Photon-Induced Superior Antibacterial Activity of Palladium-Decorated, Magnetically Separable Fe3O4/Pd/mpg-C3N4 Nanocomposites
Abstract
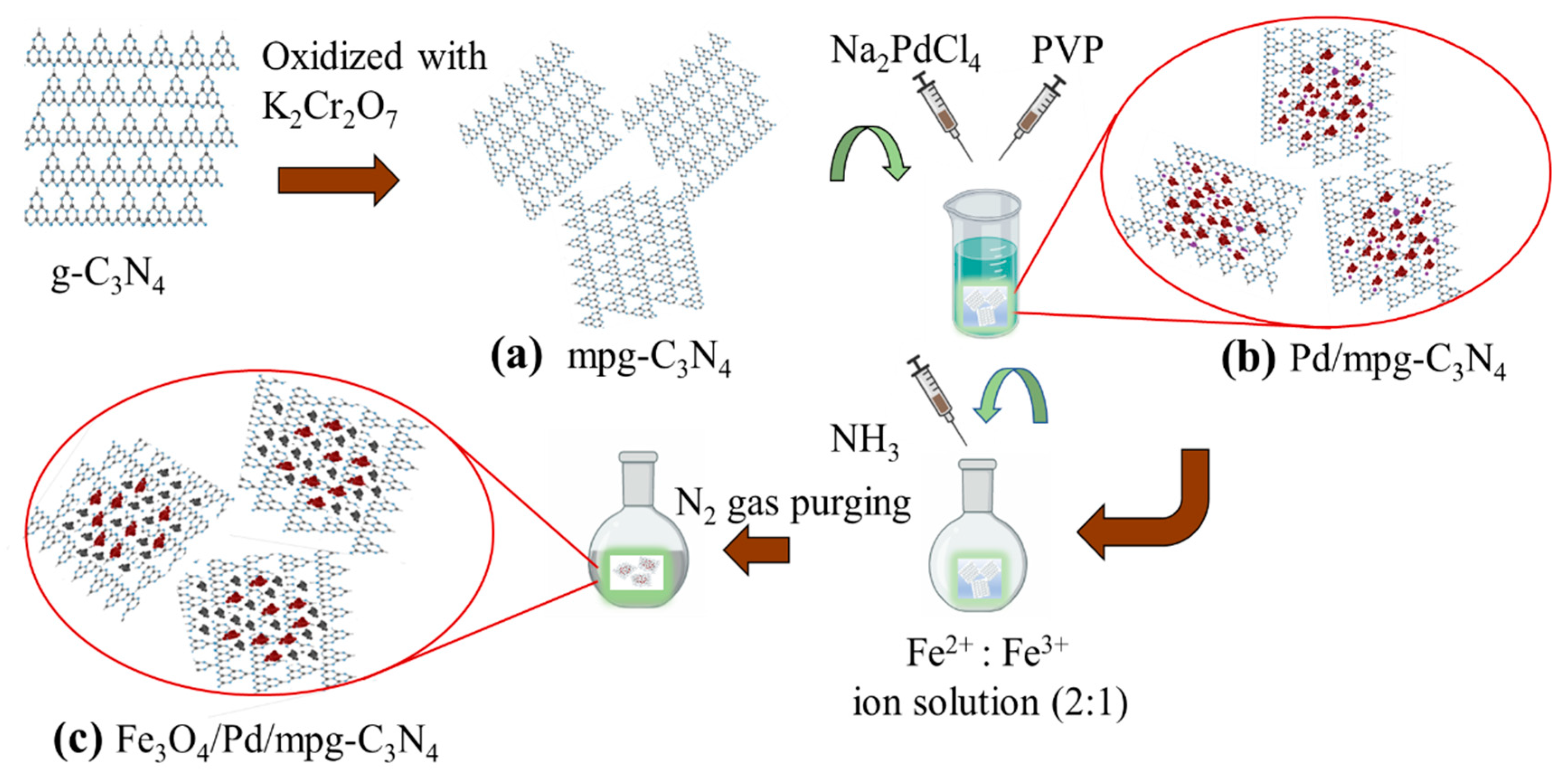
1. Introduction
2. Results and Discussion
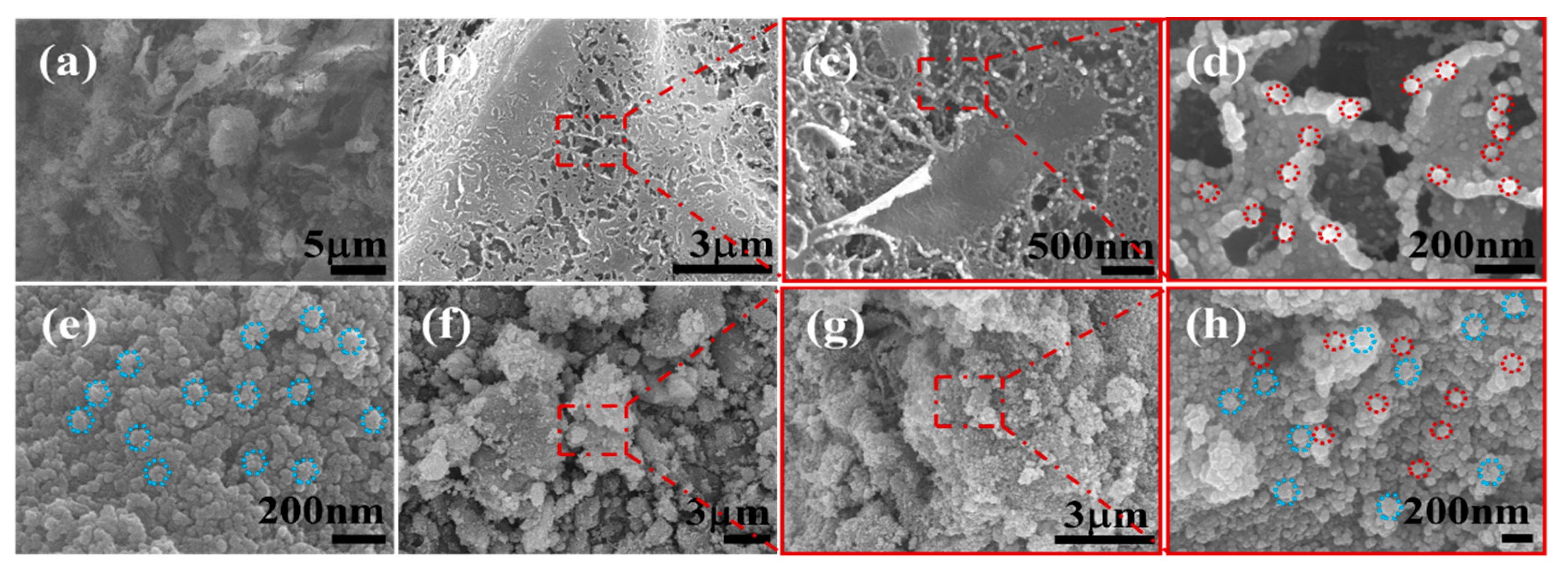
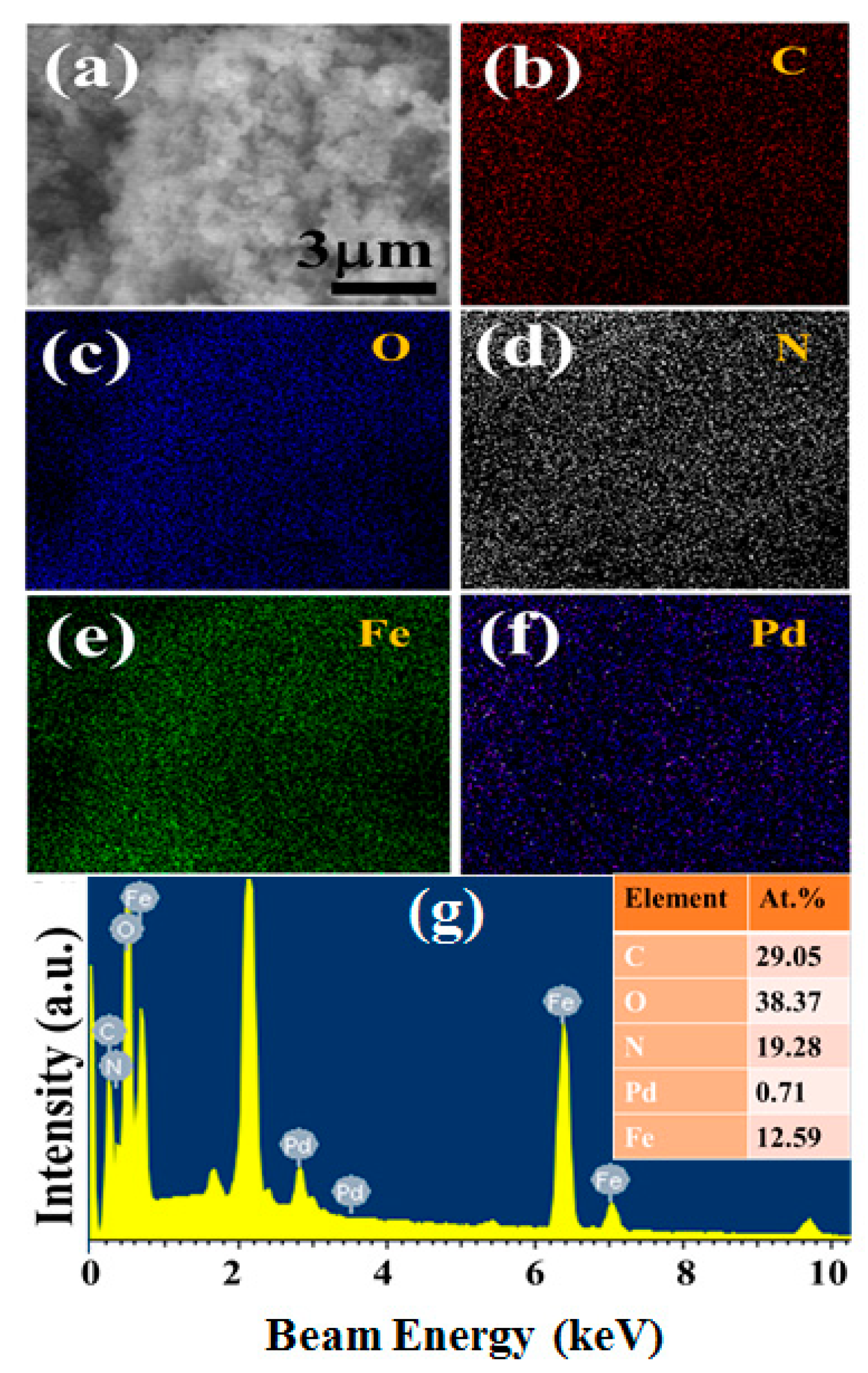
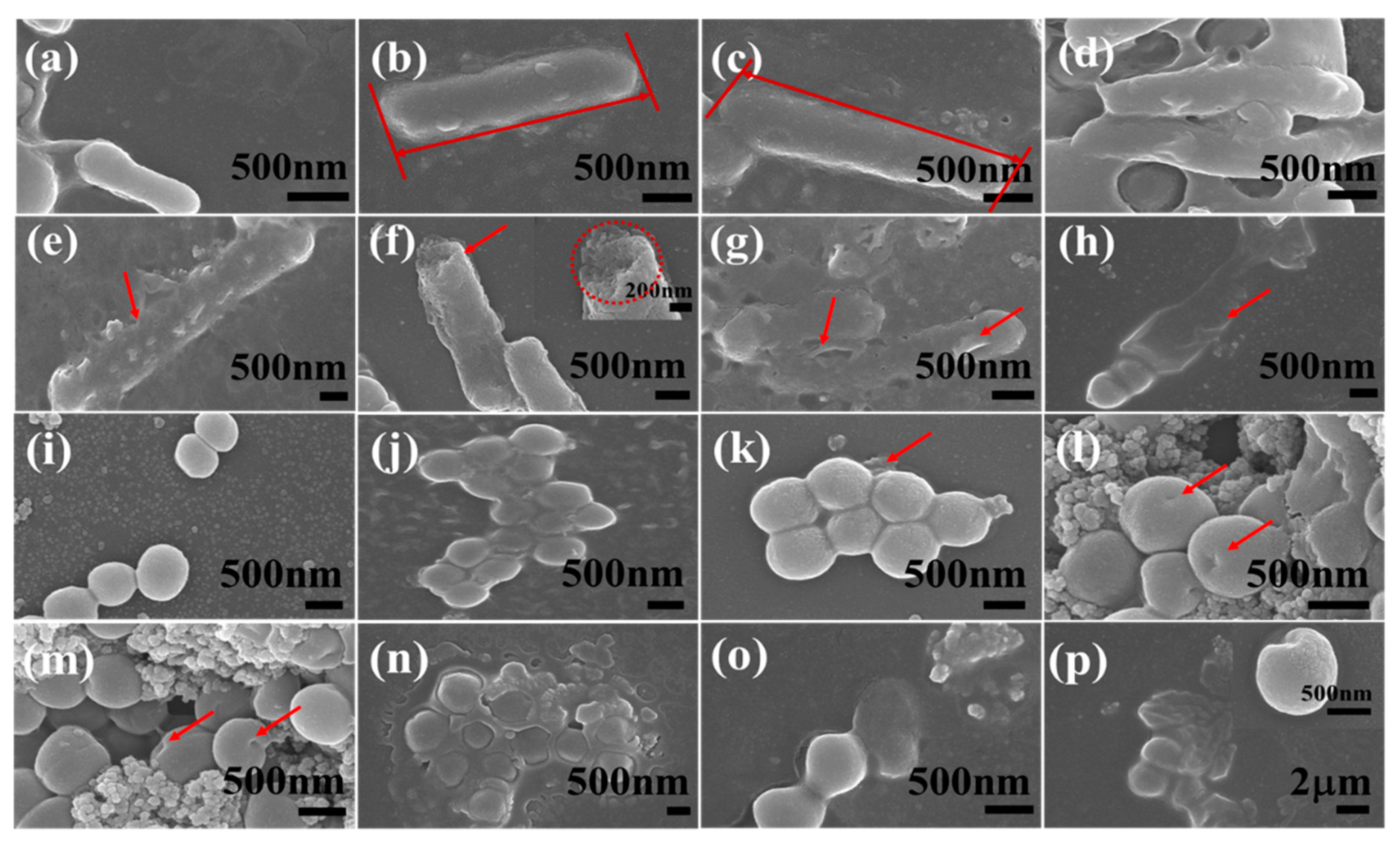
2.1. Morphological Characterization of Nanocomposites
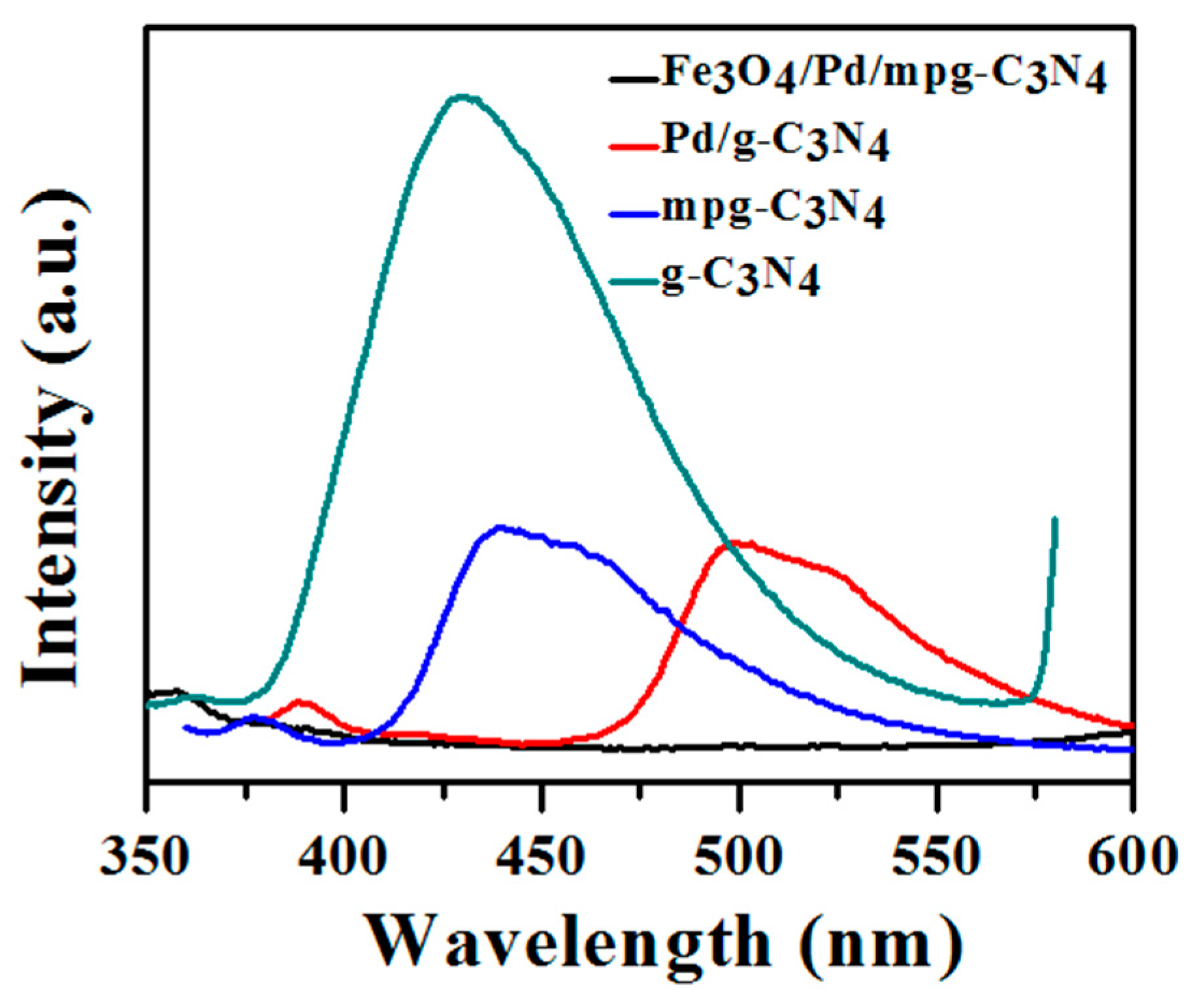
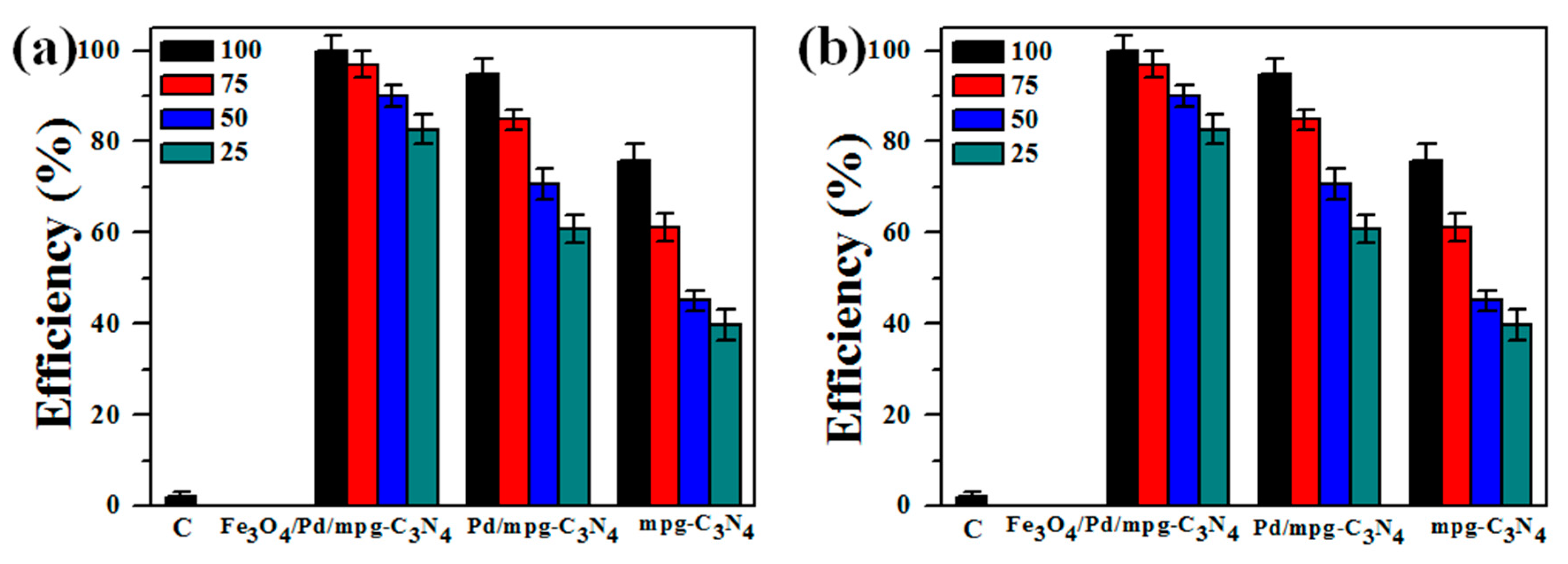
2.2. Carrier Separation and Bacterial Degradation
2.3. Photocatalytic Cell Destruction Analysis
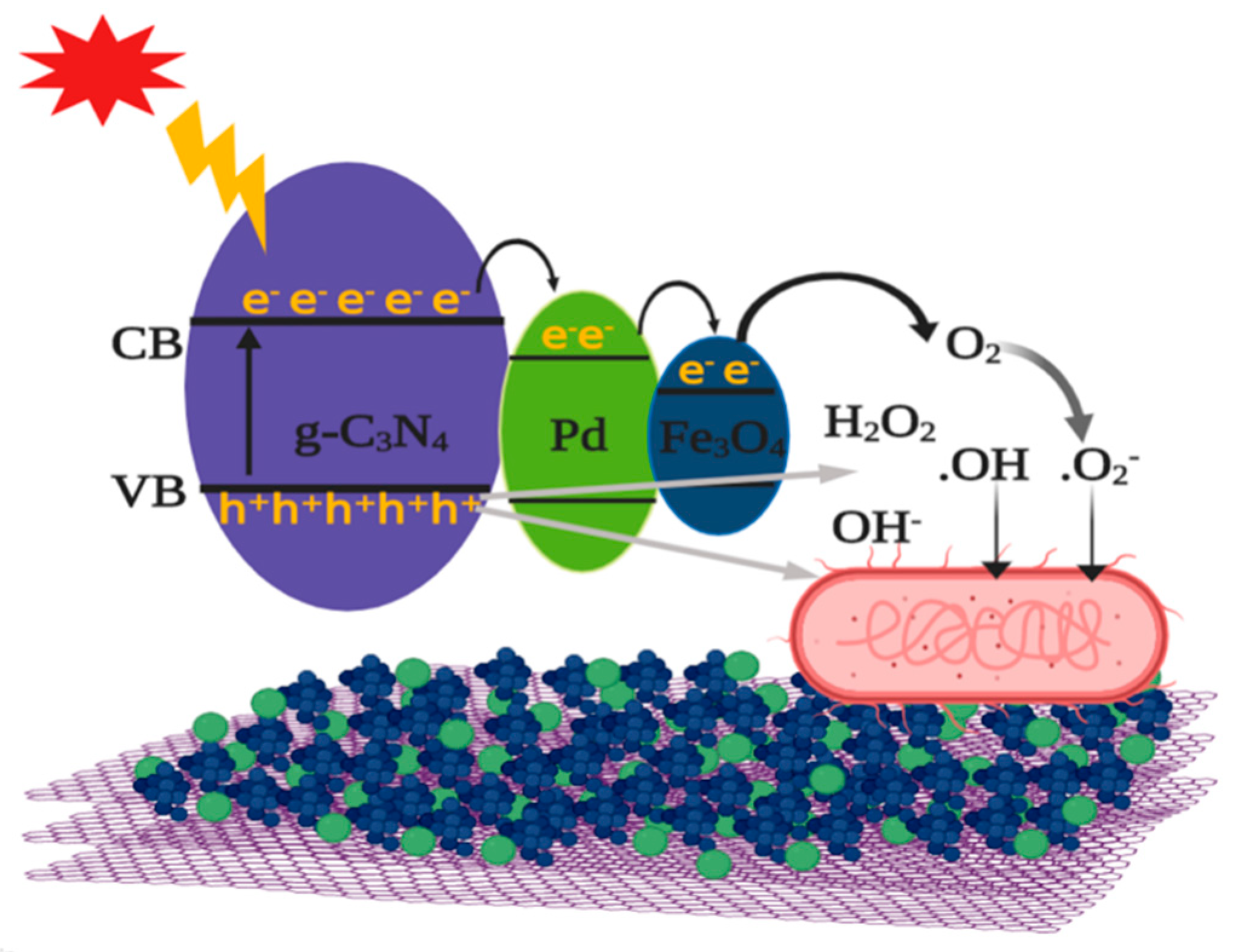
2.4. Mechanism of Photocatalytic Antibacterial Activity
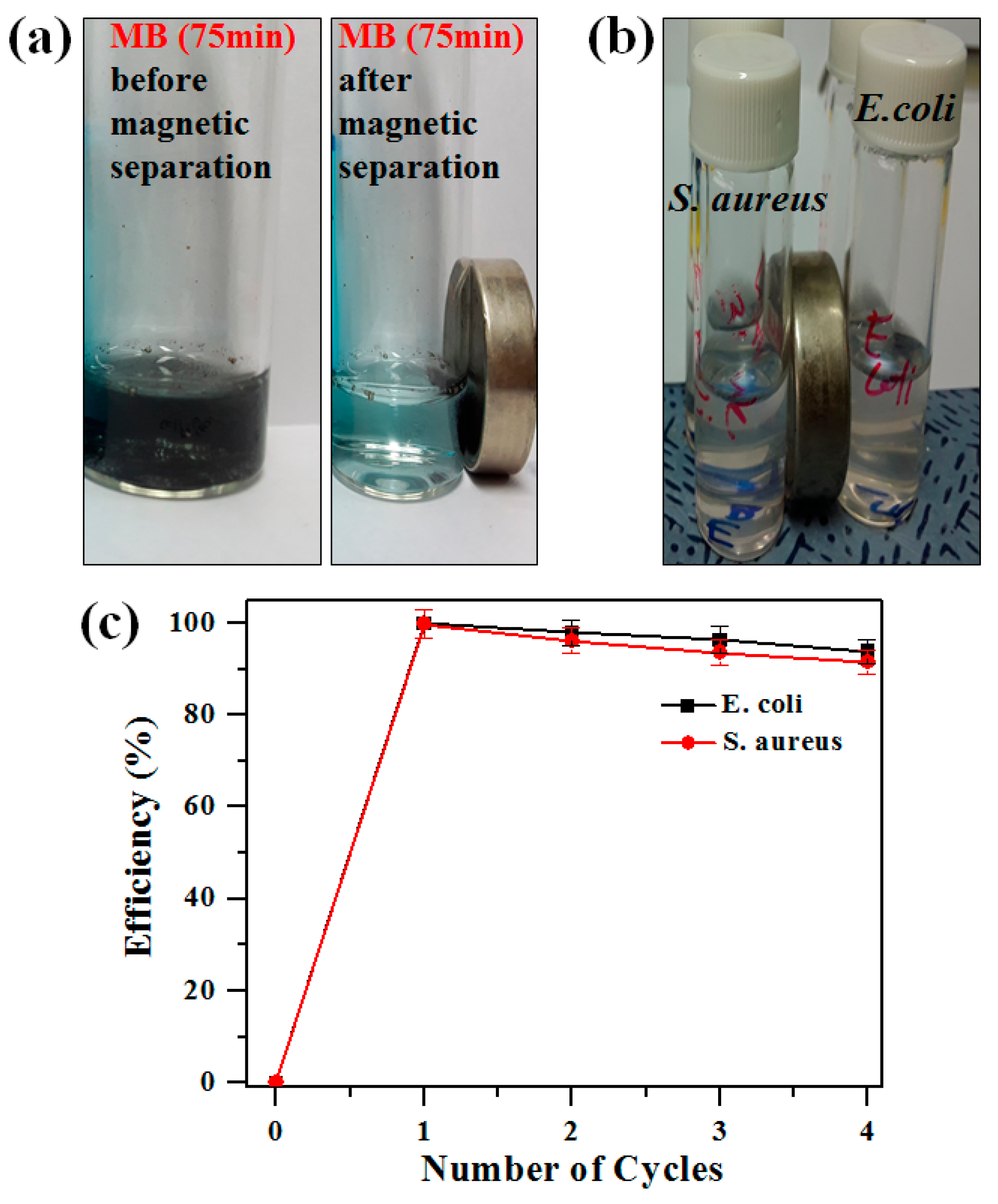
2.5. Magnetic Separation and Recyclability of Photocatalysts
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Nanocomposite Preparation
3.3. Antibacterial Activity
3.4. SEM Observation of Bacterial Morphological Changes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Fakhrul Ridhwan Samsudin, M.; Bacho, N.; Sufian, S. Recent Development of Graphitic Carbon Nitride-Based Photocatalyst for Environmental Pollution Remediation. In Nanocatalysts; IntechOpen: London, UK, 2019. [Google Scholar]
- Oliveira, M.M.; Ugarte, D.; Zanchet, D.; Zarbin, A.J.G. Influence of synthetic parameters on the size, structure, and stability of dodecanethiol-stabilized silver nanoparticles. J. Colloid Interface Sci. 2005, 292, 429–435. [Google Scholar] [CrossRef]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 7104. [Google Scholar] [CrossRef]
- Wu, M.; Gong, Y.; Nie, T.; Zhang, J.; Wang, R.; Wang, H.; He, B. Template-free synthesis of nanocage-like g-C3N4 with high surface area and nitrogen defects for enhanced photocatalytic H2 activity. J. Mater. Chem. A 2019, 7, 5324–5332. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A reveiw. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Samanta, S.; Martha, S.; Parida, K. Facile Synthesis of Au/g-C3N4 Nanocomposites: An Inorganic/Organic Hybrid Plasmonic Photocatalyst with Enhanced Hydrogen Gas Evolution Under Visible-Light Irradiation. ChemCatChem 2014, 6, 1453–1462. [Google Scholar]
- Li, Y.; Yan, Y.; Li, Y.; Zhang, H.; Li, D.; Yang, D. Size-controlled synthesis of Pd nanosheets for tunable plasmonic properties. CrystEngComm 2015, 17, 1833–1838. [Google Scholar] [CrossRef]
- Le, S.; Jiang, T.; Zhao, Q.; Liu, X.; Li, Y.; Fang, B.; Gong, M. Cu-doped mesoporous graphitic carbon nitride for enhanced visible-light driven photocatalysis. RSC Adv. 2016, 6, 38811–38819. [Google Scholar] [CrossRef]
- Wang, N.; Wang, J.; Hu, J.; Lu, X.; Sun, J.; Shi, F.; Liu, Z.-H.; Lei, Z.; Jiang, R. Design of Palladium-Doped g-C3N4 for Enhanced Photocatalytic Activity toward Hydrogen Evolution Reaction. ACS Appl. Energy Mater. 2018, 1, 2866–2873. [Google Scholar] [CrossRef]
- Cai, J.; Wu, M.; Wang, Y.; Zhang, H.; Meng, M.; Tian, Y.; Li, X.; Zhang, J.; Zheng, L.; Gong, J. Synergetic Enhancement of Light Harvesting and Charge Separation over Surface-Disorder-Engineered TiO2 Photonic Crystals. Chem 2017, 2, 877–892. [Google Scholar] [CrossRef]
- Sun, S.; Liang, S. Recent advances in functional mesoporous graphitic carbon nitride (mpg-C3N4) polymers. Nanoscale 2017, 9, 10544–10578. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-J.; Sun, B.-W.; Sui, L.; Qian, D.-J.; Chen, M. Preparation of water-dispersible porous g-C3N4 with improved photocatalytic activity by chemical oxidation. Phys. Chem. Chem. Phys. 2015, 17, 3309–3315. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-N.; Wang, H.-L. Preparation of magnetic g-C3N4/Fe3O4/TiO2 photocatalyst for visible light photocatalytic application. J. Alloys Compd. 2018, 763, 844–853. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Gao, J.; Yan, T.; Zhou, F.; Cui, S.; Bi, W. Synthesis of Fe3O4/g-C3N4 nanocomposites and their application in the photodegradation of 2,4,6-trichlorophenol under visible light. Mater. Lett. 2016, 164, 183–189. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Liu, X.; Yang, X.; Yang, Y. A Ternary Magnetic Recyclable ZnO/Fe3O4/g-C3N4 Composite Photocatalyst for Efficient Photodegradation of Monoazo Dye. Nanoscale Res. Lett. 2019, 14, 147. [Google Scholar] [CrossRef]
- Fei, B.; Tang, Y.; Wang, X.; Dong, X.; Liang, J.; Fei, X.; Xu, L.; Song, Y.; Zhang, F. One-pot synthesis of porous g-C3N4 nanomaterials with different morphologies and their superior photocatalytic performance. Mater. Res. Bull. 2018, 102, 209–217. [Google Scholar] [CrossRef]
- Zou, H.; Yan, X.; Ren, J.; Wu, X.; Dai, Y.; Sha, D.; Pan, J.; Liu, J. Photocatalytic activity enhancement of modified g-C3N4 by ionothermal copolymerization. J. Mater. 2015, 1, 340–347. [Google Scholar] [CrossRef]
- Ma, S.; Zhan, S.; Jia, Y.; Zhou, Q. Superior Antibacterial Activity of Fe3O4-TiO2 Nanosheets under Solar Light. ACS Appl. Mater. Interfaces 2015, 7, 21875–21883. [Google Scholar] [CrossRef]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic basis of antimicrobial actions of silver nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Cegelski, L.; Romaniuk, J.A.H. Bacterial cell wall composition and the influence of antibiotics by cell-wall and whole-cell NMR. Philos. Trans. R. Soc. B 2015, 370, 20150024. [Google Scholar]
- Zhao, H.; Yu, H.; Quan, X.; Chen, S.; Zhang, Y.; Zhao, H.; Wang, H. Fabrication of atomic single layer graphitic-C3N4 and its high performance of photocatalytic disinfection under visible light irradiation. Appl. Catal. B Environ. 2014, 152–153, 46–50. [Google Scholar] [CrossRef]
- Sadjadi, S.; Malmir, M.; Heravi, M.M.; Kahangi, F.G. Magnetic covalent hybrid of graphitic carbon nitride and graphene oxide as an efficient catalyst support for immobilization of Pd nanoparticles. Inorg. Chim. Acta 2019, 488, 62–70. [Google Scholar] [CrossRef]
- Long, N.V.; Hien, T.D.; Asaka, T.; Ohtaki, M.; Nogami, M. Synthesis and characterization of Pt–Pd nanoparticles with core-shell morphology: Nucleation and overgrowth of the Pd shells on the as-prepared and defined Pt seeds. J. Alloys Compd. 2011, 509, 7702–7709. [Google Scholar] [CrossRef]
- Nemati, F.; Heravi, M.M.; Saeedi Rad, R. Nano-Fe3O4 Encapsulated-Silica Particles Bearing Sulfonic Acid Groups as a Magnetically Separable Catalyst for Highly Efficient Knoevenagel Condensation and Michael Addition Reactions of Aromatic Aldehydes with 1,3-Cyclic Diketones. Chin. J. Catal. 2012, 33, 1825–1831. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakur, D.; Ta, Q.T.H.; Noh, J.-S. Photon-Induced Superior Antibacterial Activity of Palladium-Decorated, Magnetically Separable Fe3O4/Pd/mpg-C3N4 Nanocomposites. Molecules 2019, 24, 3888. https://doi.org/10.3390/molecules24213888
Thakur D, Ta QTH, Noh J-S. Photon-Induced Superior Antibacterial Activity of Palladium-Decorated, Magnetically Separable Fe3O4/Pd/mpg-C3N4 Nanocomposites. Molecules. 2019; 24(21):3888. https://doi.org/10.3390/molecules24213888
Chicago/Turabian StyleThakur, Deepika, Qui Thanh Hoai Ta, and Jin-Seo Noh. 2019. "Photon-Induced Superior Antibacterial Activity of Palladium-Decorated, Magnetically Separable Fe3O4/Pd/mpg-C3N4 Nanocomposites" Molecules 24, no. 21: 3888. https://doi.org/10.3390/molecules24213888
APA StyleThakur, D., Ta, Q. T. H., & Noh, J.-S. (2019). Photon-Induced Superior Antibacterial Activity of Palladium-Decorated, Magnetically Separable Fe3O4/Pd/mpg-C3N4 Nanocomposites. Molecules, 24(21), 3888. https://doi.org/10.3390/molecules24213888