Icariin Promotes the Osteogenic Action of BMP2 by Activating the cAMP Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. ICA Improves the BMP2-Mediated Osteogenic Differentiation of C2C12 Cells in a Dose-Dependent Manner
2.1.1. Successful Model of Osteoblast Differentiation with BMP2-Treated C2C12 Cells
2.1.2. Cytotoxic Effects of ICA
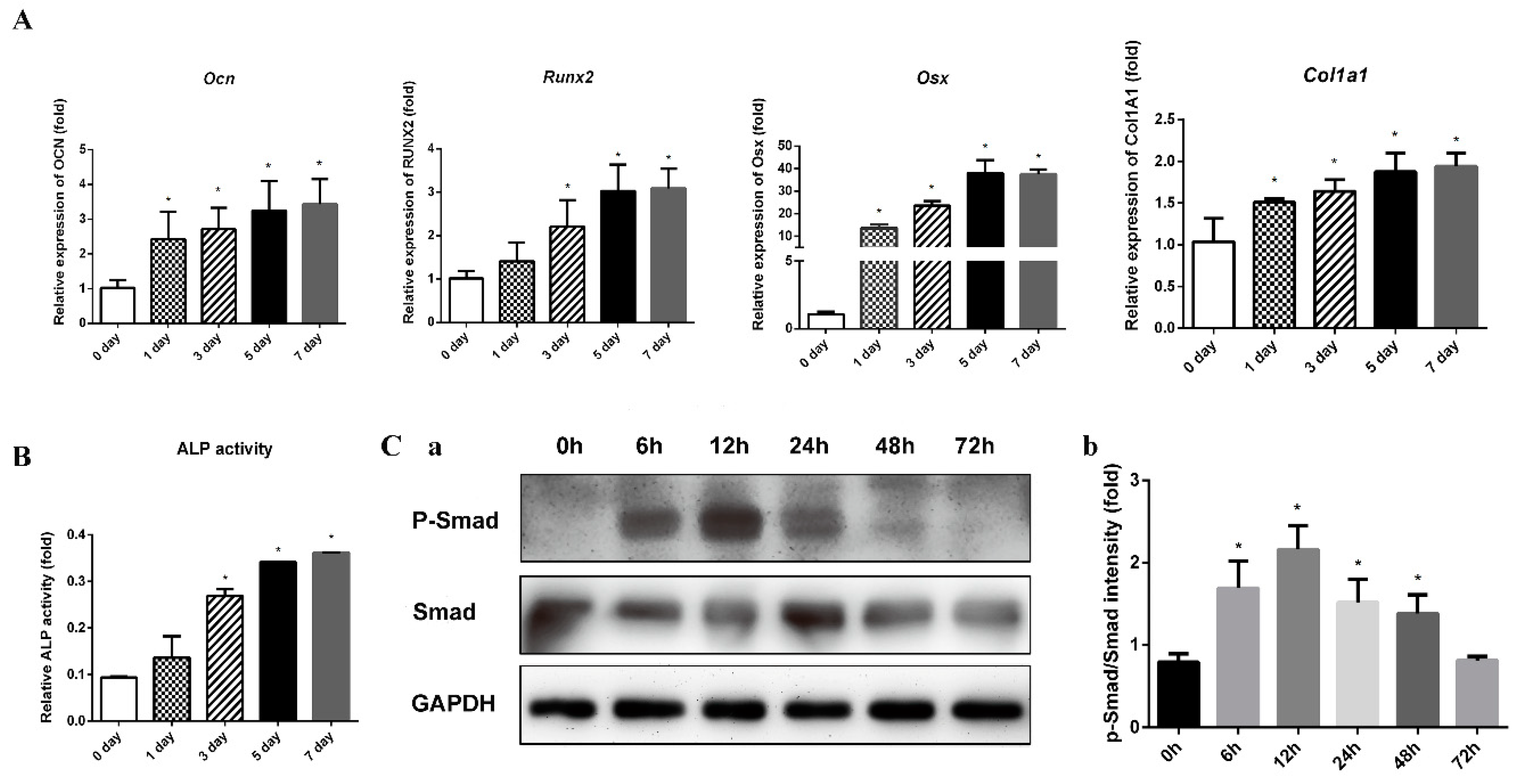
2.1.3. ICA Improves BMP2-Mediated Osteogenic Differentiation
2.2. The Osteogenesis-Stimulating Activity of ICA is Mediated through the cAMP Pathways
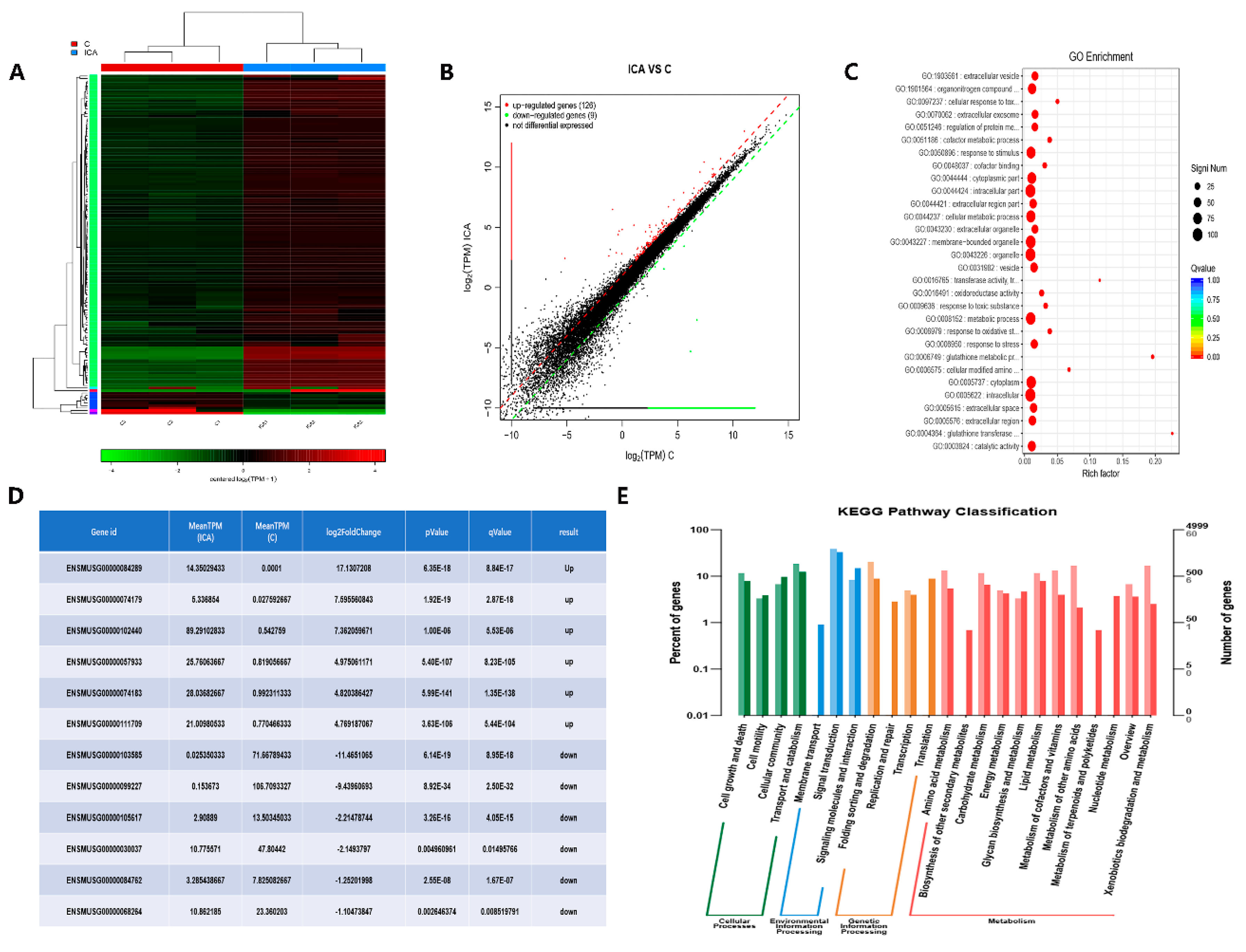
2.2.1. Differentially Expressed Gene (DEG) Analysis by RNA-Seq before and after the Addition of ICA
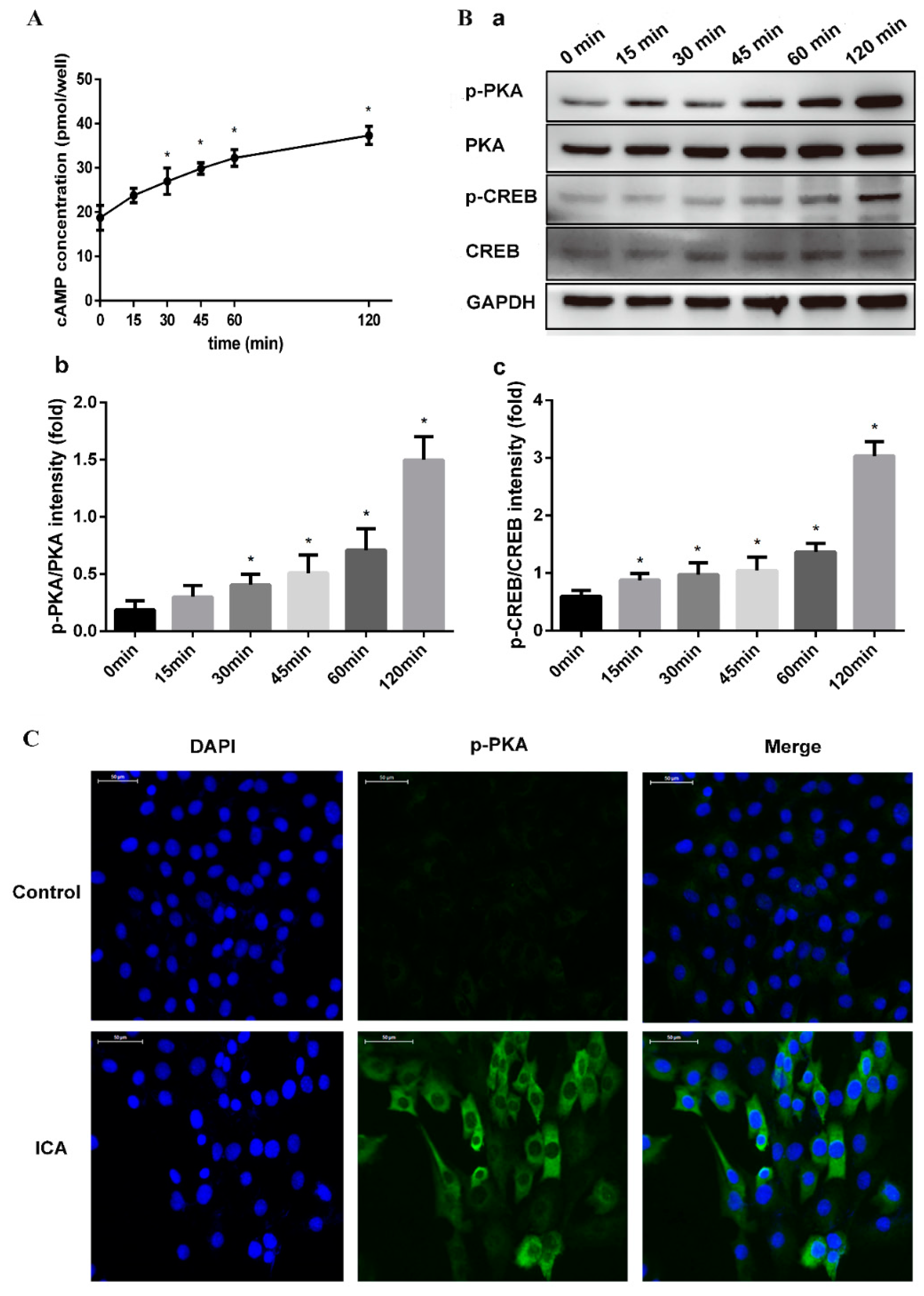
2.2.2. ICA Activated the cAMP Pathway in Promoting Bone Formation
2.3. PKA Inhibitor (H89) Abrogated ICA-Prompted Osteogenesis via a cAMP/PKA/CREB Signaling Blockade
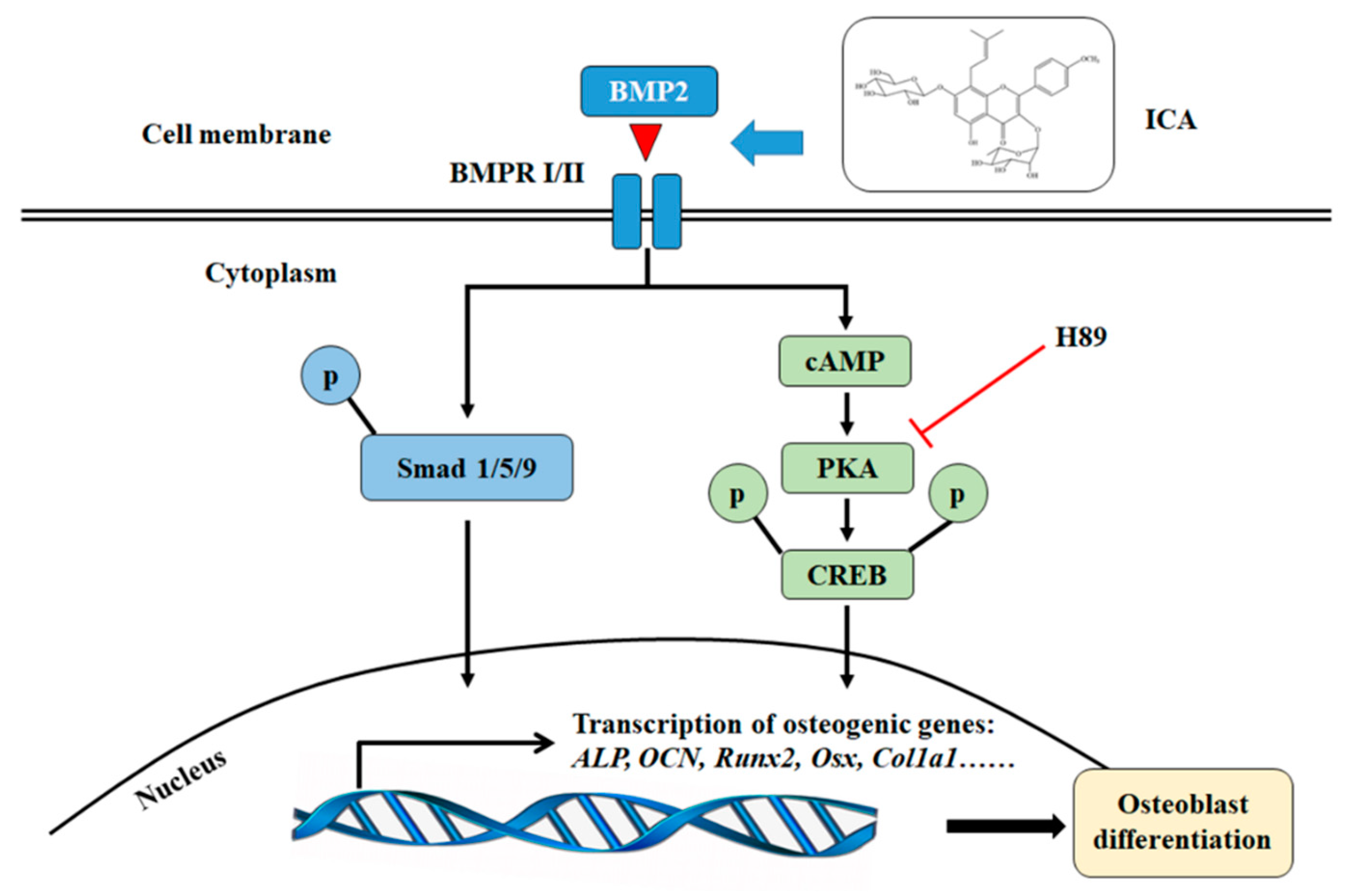
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Cytotoxicity Assay
4.4. Alkaline Phosphatase (ALP) Activity Assay
4.5. Transcriptome Sequencing
4.6. cAMP Assay
4.7. qRT-PCR Analysis
4.8. Western Blot Analysis
4.9. Immunofluorescence Staining
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Padova, G.; Borzì, G.; Incorvaia, L.; Siciliano, G.; Migliorino, V.; Vetri, M.; Tita, P. Prevalence of osteoporosis and vertebral fractures in acromegalic patients. Clin. Cases Miner. Bone Metab. Off. J. Ital. Soc. Osteoporos. Miner. Metab. Skelet. Dis. 2011, 8, 37–43. [Google Scholar]
- Lee, W.C.; Guntur, A.R.; Long, F.; Rosen, C.J. Energy Metabolism of the Osteoblast: Implications for Osteoporosis. Endocr. Rev. 2017, 38, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; Economides, A.N.; Gazzerro, E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr. Rev. 2003, 24, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Glassman, S.D.; Carreon, L.Y.; Djurasovic, M.; Campbell, M.J.; Puno, R.M.; Johnson, J.R.; Dimar, J.R. RhBMP-2 versus iliac crest bone graft for lumbar spine fusion: A randomized, controlled trial in patients over sixty years of age. Spine 2008, 33, 2843–2849. [Google Scholar] [CrossRef] [PubMed]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: Delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol. Lett. 2009, 31, 1825–1835. [Google Scholar] [CrossRef]
- Chen, N.F.; Smith, Z.A.; Stiner, E.; Armin, S.; Sheikh, H.; Khoo, L.T. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J. Neurosurg. Spine 2010, 12, 40–46. [Google Scholar] [CrossRef]
- Cahill, K.S.; Chi, J.H.; Day, A.; Claus, E.B. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA 2009, 302, 58–66. [Google Scholar] [CrossRef]
- Zeng, R.; Wang, X.; Zhou, Q.; Fu, X.; Wu, Q.; Lu, Y.; Shi, J.; Klaunig, J.E.; Zhou, S. Icariin protects rotenone-induced neurotoxicity through induction of SIRT3. Toxicol. Appl. Pharmacol. 2019, 379, 114639. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Liao, L.X.; Tu, P.F.; Li, W.W.; Zeng, K.W. Icariin Inhibits AGE-Induced Injury in PC12 Cells by Directly Targeting Apoptosis Regulator Bax. Oxidative Med. Cell. Longev. 2019, 2019, 7940808. [Google Scholar] [CrossRef]
- Kim, J.Y.; Shim, S.H. Extract and Its Flavonoids Reduced Atherosclerotic Risk via Suppressing Modification of Human HDL. Nutrients 2019, 11, 1110. [Google Scholar] [CrossRef]
- Wu, T.; Wang, S.; Wu, J.; Lin, Z.; Sui, X.; Xu, X.; Shimizu, N.; Chen, B.; Wang, X. Icaritin induces lytic cytotoxicity in extranodal NK/T-cell lymphoma. J. Exp. Clin. Cancer Res. CR 2015, 34, 17. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.F.; Liu, S.; Yang, M.; Xu, J.Q.; Li, Z.C.; Zhou, X. Effects and possible mechanism of Ruyiping formula application to breast cancer based on network prediction. Sci. Rep. 2019, 9, 5249. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, J.; Chen, X.; Fortenbery, N.; Eksioglu, E.A.; Wei, S.; Dong, J. Attenuation of LPS-induced inflammation by ICT, a derivate of icariin, via inhibition of the CD14/TLR4 signaling pathway in human monocytes. Int. Immunopharmacol. 2012, 12, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Liu, L.; Tian, X.; Guo, Y.; Cao, Y.; Mei, Y.; Wang, C. Icariin Attenuates High Glucose-Induced Apoptosis, Oxidative Stress, and Inflammation in Human Umbilical Venous Endothelial Cells. Planta Med. 2019, 85, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.B.; Ye, J.; Li, C.L.; Wang, Y.H.; Zhao, J.; Cai, S.Q. Antiaging effect of Cordyceps sinensis extract. Phytother. Res. PTR 2009, 23, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Hou, Z.; Xie, Y.; Yan, F.; Li, S.; Zhu, X.; Cai, L. Icariin promotes osteogenic differentiation of bone marrow stromal cells and prevents bone loss in OVX mice via activating autophagy. J. Cell. Biochem. 2019, 120, 13121–13132. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.N.; Zhou, J.; Ge, B.F.; Zhen, P.; Ma, H.P.; Shi, W.G.; Cheng, K.; Xian, C.J.; Chen, K.M. Icariin induces osteoblast differentiation and mineralization without dexamethasone in vitro. Planta Med. 2013, 79, 1501–1508. [Google Scholar] [CrossRef]
- Hsieh, T.P.; Sheu, S.Y.; Sun, J.S.; Chen, M.H. Icariin inhibits osteoclast differentiation and bone resorption by suppression of MAPKs/NF-κB regulated HIF-1α and PGE(2) synthesis. Phytomedicine Int. J. Phytother. Phytopharm. 2011, 18, 176–185. [Google Scholar] [CrossRef]
- Wang, B.L.; Dai, C.L.; Quan, J.X.; Zhu, Z.F.; Zheng, F.; Zhang, H.X.; Guo, S.Y.; Guo, G.; Zhang, J.Y.; Qiu, M.C. Parathyroid hormone regulates osterix and Runx2 mRNA expression predominantly through protein kinase A signaling in osteoblast-like cells. J. Endocrinol. Investig. 2006, 29, 101–108. [Google Scholar] [CrossRef]
- Nakao, Y.; Koike, T.; Ohta, Y.; Manaka, T.; Imai, Y.; Takaoka, K. Parathyroid hormone enhances bone morphogenetic protein activity by increasing intracellular 3’, 5’-cyclic adenosine monophosphate accumulation in osteoblastic MC3T3-E1 cells. Bone 2009, 44, 872–877. [Google Scholar] [CrossRef]
- Siddappa, R.; Martens, A.; Doorn, J.; Leusink, A.; Olivo, C.; Licht, R.; van Rijn, L.; Gaspar, C.; Fodde, R.; Janssen, F.; et al. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 7281–7286. [Google Scholar] [CrossRef] [PubMed]
- Doorn, J.; Siddappa, R.; van Blitterswijk, C.A.; de Boer, J. Forskolin enhances in vivo bone formation by human mesenchymal stromal cells. Tissue Eng. Part A 2012, 18, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Sugama, R.; Koike, T.; Imai, Y.; Nomura-Furuwatari, C.; Takaoka, K. Bone morphogenetic protein activities are enhanced by 3’,5’-cyclic adenosine monophosphate through suppression of Smad6 expression in osteoprogenitor cells. Bone 2006, 38, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, T.; Yamaguchi, A.; Komaki, M.; Abe, E.; Takahashi, N.; Ikeda, T.; Rosen, V.; Wozney, J.M.; Fujisawa-Sehara, A.; Suda, T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 1994, 127, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Chen, D. The BMP signaling and in vivo bone formation. Gene 2005, 357, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Myneni, V.D.; Mezey, E. Regulation of bone remodeling by vitamin K2. Oral Dis. 2017, 23, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Rosenberg, E.; de Papp, A.E.; Duong, L.T. The osteoclast, bone remodelling and treatment of metabolic bone disease. Eur. J. Clin. Investig. 2012, 42, 1332–1341. [Google Scholar] [CrossRef]
- Xie, F.; Wu, C.F.; Lai, W.P.; Yang, X.J.; Cheung, P.Y.; Yao, X.S.; Leung, P.C.; Wong, M.S. The osteoprotective effect of Herba epimedii (HEP) extract in vivo and in vitro. Evid. Based Complementary Altern. Med. ECAM 2005, 2, 353–361. [Google Scholar] [CrossRef]
- Shen, P.; Guo, B.L.; Gong, Y.; Hong, D.Y.; Hong, Y.; Yong, E.L. Taxonomic, genetic, chemical and estrogenic characteristics of Epimedium species. Phytochemistry 2007, 68, 1448–1458. [Google Scholar] [CrossRef]
- Li, G.W.; Xu, Z.; Chang, S.X.; Nian, H.; Wang, X.Y.; Qin, L.D. Icariin prevents ovariectomy-induced bone loss and lowers marrow adipogenesis. Menopause 2014, 21, 1007–1016. [Google Scholar] [CrossRef]
- Feng, R.; Feng, L.; Yuan, Z.; Wang, D.; Wang, F.; Tan, B.; Han, S.; Li, T.; Li, D.; Han, Y. Icariin protects against glucocorticoid-induced osteoporosis in vitro and prevents glucocorticoid-induced osteocyte apoptosis in vivo. Cell Biochem. Biophys. 2013, 67, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; He, J.; Zheng, H.; Chen, C.; Lan, S. Icariin Prevents Diabetes-Induced Bone Loss in Rats by Reducing Blood Glucose and Suppressing Bone Turnover. Molecules 2019, 24, 1871. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Xu, H.; Zhao, Y.J.; Tang, D.Z.; Xu, G.H.; Holz, J.; Wang, J.; Cheng, S.D.; Shi, Q.; Wang, Y.J. Icariin Augments Bone Formation and Reverses the Phenotypes of Osteoprotegerin-Deficient Mice through the Activation of Wnt/β -Catenin-BMP Signaling. Evid. Based Complementary Altern. Med. ECAM 2013, 2013, 652317. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.J.; Cao, L.G.; Wu, T.; Wang, D.X.; Jin, D.; Jiang, S.; Zhang, Z.Y.; Bi, L.; Pei, G.X. The dose-effect of icariin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cells. Molecules 2011, 16, 10123–10133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ohba, S.; Shinkai, M.; Chung, U.I.; Nagamune, T. Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner. Biochem. Biophys. Res. Commun. 2008, 369, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Lin, M.; Li, X.; Li, C.; Gao, B.; Gan, H.; Yang, Z.; Lin, X.; Liao, L.; Yang, M. Icariin promotes bone formation via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19 human osteoblastic cell line. Int. J. Mol. Med. 2012, 30, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yuan, L.; Wang, X.; Zhang, T.L.; Wang, K. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci. 2007, 81, 832–840. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Fong, C.; Yao, X.; Yang, M. Herba epimedii flavonoids suppress osteoclastic differentiation and bone resorption by inducing G2/M arrest and apoptosis. Biochimie 2012, 94, 2514–2522. [Google Scholar] [CrossRef]
- Chen, K.M.; Ge, B.F.; Liu, X.Y.; Ma, P.H.; Lu, M.B.; Bai, M.H.; Wang, Y. Icariin inhibits the osteoclast formation induced by RANKL and macrophage-colony stimulating factor in mouse bone marrow culture. Die Pharm. 2007, 62, 388–391. [Google Scholar]
- Zhai, Y.K.; Guo, X.Y.; Ge, B.F.; Zhen, P.; Ma, X.N.; Zhou, J.; Ma, H.P.; Xian, C.J.; Chen, K.M. Icariin stimulates the osteogenic differentiation of rat bone marrow stromal cells via activating the PI3K-AKT-eNOS-NO-cGMP-PKG. Bone 2014, 66, 189–198. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, J.; Hong, G.; Chen, Z.; Deng, W.; He, W.; Chen, M.H. Icariin promotes osteogenic differentiation of rat bone marrow stromal cells by activating the ERα-Wnt/β-catenin signaling pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2016, 84, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhao, J.; Zhang, X.; Li, H.; Zhou, Y. Icariin induces osteoblast proliferation, differentiation and mineralization through estrogen receptor-mediated ERK and JNK signal activation. Eur. J. Pharmacol. 2013, 714, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Z.; Lin, S.; Lu, H.; Xu, J. Icariin enhances the healing of rapid palatal expansion induced root resorption in rats. Phytomedicine Int. J. Phytother. Phytopharm. 2012, 19, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Gao, Y.; Wang, Y.; Zhou, J.; Wei, Z.; Ma, X.; Ma, H.; Xian, C.J.; Wang, J.; Chen, K. The flavonol glycoside icariin promotes bone formation in growing rats by activating the cAMP signaling pathway in primary cilia of osteoblasts. J. Biol. Chem. 2017, 292, 20883–20896. [Google Scholar] [CrossRef] [PubMed]
- Pettway, G.J.; Meganck, J.A.; Koh, A.J.; Keller, E.T.; Goldstein, S.A.; McCauley, L.K. Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone 2008, 42, 806–818. [Google Scholar] [CrossRef]
- Chen, B.; Lin, T.; Yang, X.; Li, Y.; Xie, D.; Cui, H. Intermittent parathyroid hormone (1-34) application regulates cAMP-response element binding protein activity to promote the proliferation and osteogenic differentiation of bone mesenchymal stromal cells, via the cAMP/PKA signaling pathway. Exp. Ther. Med. 2016, 11, 2399–2406. [Google Scholar] [CrossRef]
- Tintut, Y.; Parhami, F.; Boström, K.; Jackson, S.M.; Demer, L.L. cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J. Biol. Chem. 1998, 273, 7547–7553. [Google Scholar] [CrossRef]
- Tsutsumimoto, T.; Wakabayashi, S.; Kinoshita, T.; Horiuchi, H.; Takaoka, K. A phosphodiesterase inhibitor, pentoxifylline, enhances the bone morphogenetic protein-4 (BMP-4)-dependent differentiation of osteoprogenitor cells. Bone 2002, 31, 396–401. [Google Scholar] [CrossRef]
- Ohta, Y.; Nakagawa, K.; Imai, Y.; Katagiri, T.; Koike, T.; Takaoka, K. Cyclic AMP enhances Smad-mediated BMP signaling through PKA-CREB pathway. J. Bone Miner. Metab. 2008, 26, 478–484. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Morita, I.; Zhang, L.; Shao, G.; Yao, X.S.; Murota, S. Screening of anti-hypoxia/reoxygenation agents by an in vitro method. Part 2: Inhibition of tyrosine kinase activation prevented hypoxia/reoxygenation-induced injury in endothelial gap junctional intercellular communication. Planta Med. 2000, 66, 119–123. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


| Name | Sequence |
|---|---|
| Alp | 3′→5′: GGCTCTGCCTTTATTCCCTAGT 5′→3′: AAATAAGGTGCTTTGGGAATCTGT |
| Ocn | 3′→5′: GCCATCACCCTGTCTCCTAA 5′→3′: GCTGTGGAGAAGACACACGA |
| Runx2 | 3′→5′: GCCGGGAATGATGAGAACTA 5′→3′: GGTGAAACTCTTGCCTCGTC |
| Osx | 3′→5′: AGGCCTTTGCCAGTGCCTA 5′→3′:GCCAGATGGAAGCTGTGAAGA |
| Col1a1 | 3′→5′: GACATGTTCAGCTTTGTGGACCTC 5′→3′:GGGACCCTTAGGCCATTGTGTA |
| Gapdh | 3′→5′: CATCCCAGAGCTGAACG 5′→3′: CTGGTCCTCAGTGTAGCC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Cui, Y.; Li, H.; Luan, J.; Zhou, X.; Han, J. Icariin Promotes the Osteogenic Action of BMP2 by Activating the cAMP Signaling Pathway. Molecules 2019, 24, 3875. https://doi.org/10.3390/molecules24213875
Chen M, Cui Y, Li H, Luan J, Zhou X, Han J. Icariin Promotes the Osteogenic Action of BMP2 by Activating the cAMP Signaling Pathway. Molecules. 2019; 24(21):3875. https://doi.org/10.3390/molecules24213875
Chicago/Turabian StyleChen, Meng, Yazhou Cui, Hui Li, Jing Luan, Xiaoyan Zhou, and Jinxiang Han. 2019. "Icariin Promotes the Osteogenic Action of BMP2 by Activating the cAMP Signaling Pathway" Molecules 24, no. 21: 3875. https://doi.org/10.3390/molecules24213875
APA StyleChen, M., Cui, Y., Li, H., Luan, J., Zhou, X., & Han, J. (2019). Icariin Promotes the Osteogenic Action of BMP2 by Activating the cAMP Signaling Pathway. Molecules, 24(21), 3875. https://doi.org/10.3390/molecules24213875




