Antiproliferative Aspect of Benzimidazole Derivatives’ Activity and Their Impact on NF-κB Expression
Abstract
1. Introduction
2. Results and Discussion
2.1. Tested Compounds
2.2. Cytotoxic Activity
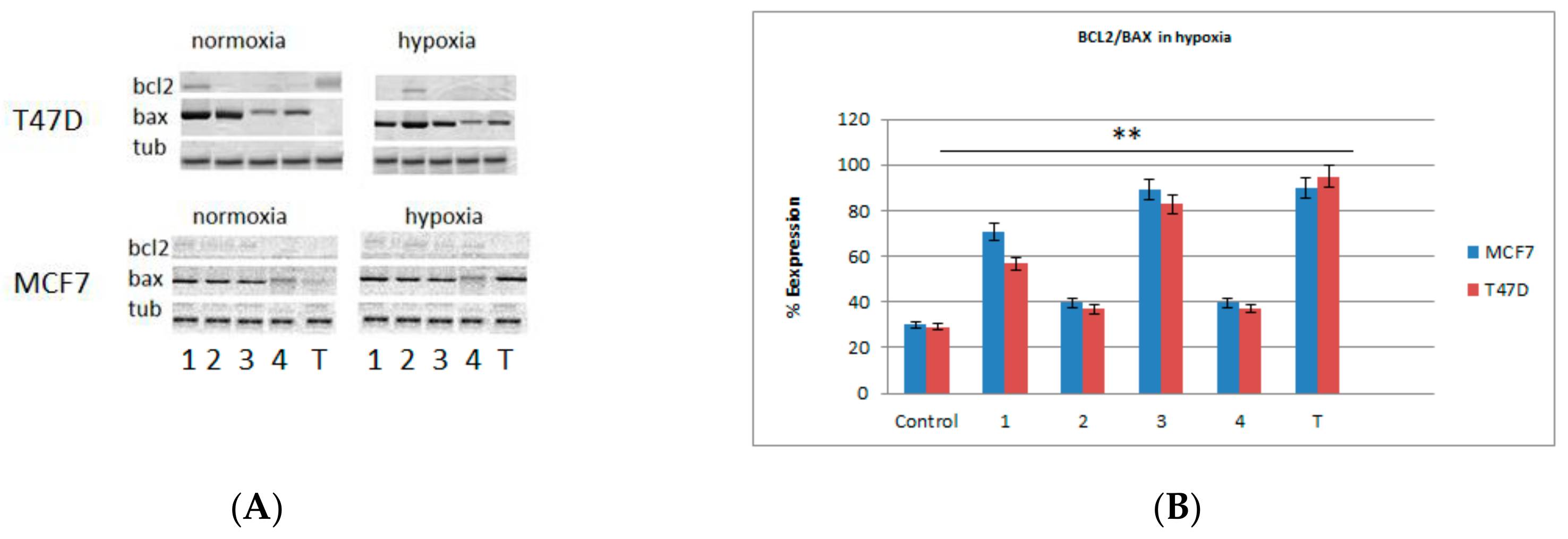
2.3. Western Blots and Real-Time PCR Analysis for Bax and bcl2 Genes
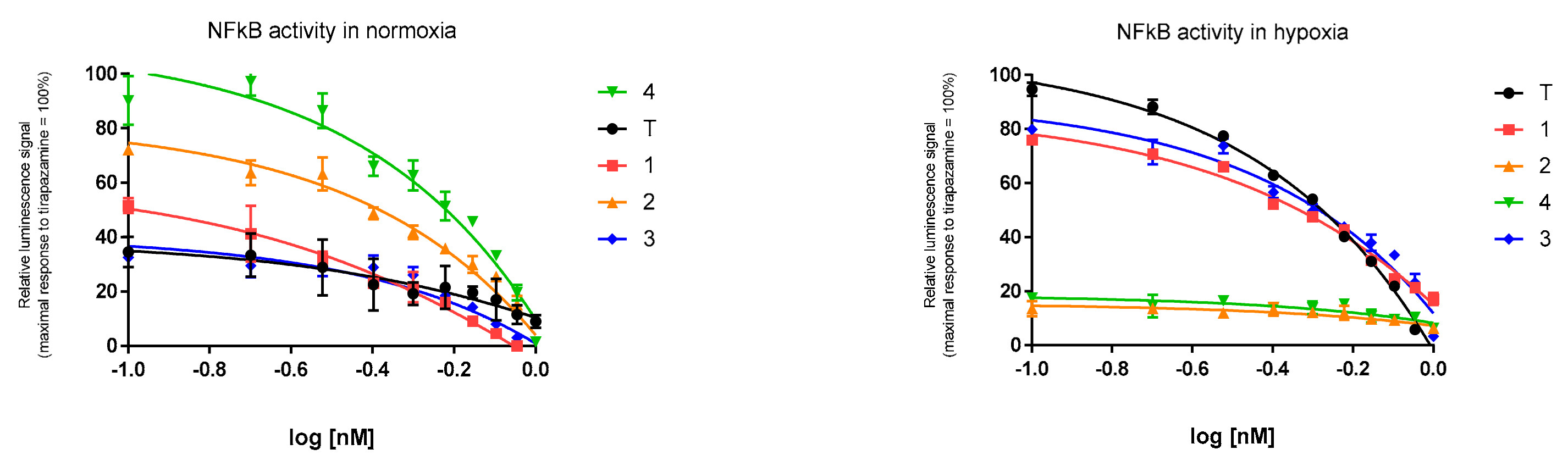
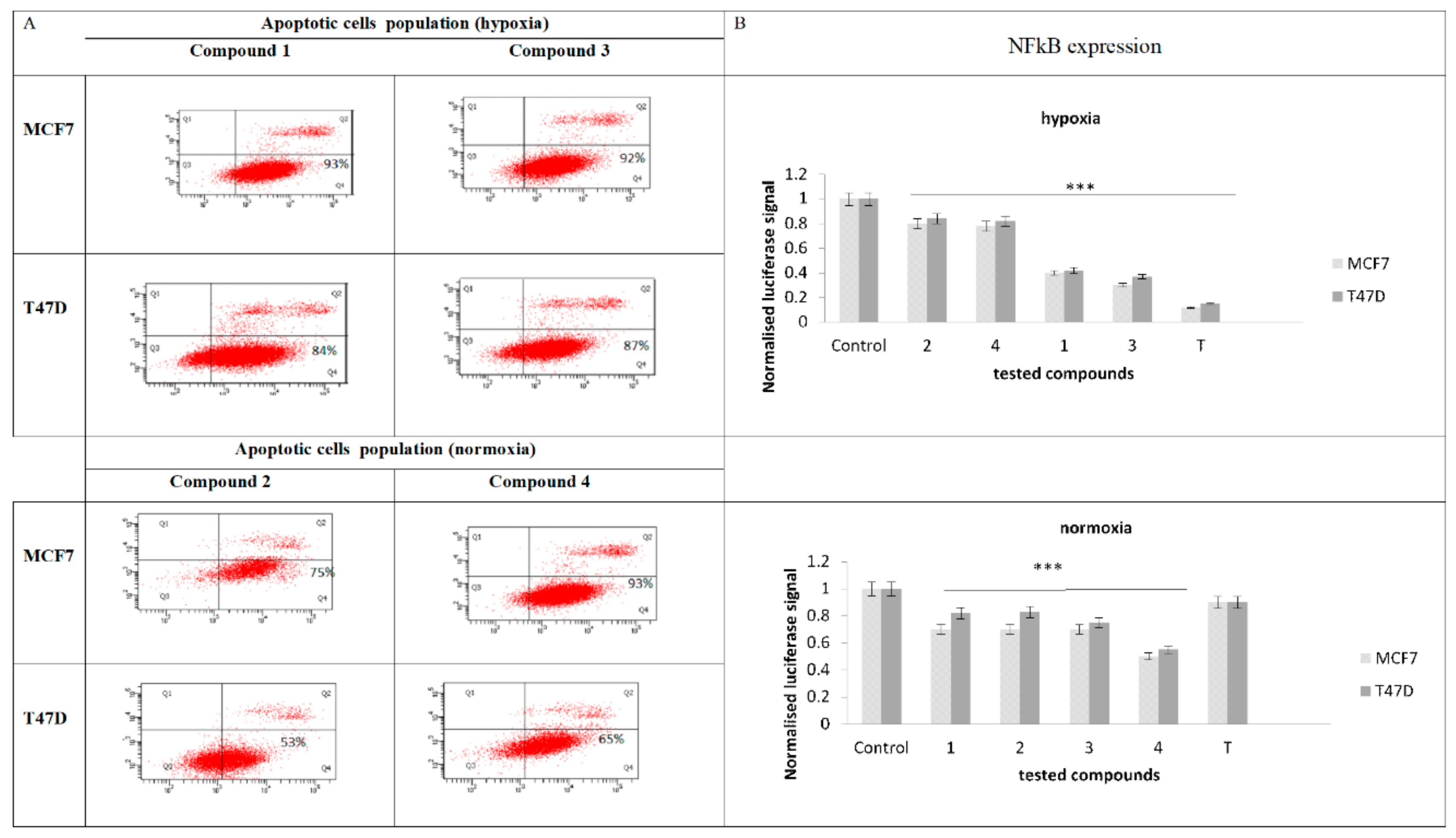
2.4. Expresion of NF-κB
2.5. Evaluation of Apoptotic Cell Population and NF-κB Expression
3. Experimental Section
3.1. Materials and Methods
3.2. Cell Culture
3.3. WST Cytotoxic Assay
3.4. Western Blot Analysis of the Expression of Bax and bcl2 Proteins
Real-Time PCR Analysis of Bax and bcl2 Gene Expression
3.5. Transfection of T47D and MCF7 Cells with NF-κB Gene and Reporter Vector LUC
3.6. Transactivation Assay
3.7. Flow Cytometry Analysis of Apoptosis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yip, N.C.; Fombon, I.S.; Liu, P.; Brown, S.; Kannappan, V.; Armesilla, A.L.; Xu, B.; Cassidy, J.; Darling, J.L.; Wang, W. Disulfiram modulated ROS-MAPK and NFκB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br. J. Cancer 2011, 104, 1564–1574. [Google Scholar] [CrossRef] [PubMed]
- Biswas, D.K.; Shi, Q.; Baily, S.; Strickland, I.; Ghosh, S.; Pardee, A.B.; Iglehart, J.D. NF-kB activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc. Nat. Acad. Sci. USA 2004, 101, 10137–10142. [Google Scholar] [CrossRef] [PubMed]
- Dutta, J.; Fan, Y.; Gupta, N.; Fan, G.; Gelinas, C. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene 2006, 25, 6800–6816. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef] [PubMed]
- McKay, L.I.; Cidlowski, J.A. Molecular control of immune/inflammatoryresponses: Interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr. Rev. 1999, 20, 435–459. [Google Scholar] [PubMed]
- Ghosh, S.; May, M.J.; Kopp, E.B. NF-κB and Rel proteins: Evolutionarily conserved mediators of immune responses. Ann. Rev. Immunol. 1998, 16, 225–260. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xu, B.; Pandey, S.; Goessl, E.; Brown, J.; Armesilla, A.L.; Darling, J.L.; Wang, W. Disulfiram/copper complex inhibiting NFκB activity and potentiating cytotoxic effect of gemcitabine on colon and breast cancer cell lines. Cancer Lett. 2009, 290, 104–113. [Google Scholar] [CrossRef]
- Wang, C.Y.; Cusack, J.C.; Liu, R., Jr.; Baldwin, A.S., Jr. Control of inducible chemoresistance: Enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-κB. Nat. Med. 1999, 5, 412–417. [Google Scholar] [CrossRef]
- Wang, F.; He, Y.L.; Peng, D.X.; Liu, M.B. Expressions of nuclear factor-κB and intercellular adhesion molecule-1 in endometriosis. Di Yi Jun Yi Da Xue Xue Bao 2005, 25, 703–705. [Google Scholar]
- Israe, l.A. The IKK complex: An integrator of all signals that activate NF-κB. Trends Cell Biol. 2000, 10, 129–133. [Google Scholar] [CrossRef]
- D’Acquisto, F.; May, M.J.; Ghosh, S. Inhibition of nuclear factor κB (NF-B): An emerging theme in anti-inflammatory therapies. Mol. Interv. 2002, 2, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Błaszczak-Świątkiewicz, K.; Sikora, J.; Szymański, J.; Danilewicz, M.; Mikiciuk-Olasik, E. Biological evaluation of the toxicity and cell cycle interruption by some benzimidazole derivatives. Tumor Biol. 2016, 37, 11135–11145. [Google Scholar] [CrossRef] [PubMed]
- Błaszczak-Świątkiewicz, K.; Mirowski, M.; Kaplińska, K.; Kruszyński, R.; Trzęsowska-Kruszyńska, A.; Mikiciuk-Olasik, E. New benzimidazole derivatives with potential cytotoxic activity—Study of their stability by RP-HPLC. Acta Biochim. Pol. 2012, 59, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Sas, L.; Lardon, F.; Vermeulen, P.B.; Hauspy, J.; Van Dam, P.; Pauwels, P.; Dirix, L.Y.; Van Laere, S.J. The interaction between ER and NFкB in resistance to endocrine therapy. Breast Cancer Res. 2012, 14, 212. [Google Scholar] [CrossRef]
- Kabel, A.M.; Altalhi, D.; Alsharabi, H.; Qadi, O.; Ad Khan, M. Tamoxifen-resistant Breast Cancer: Causes of resistance and Possible Management. J. Cancer Res. Treat. 2016, 4, 37–40. [Google Scholar]
- Yager, J.D.; Davidson, N.E. Estrogen carcinogenesis in breast cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar] [CrossRef]
- Zaks-Zilberman, M.; Zaks, T.Z.; Vogel, S.N. Induction of proinflammatory and chemokine genes by lipopolysaccharide and paclitaxel (Taxol) in murine and human breast cancer cell lines. Cytokine 2001, 15, 156–165. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Hirsch, H.A.; Struhl, K. An epigenetic switch involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 2009, 139, 693–706. [Google Scholar] [CrossRef]
- Van Laere, S.; Limame, R.; Van Marck, E.A.; Vermeulen, P.B.; Dirix, L.Y. Is there a role for mammary stem cells in inflammatory breast carcinoma: A review of evidence from cell line, animal model, and human tissue sample experments. Cancer 2010, 116, 2794–2805. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, X.; Guo, X.; Zhao, C.; Wu, X.; Zhang, Y. Toll-like receptor 9 agonist inhibits ER-mediated transactivation by activating NF-κB in breast cancer cell lines. Oncol. Rep. 2009, 22, 935–941. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matthews, J.; Wihlén, B.; Tujague, M.; Wan, J.; Ström, A.; Gustafsson, J.A. Estrogen receptor (ER) beta modulates ERα mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol. Endocrinol. 2006, 20, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Eckert, A.; Lai, K.; Adelman, S.J.; Harnish, D.C. Reciprocal antagonism between estrogen receptor and NF-κB activity in vivo. Circ. Res. 2001, 89, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Shao, L.; An, N.; Wang, J.; Pazhanisamy, S.; Feng, W.; Hauer-Jensen, M.; Miyamoto, S.; Zhou, D. IKKβ regulates the repair of DNA double-strand breaks induced by ionizing radiation in MCF-7 breast cancer cells. PLoS ONE 2011, 6, e18447. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, M.; Pastuch-Gawolek, G.; Mrozek-Wilczkiewicz, A.; Kuczak, M.; Skonieczna, M.; Musiol, R. Synthesis of 8-hydroxyquinoline glycoconjugates and preliminary assay of their β1,4-GalT inhibitory and anti-cancer properties. Bioorganic Chem. 2019, 84, 326–338. [Google Scholar] [CrossRef]
- Szewczuk, M.; Boguszewska, K.; Żebrowska, M.; Balcerczak, E.; Stasiak, M.; Świątkowska, M.; Błaszczak-Świątkiewicz, K. Virus-directed enzyme prodrug therapy and the assessment of the cytotoxic impact of some benzimidazole derivatives. Tumor Biol. 2017, 39, 1010428317713675. [Google Scholar] [CrossRef]
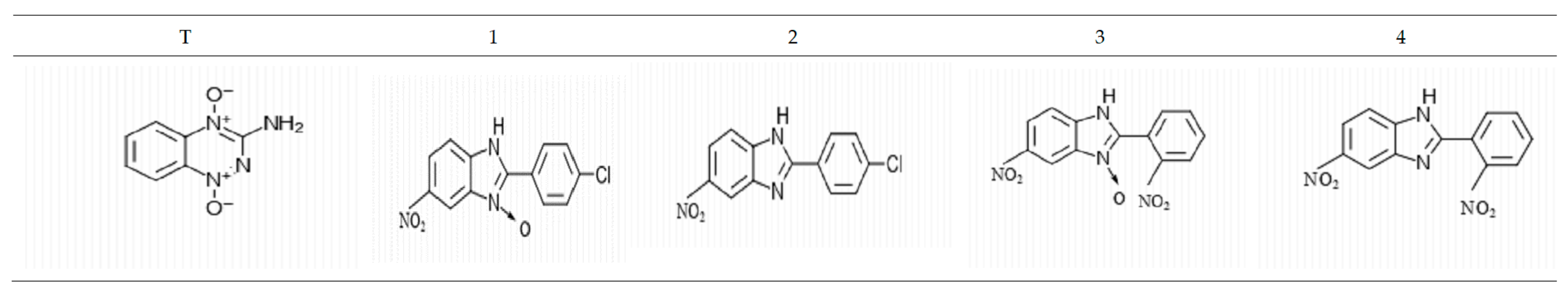
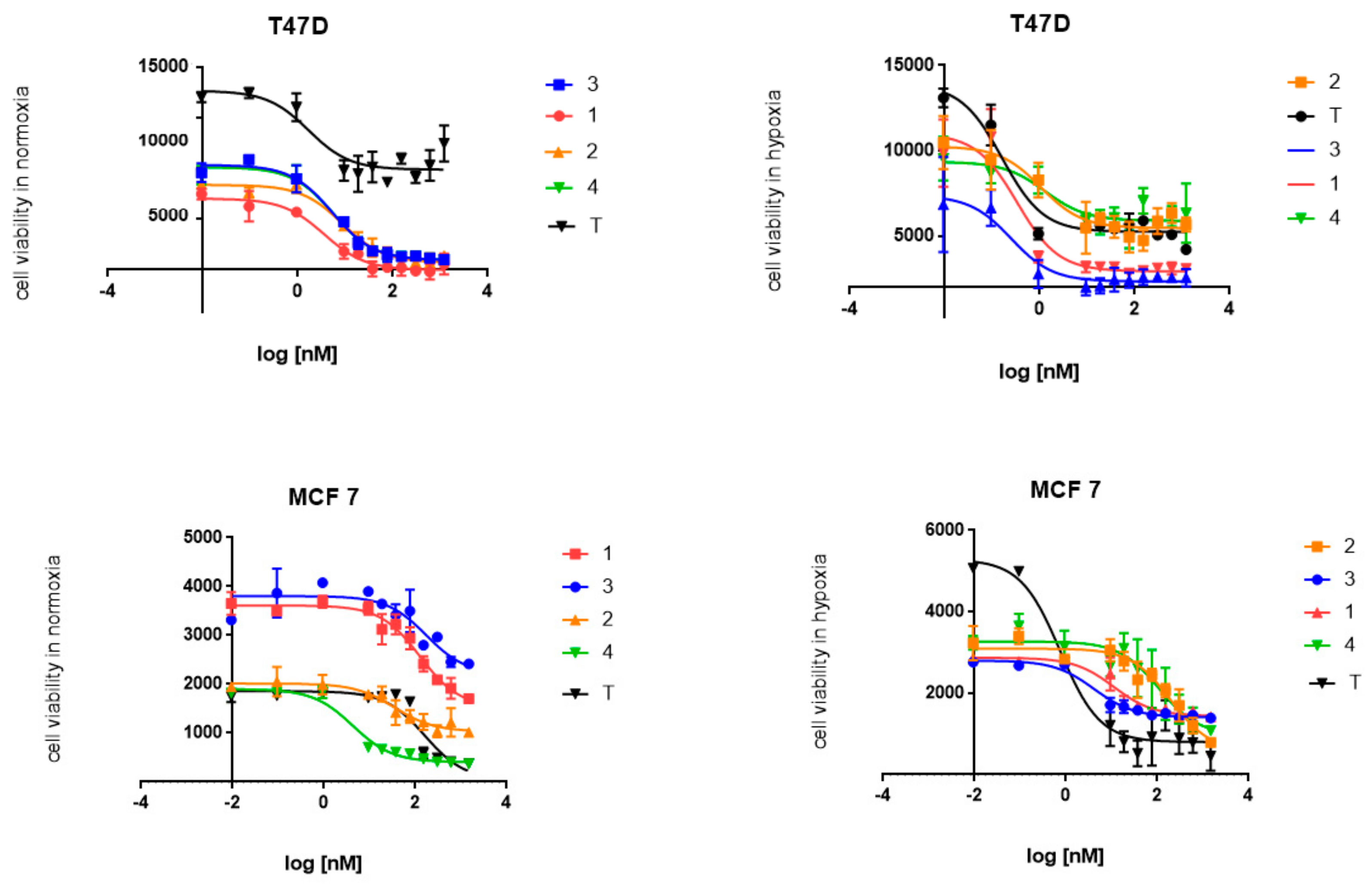
| IC50 (nM) Normoxia [N] | |||||
| 1 | 2 | 3 | 4 | T | |
| T47D | 4 ± 0.05 | 4.3 ± 0.02 | 3.2 ± 0.01 | 3.5 ± 0.07 | 1.7 ± 0.06 |
| MCF7 | 162.45 ± 2.2 | 13.5 ± 0.5 | 191.2 ± 3.3 | 8.5 ± 2.4 | 155.4 ± 1.2 |
| IC50 (nM) hypoxia [H] | |||||
| 1 | 2 | 3 | 4 | T | |
| T47D | 0.33 ± 0.03 | 0.94 ± 0.05 | 0.31 ± 0.06 | 0.75 ± 0.02 | 0.12 ± 0.002 |
| MCF7 | 10.1 ± 3.2 | 152.9 ± 2.7 | 5.5 ± 1.8 | 67.5 ± 1.9 | 2.45 ± 2.0 |
| ratio [N/H] | |||||
| 1 | 2 | 3 | 4 | T | |
| T47D | 12.1 | 4.6 | 10.3 | 4.7 | 14.2 |
| MCF7 | 16.1 | 0.09 | 34.7 | 0.12 | 63.4 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błaszczak-Świątkiewicz, K. Antiproliferative Aspect of Benzimidazole Derivatives’ Activity and Their Impact on NF-κB Expression. Molecules 2019, 24, 3902. https://doi.org/10.3390/molecules24213902
Błaszczak-Świątkiewicz K. Antiproliferative Aspect of Benzimidazole Derivatives’ Activity and Their Impact on NF-κB Expression. Molecules. 2019; 24(21):3902. https://doi.org/10.3390/molecules24213902
Chicago/Turabian StyleBłaszczak-Świątkiewicz, Katarzyna. 2019. "Antiproliferative Aspect of Benzimidazole Derivatives’ Activity and Their Impact on NF-κB Expression" Molecules 24, no. 21: 3902. https://doi.org/10.3390/molecules24213902
APA StyleBłaszczak-Świątkiewicz, K. (2019). Antiproliferative Aspect of Benzimidazole Derivatives’ Activity and Their Impact on NF-κB Expression. Molecules, 24(21), 3902. https://doi.org/10.3390/molecules24213902




