Anti-Inflammatory Effects of Formononetin 7-O-phosphate, a Novel Biorenovation Product, on LPS-Stimulated RAW 264.7 Macrophage Cells
Abstract
1. Introduction
2. Results
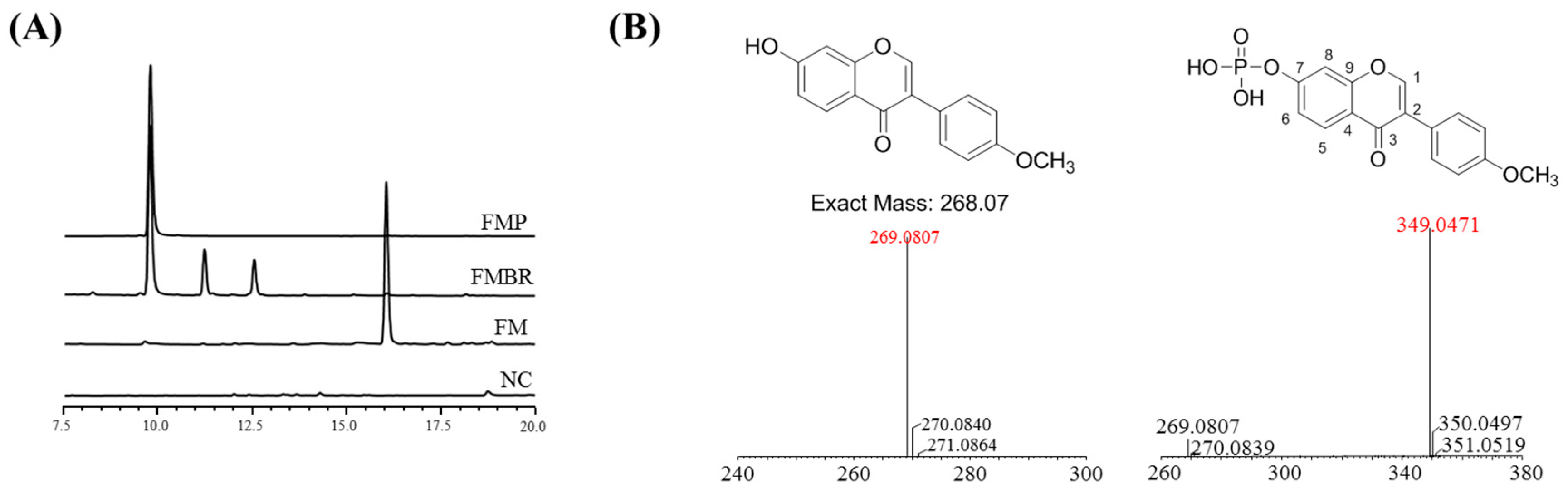
2.1. Analysis and Identification of the Biorenovation Product of Formononetin
2.2. NMR Results
2.3. Cytotoxic Effects of Compounds on RAW 264.7 Cells
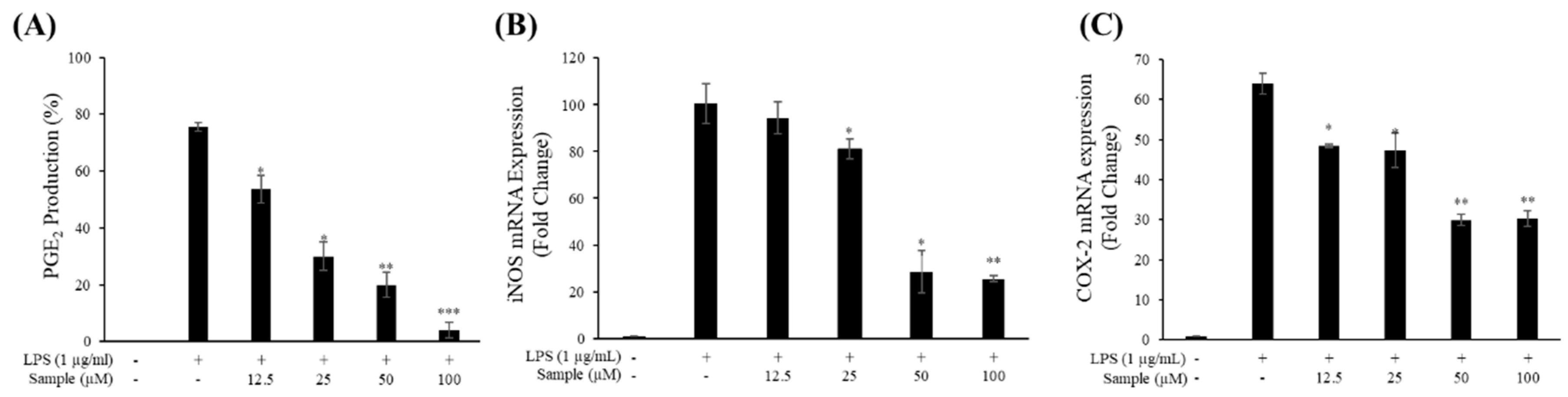
2.4. Production of NO and PGE2
2.5. mRNA Levels of iNOS and COX-2
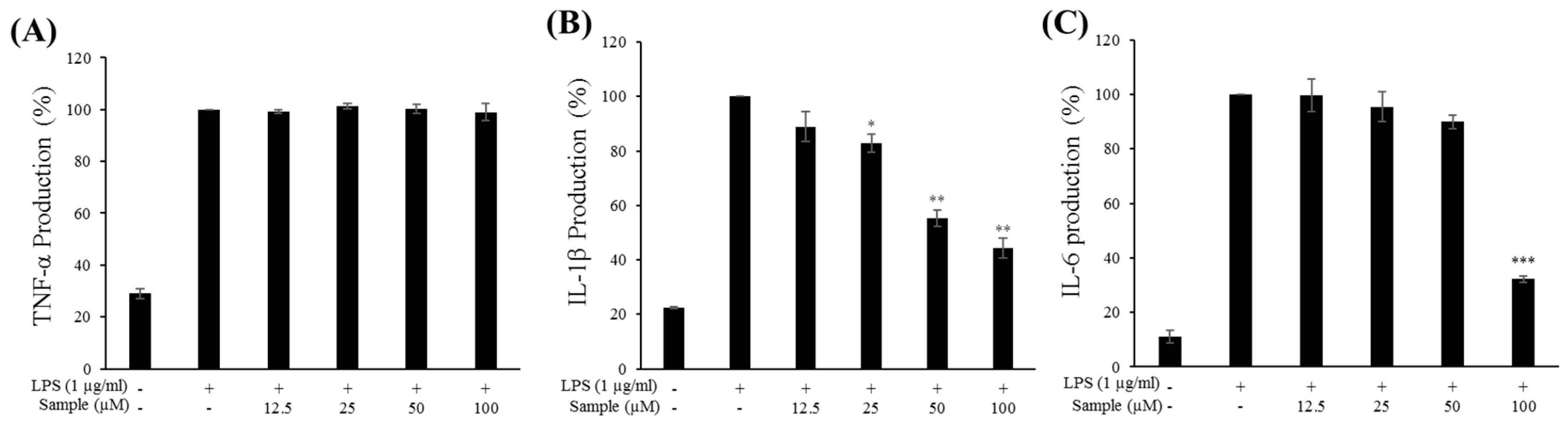
2.6. Production of Proinflammatory Cytokines
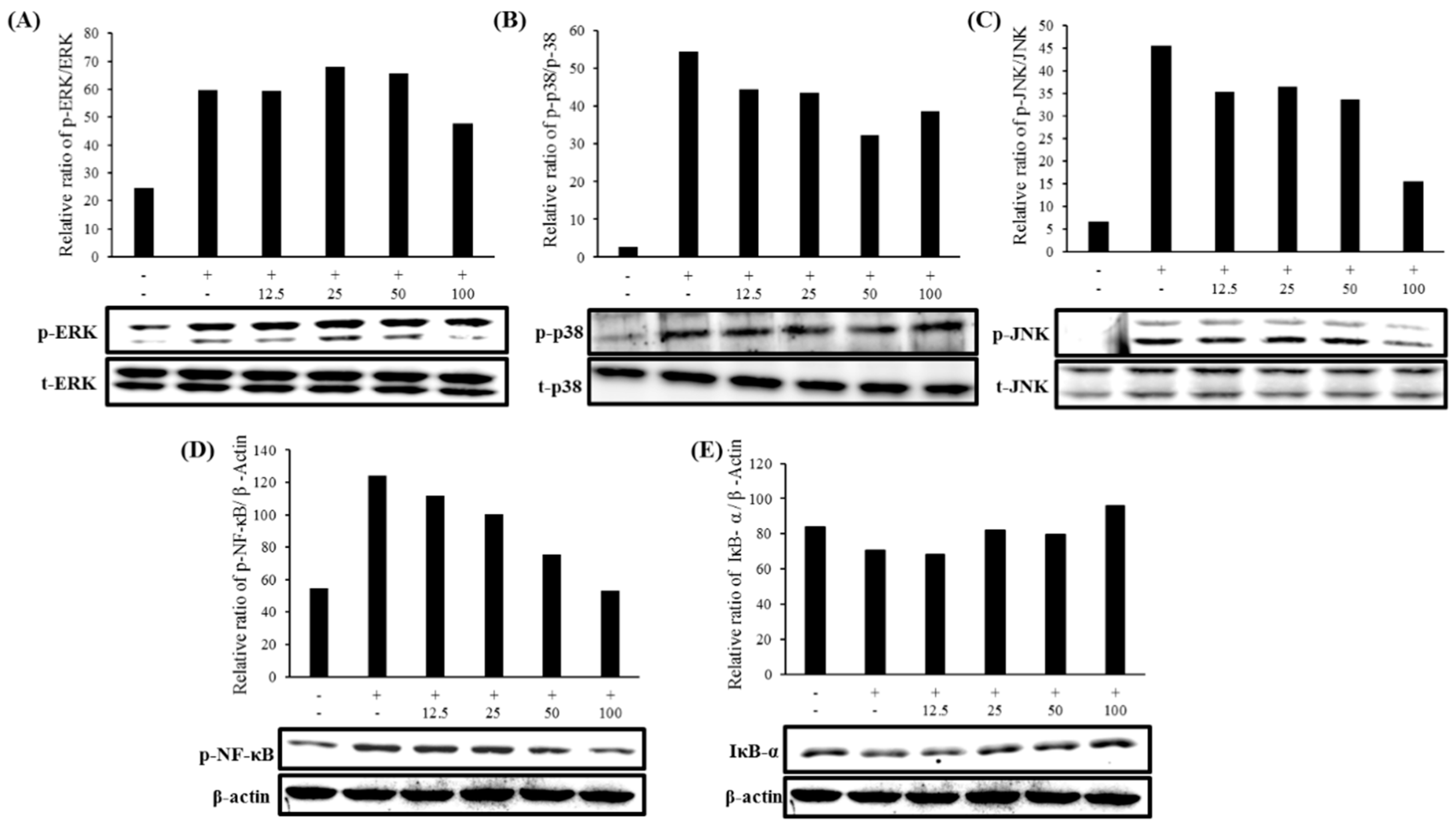
2.7. Expression of MAPK and NF-κB Pathways
3. Discussion
4. Materials and Methods
4.1. Reagents and Bacterial Strain Used in Biorenovation
4.2. Biorenovation of Formononetin
4.3. HPLC Analysis and Purification of Formononetin Biorenovation Product
4.4. LCMS and NMR Analysis of Formononetin Biorenovation Product
4.5. Cell Culture and Viability Assay
4.6. Determination of NO and PGE2 Production
4.7. Determination of TNF-α, IL-1β, and IL-6 Production
4.8. Quantitative Reverse-Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
4.9. Western Blot Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Becker, S.; Mundandhara, S.; Devlin, R.B.; Madden, M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: Further mechanistic studies. Toxicol. Appl. Pharm. 2005, 207, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.J.O.; Vasconcelos, C.C.; Pereira, F.A.N.; Silva, R.H.M.; Queiroz, P.F.D.S.; Fernandes, C.V.; Cartágenes, M.D.S.D.S. Anti-Inflammatory and Antinociceptive Activity of Pollen Extract Collected by Stingless Bee Melipona fasciculate. Int. J. Mol. Sci. 2019, 20, 4512. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, H.; Kawaguchi, T.; Kobayashi, T.; Kuramitsu, Y.; Wada, S.; Satomi, Y.; Kitagawa, T. Chronic inflammation-derived nitric oxide causes conversion of human colonic adenoma cells into adenocarcinoma cells. Exp. Cell Res. 2013, 319, 2835–2844. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Inflamm. Allergy Drug Targets 2005, 4, 281–286. [Google Scholar] [CrossRef]
- Lee, A.K.; Sung, S.H.; Kim, Y.C.; Kim, S.G. Inhibition of lipopolysaccharide-inducible nitric oxide synthase, TNF-α and COX-2 expression by sauchinone effects on I-κBα phosphorylation, C/EBP and AP-1 activation. Br. J. Pharmacol. 2003, 139, 11–20. [Google Scholar] [CrossRef]
- Udompong, S.; Mankhong, S.; Jaratjaroonphong, J.; Srisook, K. Involvement of p38 MAPK and ATF-2 signaling pathway in anti-inflammatory effect of a novel compound bis [(5-methyl) 2-furyl](4-nitrophenyl) methane on lipopolysaccharide-stimulated macrophages. Int. Immunopharmacol. 2017, 50, 6–13. [Google Scholar] [CrossRef]
- Lieb, K.; Engels, S.; Fiebich, B.L. Inhibition of LPS-induced iNOS and NO synthesis in primary rat microglial cells. Neurochem. Int. 2003, 42, 131–137. [Google Scholar] [CrossRef]
- Katsuyama, K.; Shichiri, M.; Marumo, F.; Hirata, Y. NO inhibits cytokine-induced iNOS expression and NF-κB activation by interfering with phosphorylation and degradation of IκB-α. Arter. Thromb. Vasc. Biol. 1998, 18, 1796–1802. [Google Scholar] [CrossRef]
- Kim, B.H.; Reddy, A.M.; Lee, K.H.; Chung, E.Y.; Cho, S.M.; Lee, H.; Kim, Y. Inhibitory mechanism of chroman compound on LPS-induced nitric oxide production and nuclear factor-κB activation. Biochem. Biophys. Res. Commun. 2004, 325, 223–228. [Google Scholar] [CrossRef]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Masferrer, J.L.; Zweifel, B.S.; Manning, P.T.; Hauser, S.D.; Leahy, K.M.; Smith, W.G.; Seibert, K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc. Natl. Acad. Sci. USA 1994, 91, 3228–3232. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Huo, H.R.; Yang, Y.X.; Li, C.H.; Liu, H.B.; Zhao, B.S. 2-methoxycinnamaldehyde reduces IL-1beta-induced prostaglandin production in rat cerebral endothelial cells. Biol. Pharm. Bull. 2006, 29, 2214–2221. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wang, W.H.; Chen, S.H.; Chang, Y.W.; Hung, L.C.; Chen, C.Y.; Chen, Y.H. Lipopolysaccharide-induced nitric oxide, prostaglandin E2, and cytokine production of mouse and human macrophages are suppressed by pheophytin-b. Int. J. Mol. Sci. 2017, 18, 2637. [Google Scholar] [CrossRef]
- Riese, J.; Hoff, T.; Nordhoff, A.; DeWitt, D.L.; Resch, K.; Kaever, V. Transient expression of prostaglandin endoperoxide synthase-2 during mouse macrophage activation. J. Leukoc. Biol. 1994, 55, 476–482. [Google Scholar] [CrossRef]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264. 7 cells through suppressing NF-κB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar] [CrossRef]
- Liew, C.Y.; Lam, K.W.; Kim, M.K.; Harith, H.H.; Tham, C.L.; Cheah, Y.K.; Israf, D.A. Effects of 3-(2-Hydroxyphenyl)-1-(5-methyl-furan-2-yl) propenone (HMP) upon signalling pathways of lipopolysaccharide-induced iNOS synthesis in RAW 264.7 cells. Int. Immunopharmacol. 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef]
- Huang, P.; Han, J.; Hui, L. MAPK signaling in inflammation-associated cancer development. Protein & Cell 2010, 1, 218–226. [Google Scholar]
- Zhao, Y.; Liu, J.; Liu, C.; Zeng, X.; Li, X.; Zhao, J. Anti-inflammatory effects of p-coumaric acid in LPS-stimulated RAW264. 7 cells: Involvement of NF-κB and MAPKs pathways. J. Med. Chem. 2016, 6, 327–330. [Google Scholar]
- Bi, W.Y.; Fu, B.D.; Shen, H.Q.; Wei, Q.; Zhang, C.; Song, Z.; Yi, P.F. Sulfated derivative of 20 (S)-ginsenoside Rh2 inhibits inflammatory cytokines through MAPKs and NF-kappa B pathways in LPS-induced RAW264. 7 macrophages. Inflammation 2012, 35, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, H.; Art, J.; Pautz, A. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide 2010, 211–267. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Gao, L.; Yin, H.; Xie, Z.; Wang, D.; Han, X. Formononetin attenuates IL-1β-induced apoptosis and NF-κB activation in INS-1 cells. Molecules 2012, 17, 10052–10064. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, J.; Xin, M.; Huang, W.; Chen, X. Formononetin induces cell cycle arrest of human breast cancer cells via IGF1/PI3K/Akt pathways in vitro and in vivo. Trends Endocrinol. Metab. 2011, 43, 681–686. [Google Scholar] [CrossRef]
- Ji, Z.N.; Zhao, W.Y.; Liao, G.R.; Choi, R.C.; Lo, C.K.; Dong, T.T.X.; Tsim, K.W.K. In vitro estrogenic activity of formononetin by two bioassay systems. Gynecol. Endocrinol. 2006, 22, 578–584. [Google Scholar] [CrossRef]
- Tao, S.U.N.; Rui, L.I.U.; Cao, Y.X. Vasorelaxant and antihypertensive effects of formononetin through endothelium-dependent and-independent mechanisms. Acta Pharmacol. Sin. 2011, 32, 1009. [Google Scholar]
- Kim, K.M.; Park, J.S.; Choi, H.R.; Kim, M.S.; Seo, J.H.; Pandey, R.P.; Kim, S.Y. Biosynthesis of novel daidzein derivatives using Bacillus amyloliquefaciens whole cells. Biocatal Biotransformation. 2018, 36, 469–475. [Google Scholar] [CrossRef]
- Choi, H.R.; Park, J.S.; Kim, K.M.; Kim, M.S.; Ko, K.W.; Hyun, C.G.; Kim, S.Y. Enhancing the antimicrobial effect of genistein by biotransformation in microbial system. Ind. Eng. Chem. 2018, 63, 255–261. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Dong, L.; Yin, L.; Zhang, Y.; Fu, X.; Lu, J. Anti-inflammatory effects of ononin on lipopolysaccharide-stimulated RAW 264.7 cells. Mol. Immunol. 2017, 83, 46–51. [Google Scholar] [CrossRef]
- Marzocchella, L.; Fantini, M.; Benvenuto, M.; Masuelli, L.; Tresoldi, I.; Modesti, A.; Bei, R. Dietary flavonoids: Molecular mechanisms of action as anti-inflammatory agents. Recent Pat. Inflamm Allergy Drug Discov. 2011, 5, 200–220. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-S.; Park, J.-S.; Chung, Y.C.; Jang, S.; Hyun, C.-G.; Kim, S.-Y. Anti-Inflammatory Effects of Formononetin 7-O-phosphate, a Novel Biorenovation Product, on LPS-Stimulated RAW 264.7 Macrophage Cells. Molecules 2019, 24, 3910. https://doi.org/10.3390/molecules24213910
Kim M-S, Park J-S, Chung YC, Jang S, Hyun C-G, Kim S-Y. Anti-Inflammatory Effects of Formononetin 7-O-phosphate, a Novel Biorenovation Product, on LPS-Stimulated RAW 264.7 Macrophage Cells. Molecules. 2019; 24(21):3910. https://doi.org/10.3390/molecules24213910
Chicago/Turabian StyleKim, Min-Seon, Jin-Soo Park, You Chul Chung, Sungchan Jang, Chang-Gu Hyun, and Seung-Young Kim. 2019. "Anti-Inflammatory Effects of Formononetin 7-O-phosphate, a Novel Biorenovation Product, on LPS-Stimulated RAW 264.7 Macrophage Cells" Molecules 24, no. 21: 3910. https://doi.org/10.3390/molecules24213910
APA StyleKim, M.-S., Park, J.-S., Chung, Y. C., Jang, S., Hyun, C.-G., & Kim, S.-Y. (2019). Anti-Inflammatory Effects of Formononetin 7-O-phosphate, a Novel Biorenovation Product, on LPS-Stimulated RAW 264.7 Macrophage Cells. Molecules, 24(21), 3910. https://doi.org/10.3390/molecules24213910






