J. 1936, 30, 1588–1591. [Google Scholar]
- Wall, E.M.; Shorland, F.B. The nature, properties and estimation of iron in blood. J. N. Z. Inst. Chem. 1936, 1, 15–21. [Google Scholar]
- Ferrari, C. Histochemical identification of organic iron. Ann. Chim. Appl. 1937, 27, 487–489. [Google Scholar]
- Leuthardt, F. Medicinal chemistry. Mitt. Geb. Lebensmittelunters. Hyg. 1939, 30, 185–190. [Google Scholar]
- Heilmeyer, L.; Plotner, H. Iron deficiency and its treatment. Klin. Wochenschr. 1936, 15, 1669–1673. [Google Scholar] [CrossRef]
- Lintzel, W. Microdetermination of iron in biological materials. Z. Gesamte Exp. Med. 1933, 86, 269–274. [Google Scholar] [CrossRef]
- Sherman, W.C.; Elvehjem, C.A.; Hart, E.B. Further studies on the availability of iron in biological material. J. Biol. Chem. 1934, 107, 383–394. [Google Scholar]
- Scharrer, K. The colorimetric estimation of iron in plant materials by means of α,α’-bipyridyl. Z. Pflanzenernaehr. Dueng. Bodenkd. 1934, 33, 336–340. [Google Scholar] [CrossRef]
- Jackson, S.H. Determination of iron in biological material. Ind. Eng. Chem. Anal. Ed. 1938, 10, 302–304. [Google Scholar] [CrossRef]
- Sherman, W.C.; Elvehjem, C.A.; Hart, E.B. Factors influencing the utilization of the iron and copper of egg yolk for hemoglobin formation. J. Biol. Chem. 1934, 107, 289–295. [Google Scholar]
- Kuhn, A. Investigations of homeopathic iron preparations. Pharm. Zentralhalle Dtschl. 1934, 75, 131–134. [Google Scholar]
- Hahn, P.F.; Whipple, G.H. Iron metabolism in experimental anemia. “Availability of iron”. J. Exp. Med. 1938, 67, 259–265. [Google Scholar] [CrossRef]
- Kuhn, R.; Sörensen, N.A.; Birkofer, L. Über die Eisenproteide der Milz; der Bauplan des Ferritins. Dtsch. Chem. Ges. (A/B Ser.) 1940, 73, 823–837. [Google Scholar] [CrossRef]
- Schulek, E.; Floderer, I. Colorimetric determination of ferrous and ferric ions in the presence of aluminum, manganese, zinc, mercury, copper, phosphoric acid and organic substances, with special reference to drug preparations. Magy. Gyogyszeresztud. Tarsasag Ert. 1939, 15, 210–233. [Google Scholar]
- Okac, A. Effect of cuprous salts on Blau’s test for iron with α,α’-dipyridyl. Collect. Czech. Chem. Commun. 1939, 11, 531–539. [Google Scholar] [CrossRef]
- Parker, W.E.; Griffin, F.P. Some observations on the determination of iron and copper in biological material by photoelectric colorimetry. Can. J. Res. Sect. B 1939, 17, 66–70. [Google Scholar] [CrossRef]
- Koenig, E.W. Direct determination of alumina in certain silicates. Ind. Eng. Chem. Anal. Ed. 1939, 11, 532–535. [Google Scholar] [CrossRef]
- McFarlane, W.D. The reduction of iron by tissue extracts and by ascorbic acid with a note on the stabilization of ascorbic acid solutions. Biochem. J. 1936, 30, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Thiel, A.; Heinrich, H.; van Hengel, E. Basis and application of absolute colorimetry. XVII. Further experiences with the absolute colorimetry of iron. Ber. Dtsch. Chem. Ges. B 1938, 71, 756–758. [Google Scholar] [CrossRef]
- Shapiro, M.Y. Sensitive test for tantalum and columbium. Zh. Prikl. Khim. (S.-Peterburg, Russ. Fed.) 1938, 11, 1028–1032. [Google Scholar]
- Kuster, W.; Erfle, E.; Roll, E.V.; Schiller, K. Complex ferro salts. Z. Physiol. Chem. 1926, 155, 157–185. [Google Scholar]
- Schulek, E.; Floderer, I. A new method for the colorimetric determination of vitamin C. Angew. Chem. 1939, 52, 615–616. [Google Scholar] [CrossRef]
- Emmerie, A.; Engel, C. Chemical structure and properties of tocopherol (vitamin E). IV. Chemical tests for the tocopherols. Soc. Chem. Ind. Food Group 1939, 14–17. [Google Scholar]
- Emmerie, A.; Engel, C. Colorimetric determination of tocopherol (vitamin E). III. Estimation of tocopherol in blood serum. Recl. Trav. Chim. Pays-Bas 1939, 58, 895–902. [Google Scholar] [CrossRef]
- Emmerie, A.; Engel, C. Colorimetric determination of tocopherol (vitamin E). II. Adsorption experiments. Recl. Trav. Chim. Pays-Bas 1939, 58, 283–289. [Google Scholar] [CrossRef]
- Emmerie, A.; Engel, C. Colorimetric determination of α-tocopherol (vitamin E). Recl. Trav. Chim. Pays-Bas 1938, 57, 1351–1355. [Google Scholar] [CrossRef]
- Emmerie, A.; Engel, C. Colorimetric determination of dl-α-tocopherol (vitamin E). Nature 1938, 142, 873. [Google Scholar] [CrossRef]
- John, W. Über das Vitamin E. Angew. Chem. 1939, 52, 413–419. [Google Scholar] [CrossRef]
- Weil, L. The activation of arginase. J. Biol. Chem. 1935, 110, 201–209. [Google Scholar]
- Lintzel, W. Demonstration of the absorption of food iron in the form of ferrous ions. Biochem. Z. 1933, 263, 173–186. [Google Scholar]
- Rossi, A. Activation of arginase. Boll. Soc. Ital. Biol. Sper. 1939, 14, 19–20. [Google Scholar]
- Beccari, E. Pharmacological studies on the ferrous tri-α,α’-bipyridyl complex. I. General toxicity. Boll. Soc. Ital. Biol. Sper. 1938, 13, 6–8. [Google Scholar]
- Beccari, E. Pharmacological studies on the ferrous tri-α,α’-bipyridyl complex. II. Distribution in the organism. Boll. Soc. Ital. Biol. Sper. 1938, 13, 8–11. [Google Scholar]
- Michaelis, L.; Stern, K.G. Influence of heavy metals and metal complexes on the proteolytic processes. Biochem. Z. 1931, 240, 192–217. [Google Scholar]
- Stern, K.G. The catalase of colorless blood cells. Z. Physiol. Chem. 1932, 204, 259–282. [Google Scholar] [CrossRef]
- Maschmann, E.; Helmert, E. Inhibition of cathepsin and activation of papain by α-thiocarboxylic acids. Z. Physiol. Chem. 1933, 222, 207–214. [Google Scholar] [CrossRef]
- Eichholtz, F. New conceptions of the pharmacology and therapeutic use of iron. Med. Klin. (Muenchen Ger.) 1939, 35, 1279–1282. [Google Scholar]
- Bonino, G.B.; Ansidei, R.M. Investigations on the Raman effect in some organic substances. arxiv 1934, 1, 1. [Google Scholar]
- Yamasaki, K. Absorptions spektren von Metallkomplexsalzen des 2,2′-Dipyridyls. I. Bull. Chem. Soc. Jpn. 1937, 12, 390–394. [Google Scholar] [CrossRef]
- Yamasaki, K. Absorptions spektren von Metallkomplexsalzen des 2,2′-Dipyridyls. II. Bull. Chem. Soc. Jpn. 1938, 13, 538–542. [Google Scholar] [CrossRef]
- Franke, I.W. Autoxidation of unsaturated fatty acids. Justus Liebigs Ann. Chem. 1932, 498, 129–165. [Google Scholar] [CrossRef]
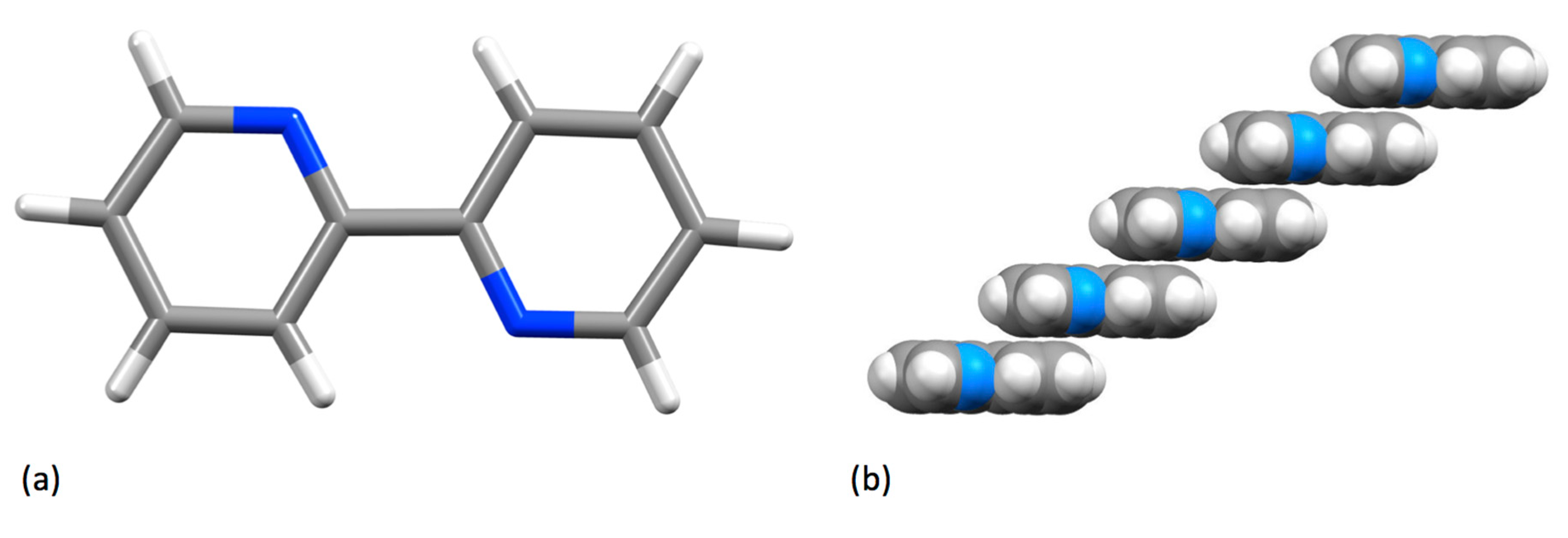
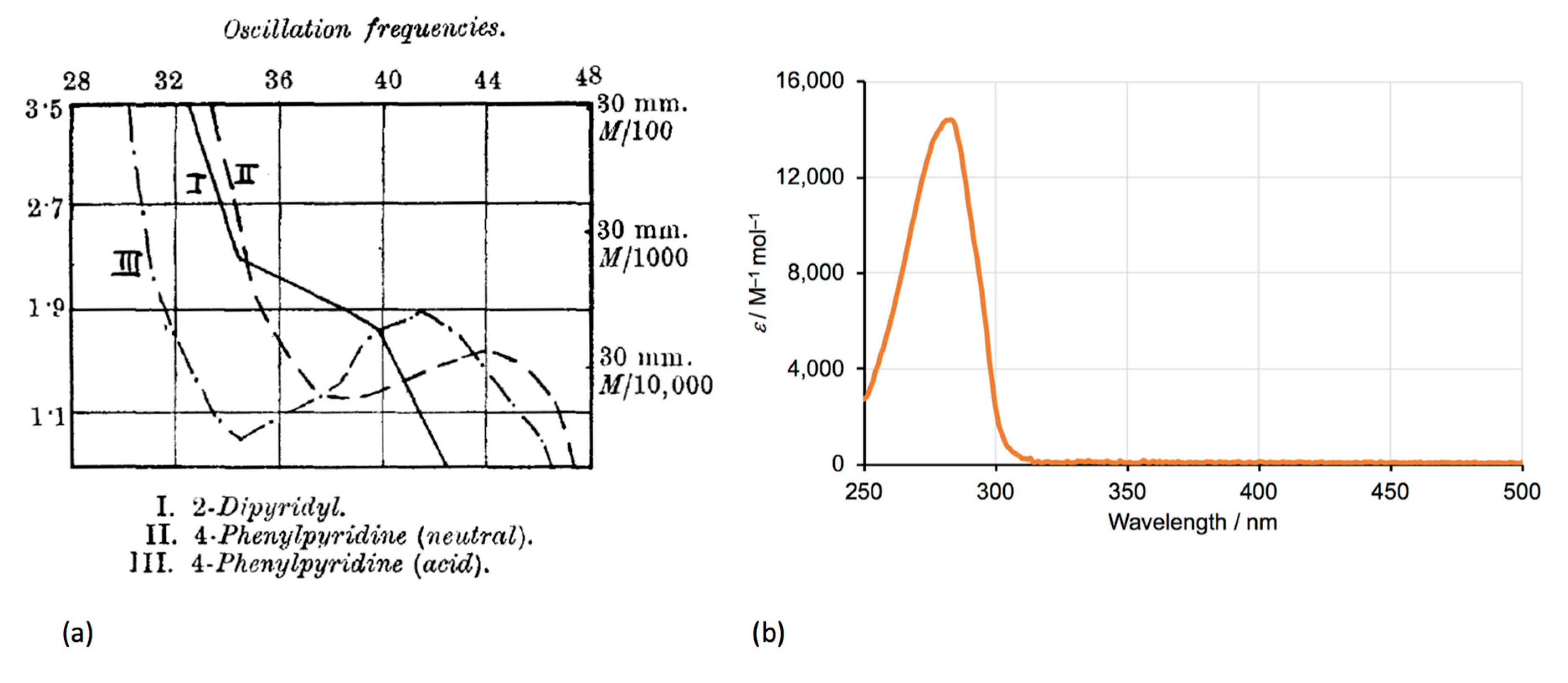

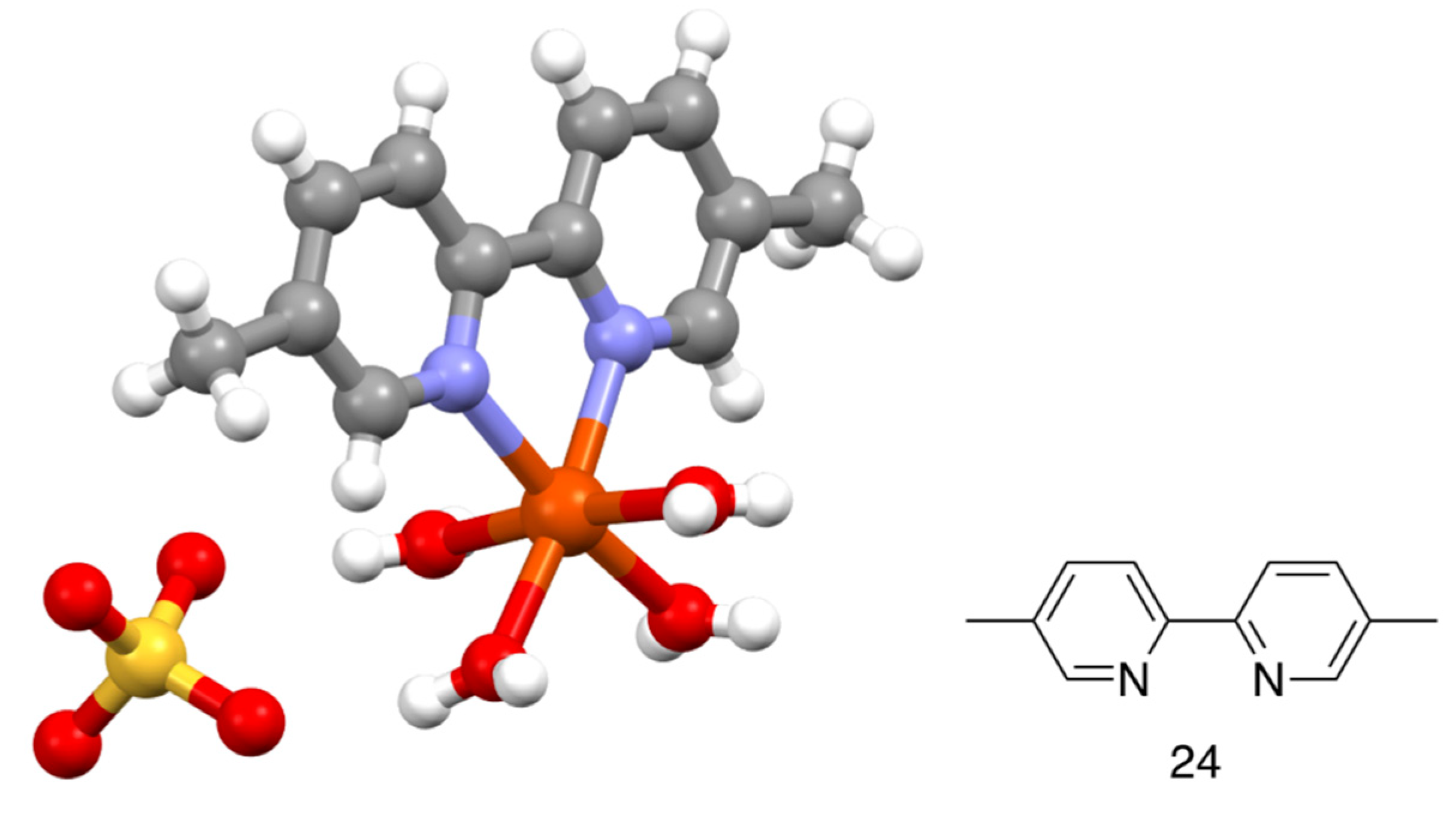
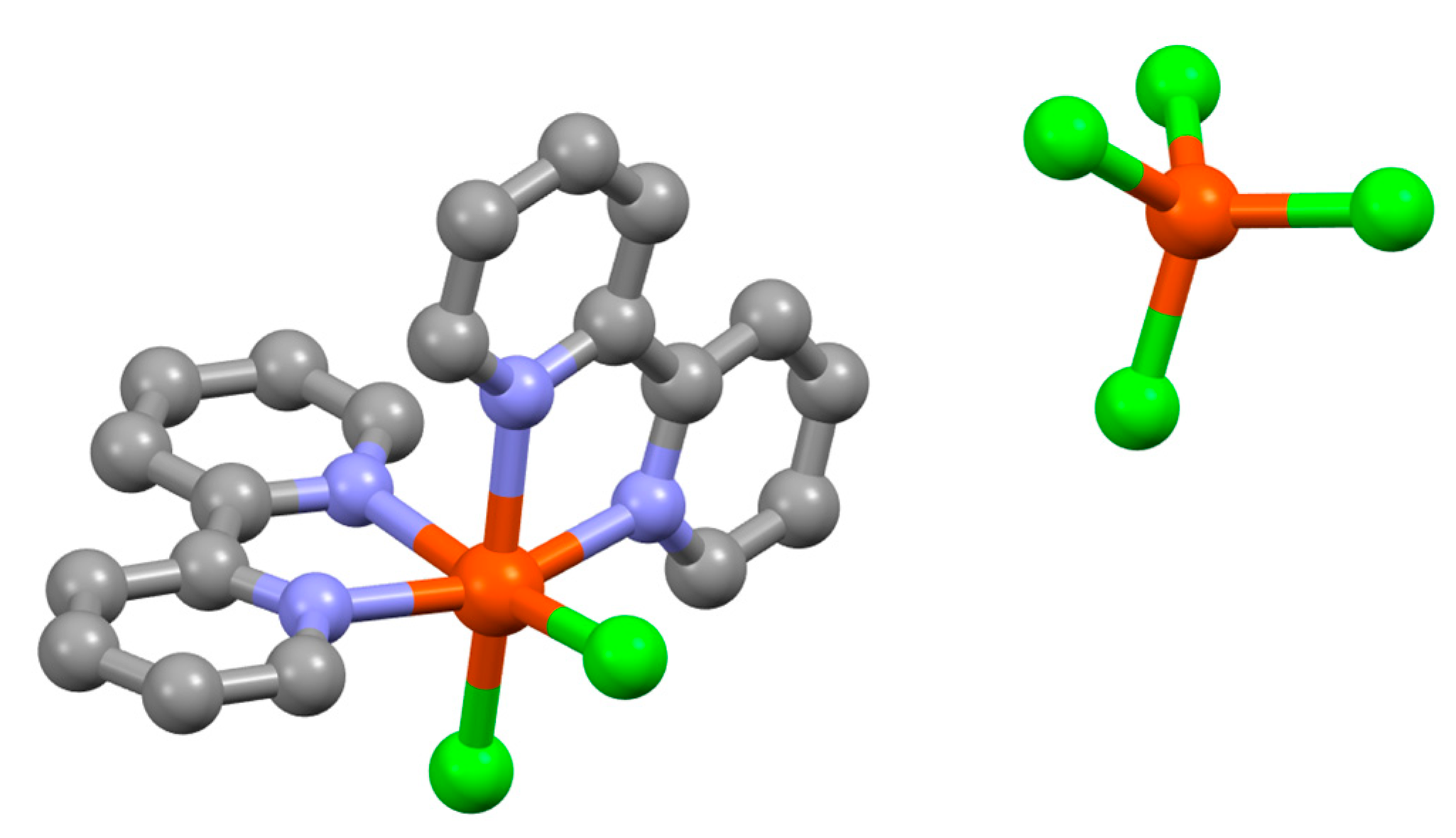
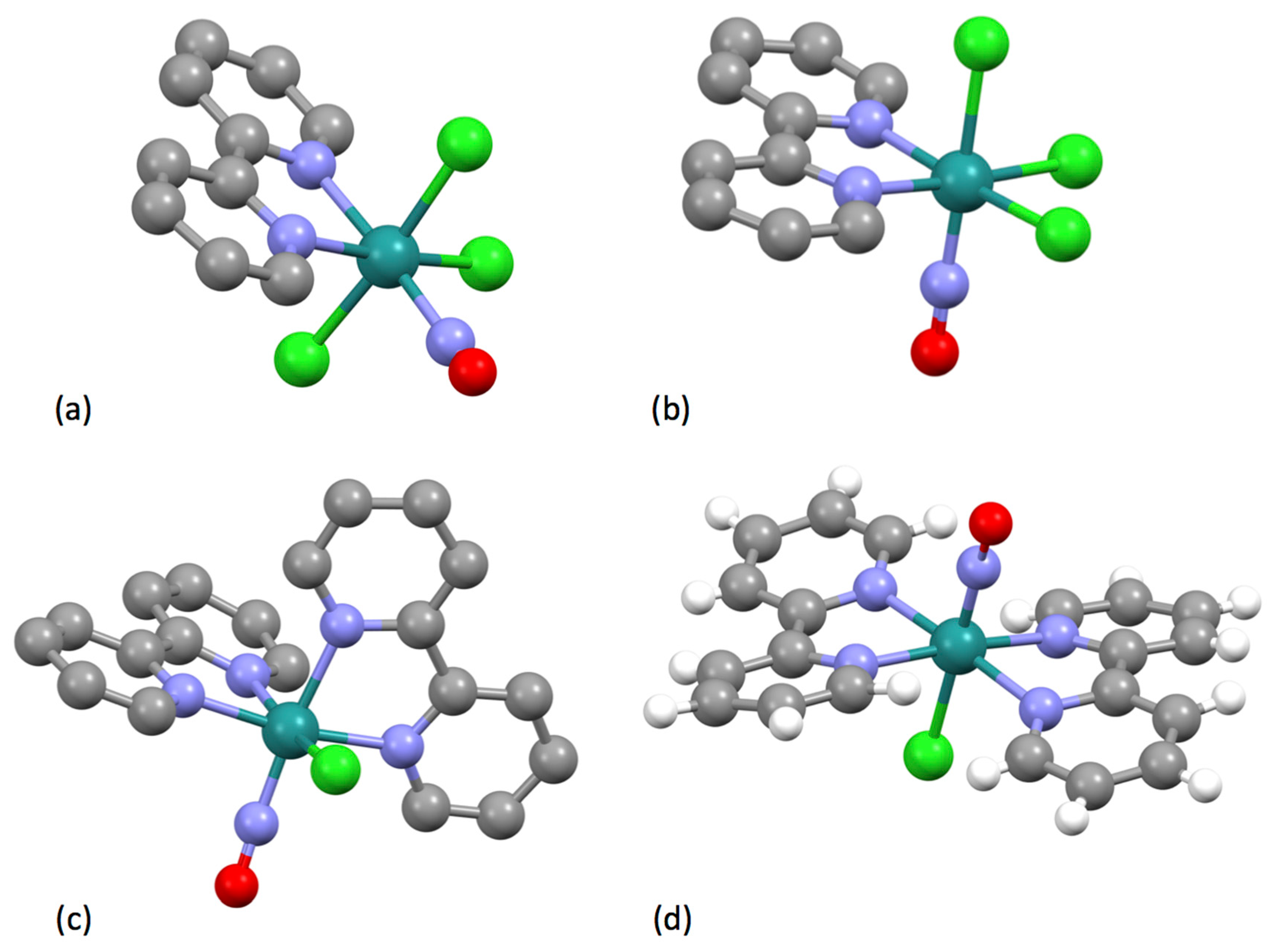
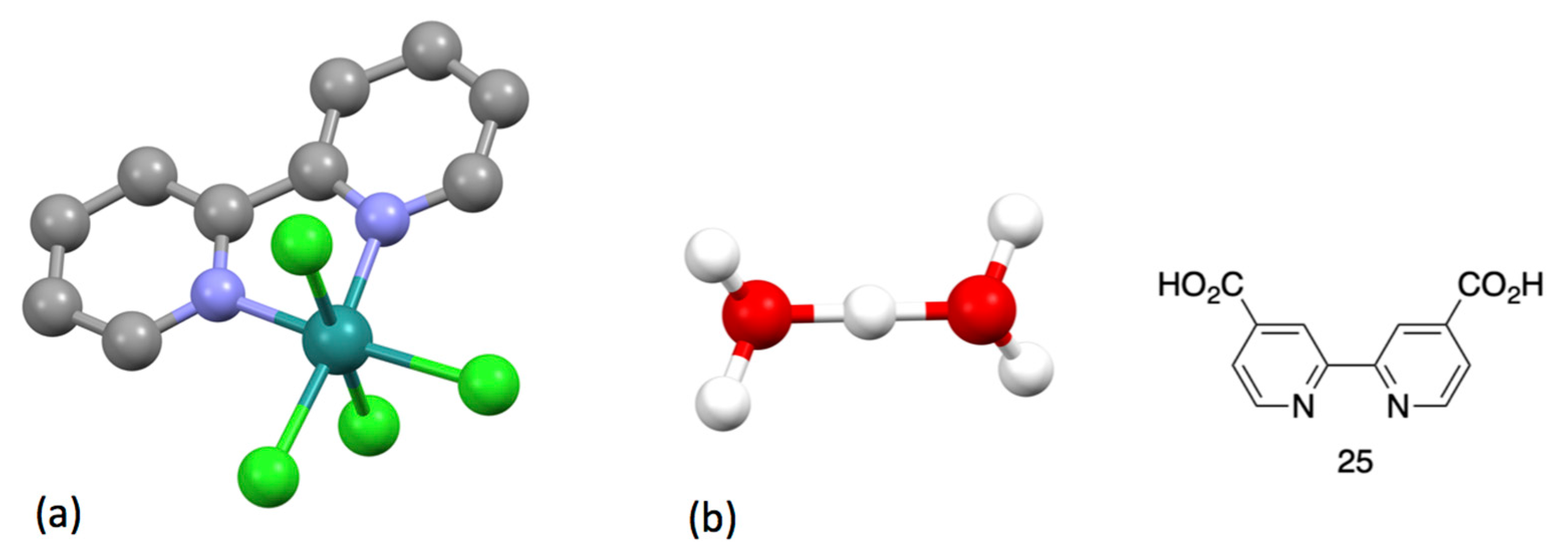
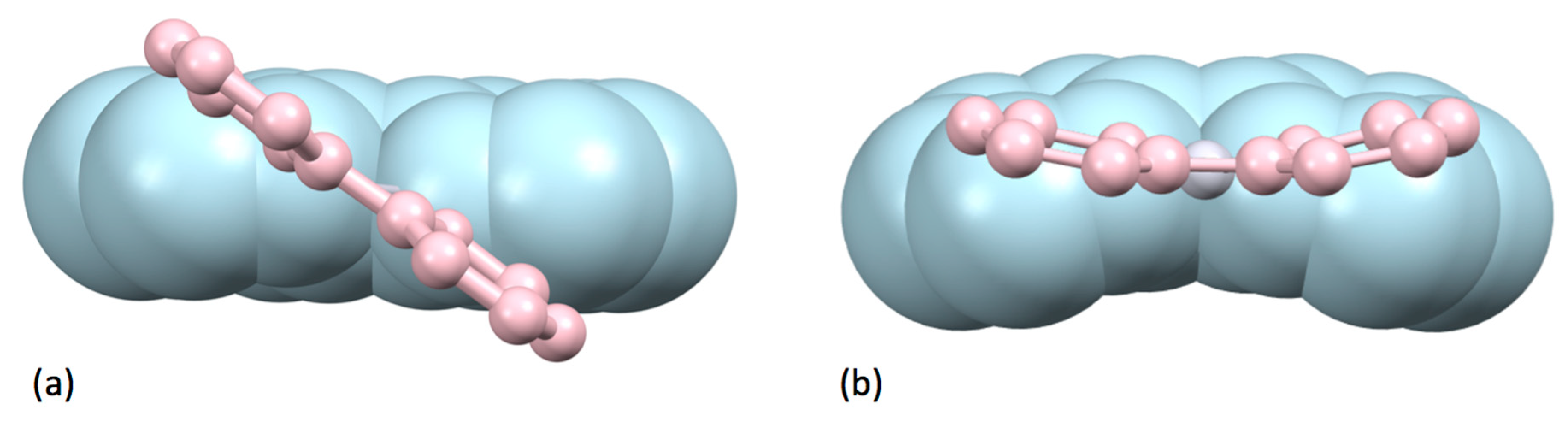
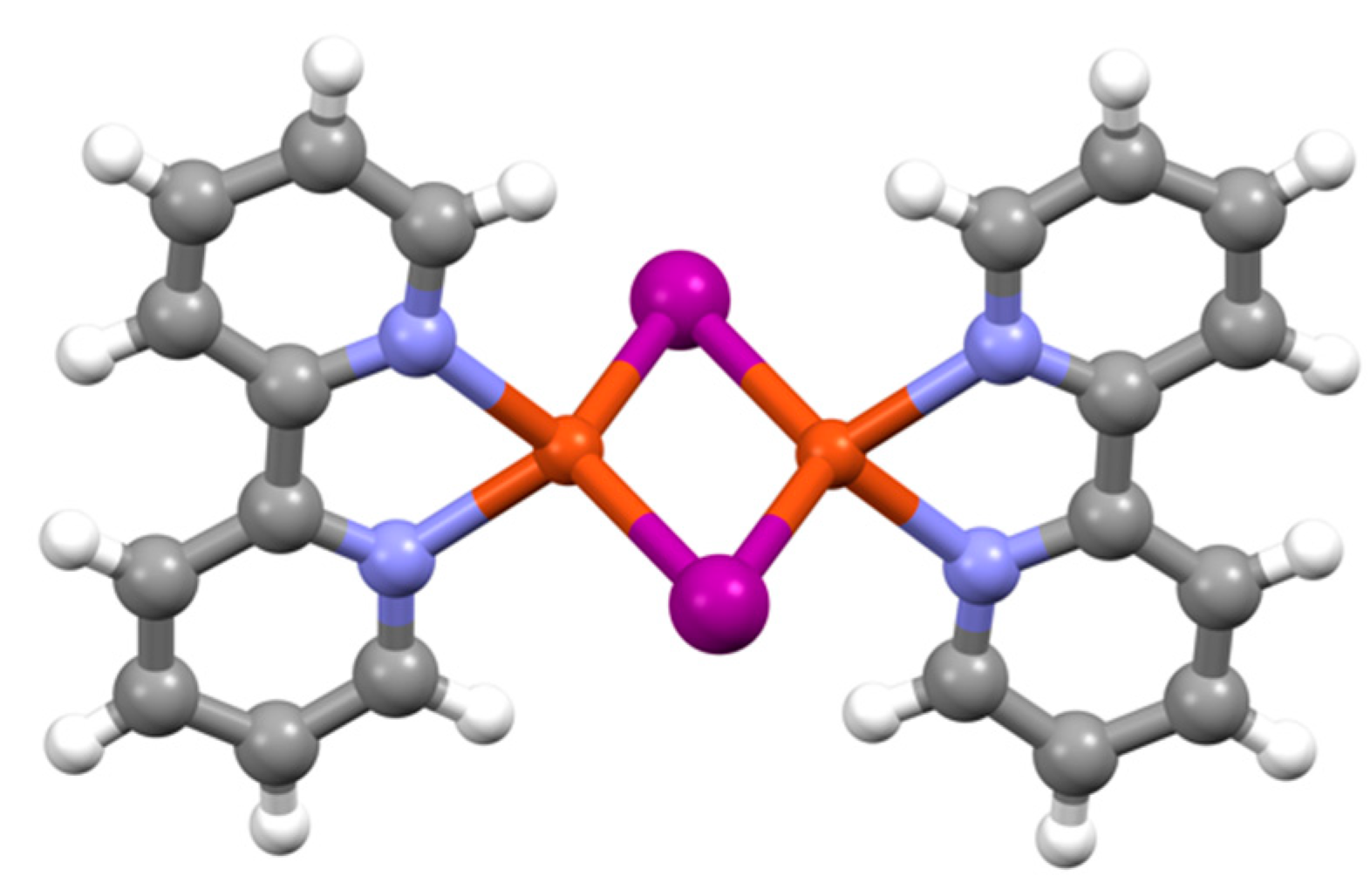
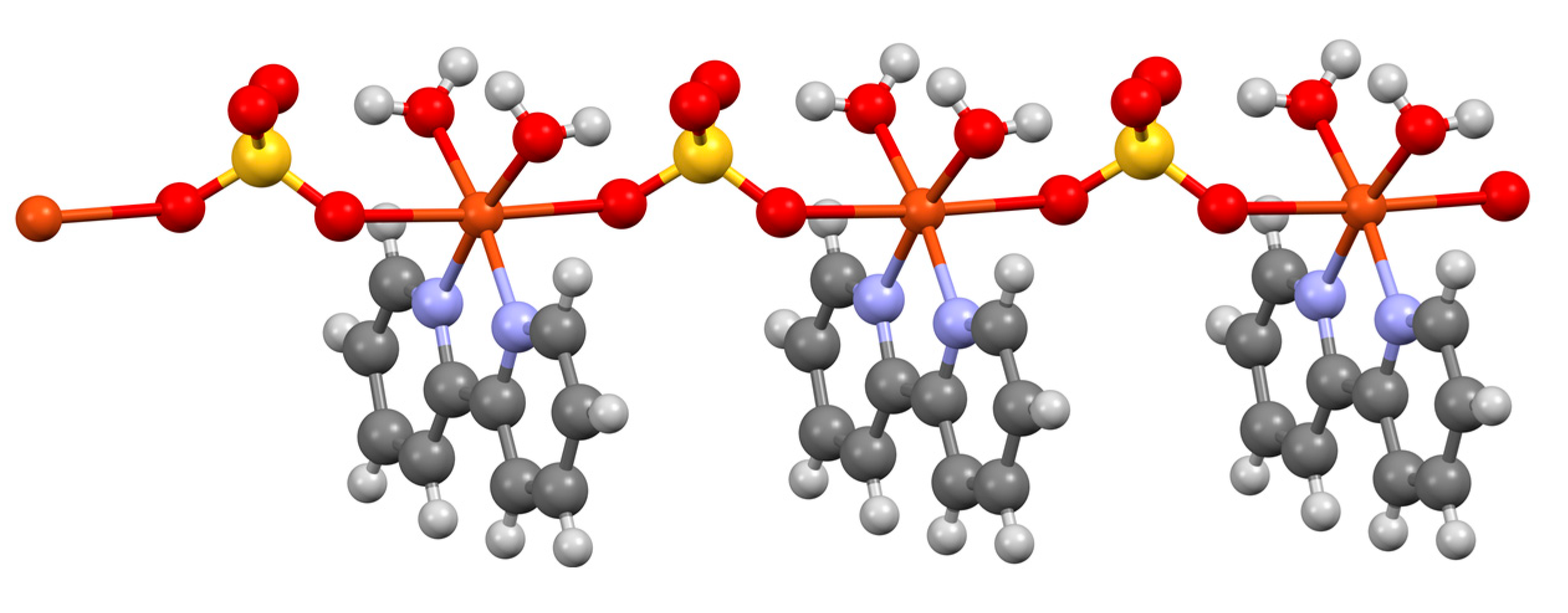
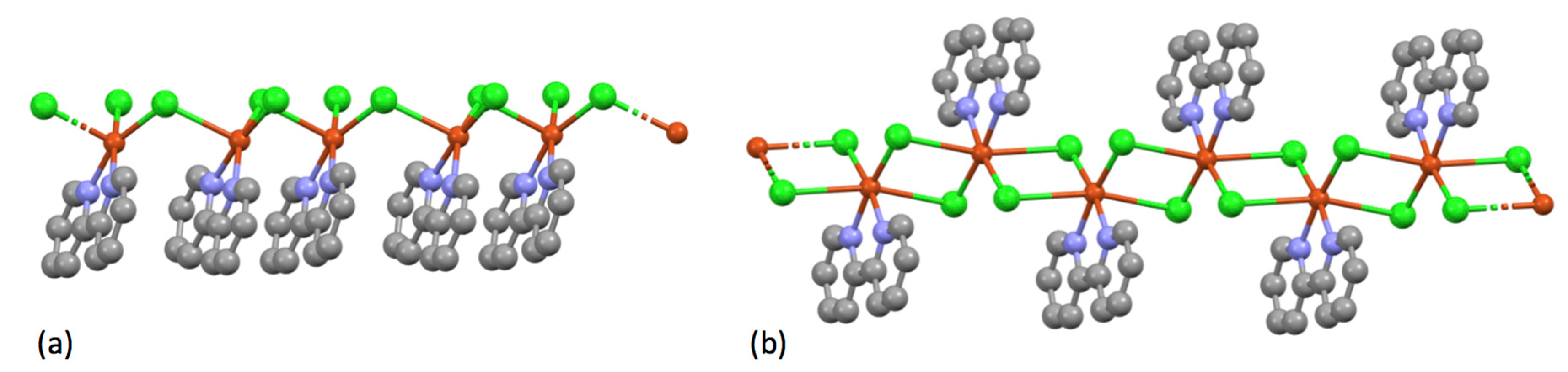
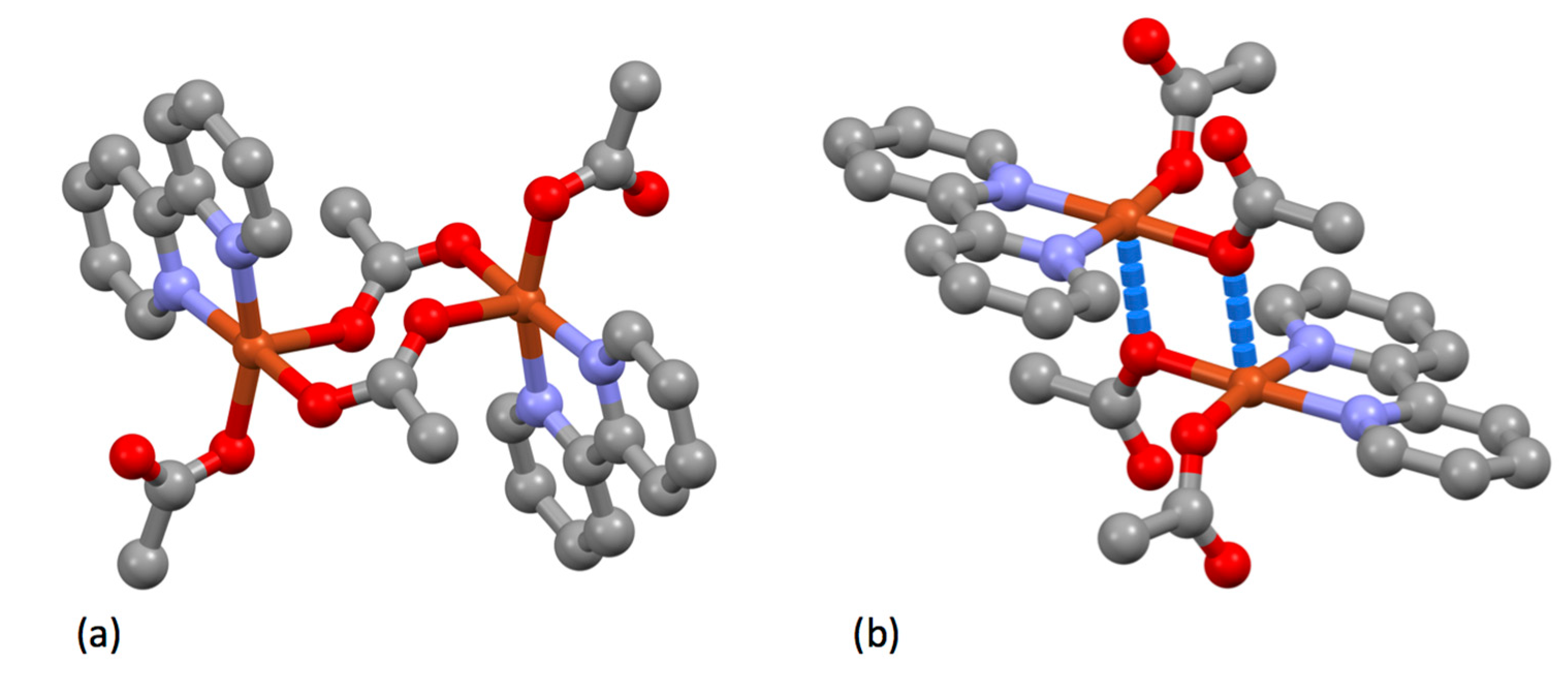
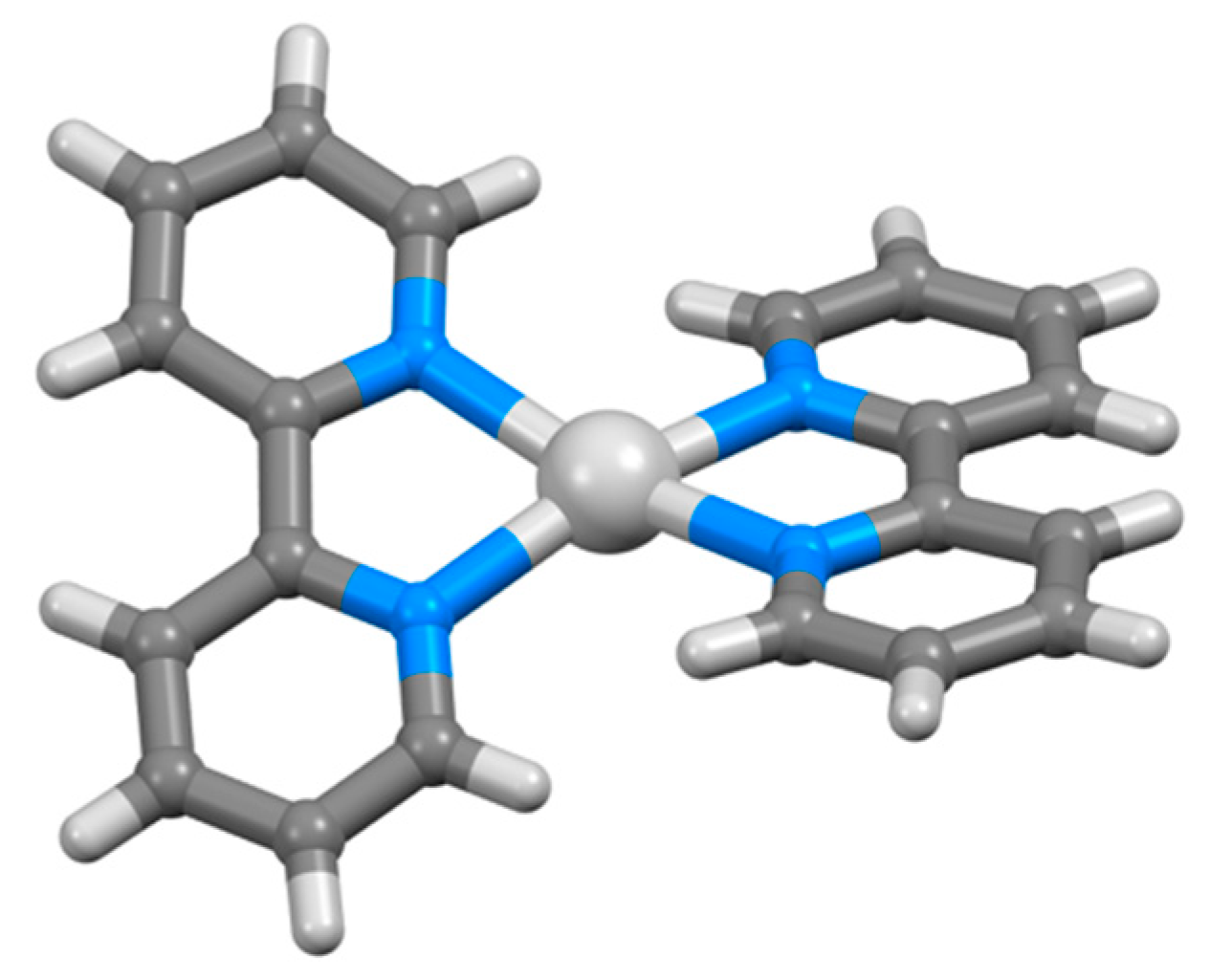
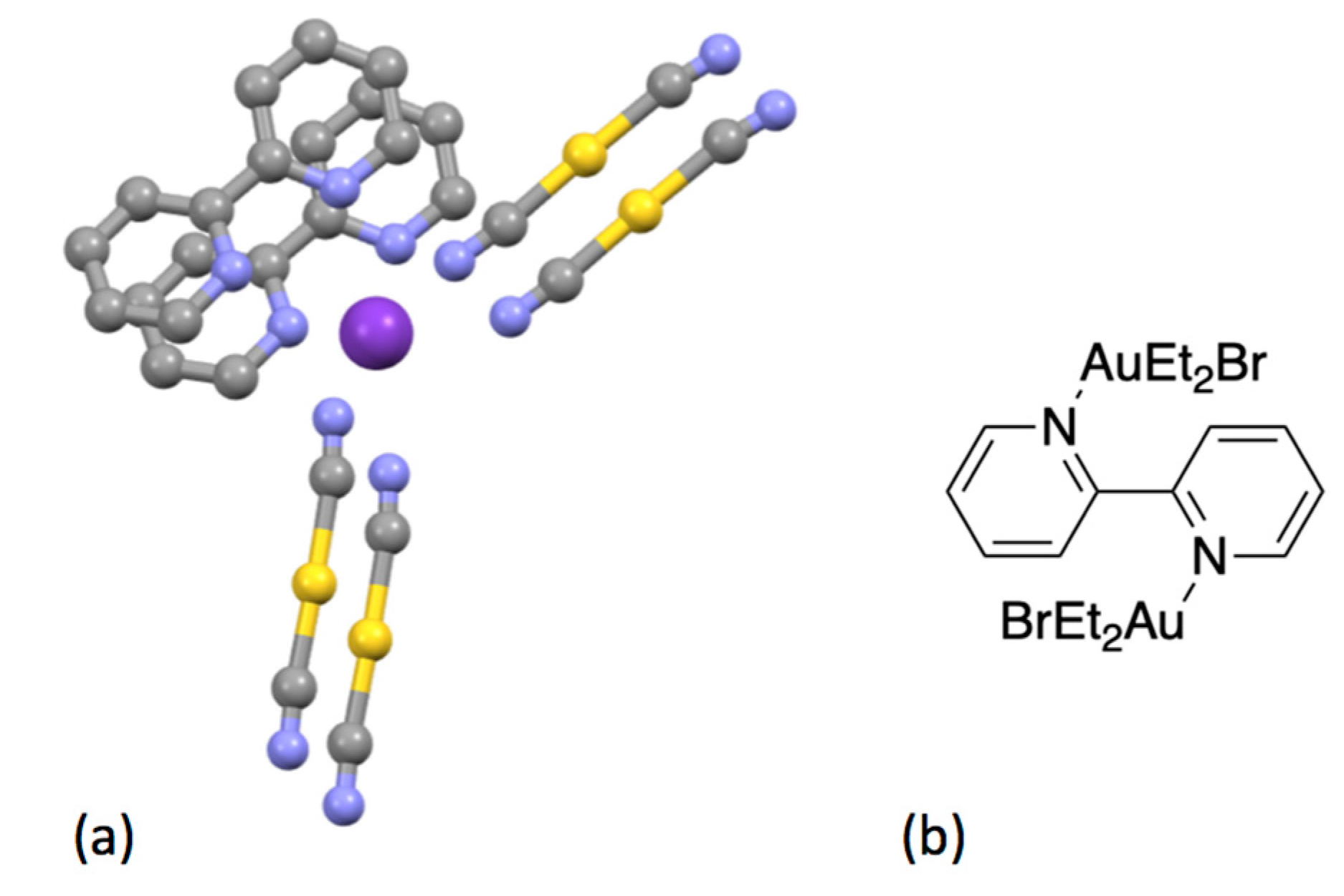
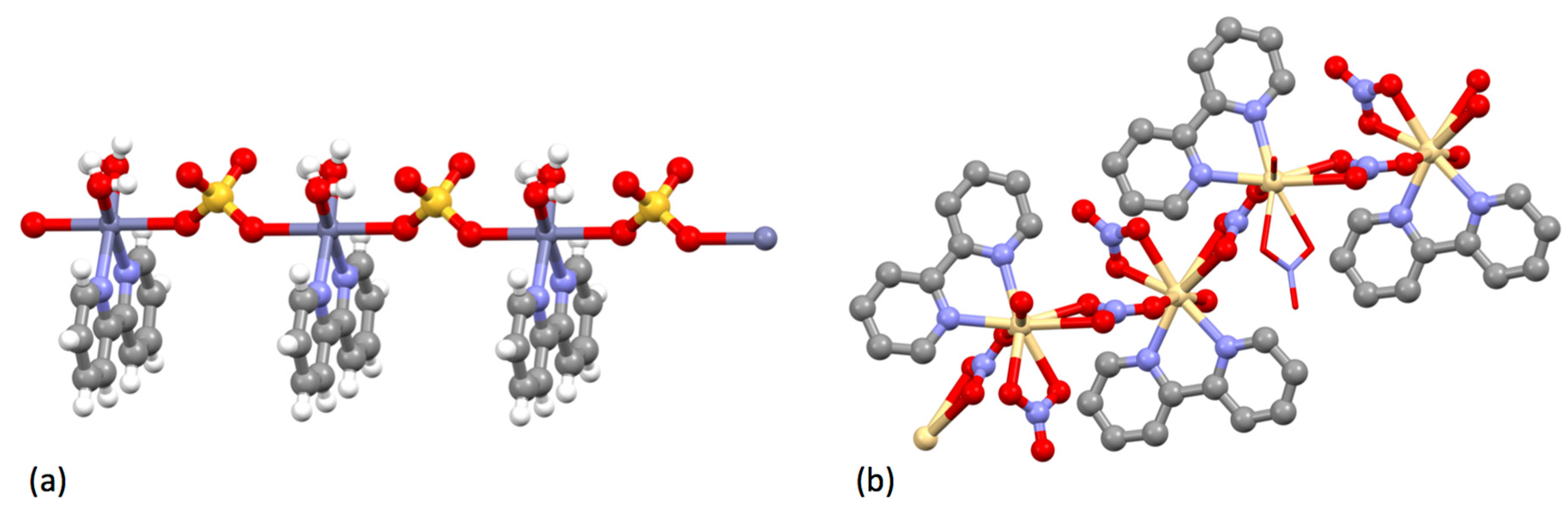
| Compound | pKa |
|---|---|
| (bpyH2)2+ | 0.05 |
| (bpyH)+ | 4.4 |
| (phenH2)2+ | −0.2 |
| (phenH)+ | 4.9 |
| (pyH)+ | 5.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constable, E.C.; Housecroft, C.E. The Early Years of 2,2′-Bipyridine—A Ligand in Its Own Lifetime. Molecules 2019, 24, 3951. https://doi.org/10.3390/molecules24213951
Constable EC, Housecroft CE. The Early Years of 2,2′-Bipyridine—A Ligand in Its Own Lifetime. Molecules. 2019; 24(21):3951. https://doi.org/10.3390/molecules24213951
Chicago/Turabian StyleConstable, Edwin C., and Catherine E. Housecroft. 2019. "The Early Years of 2,2′-Bipyridine—A Ligand in Its Own Lifetime" Molecules 24, no. 21: 3951. https://doi.org/10.3390/molecules24213951
APA StyleConstable, E. C., & Housecroft, C. E. (2019). The Early Years of 2,2′-Bipyridine—A Ligand in Its Own Lifetime. Molecules, 24(21), 3951. https://doi.org/10.3390/molecules24213951