Copper Ions Removal from Water using A2B3 Type Hyperbranched Poly(amidoamine) Hydrogel Particles
Abstract
1. Introduction
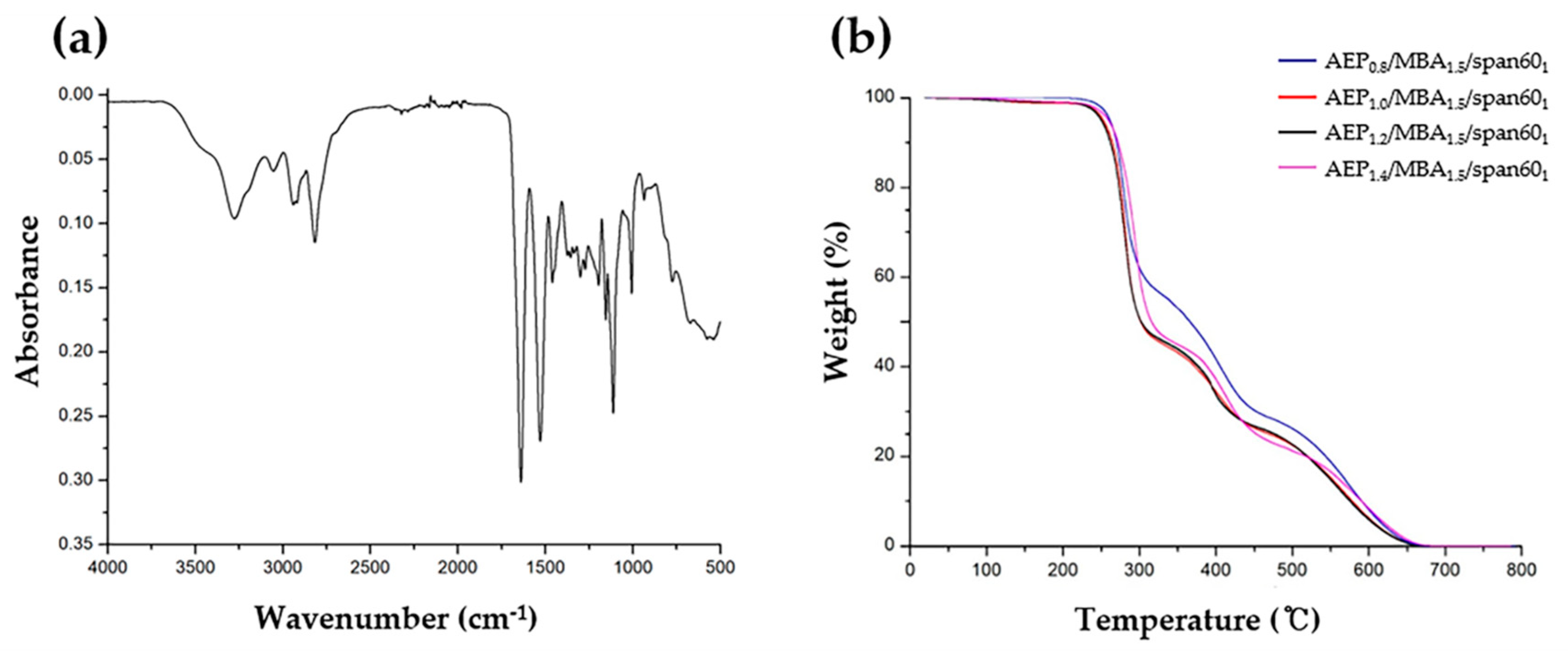
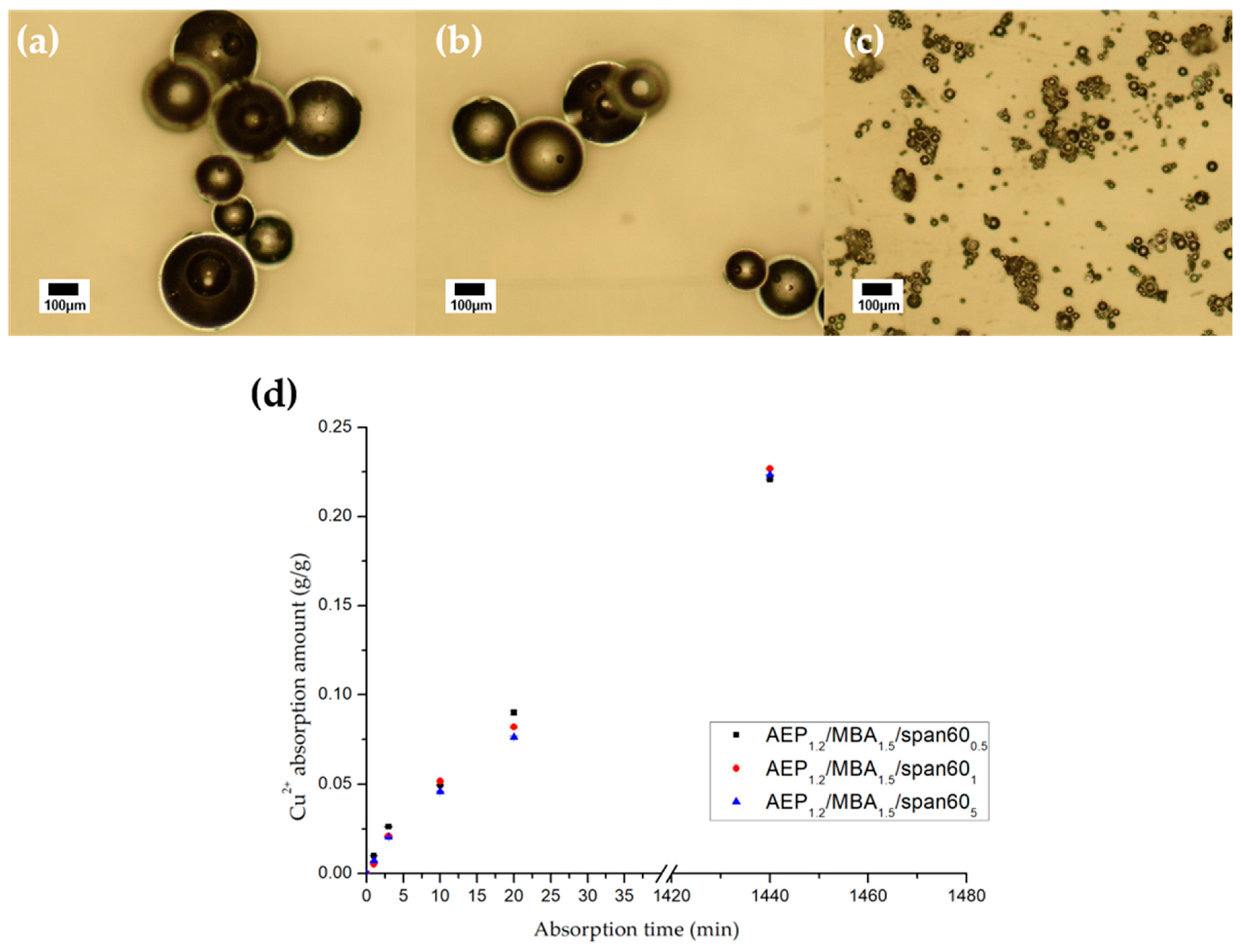
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
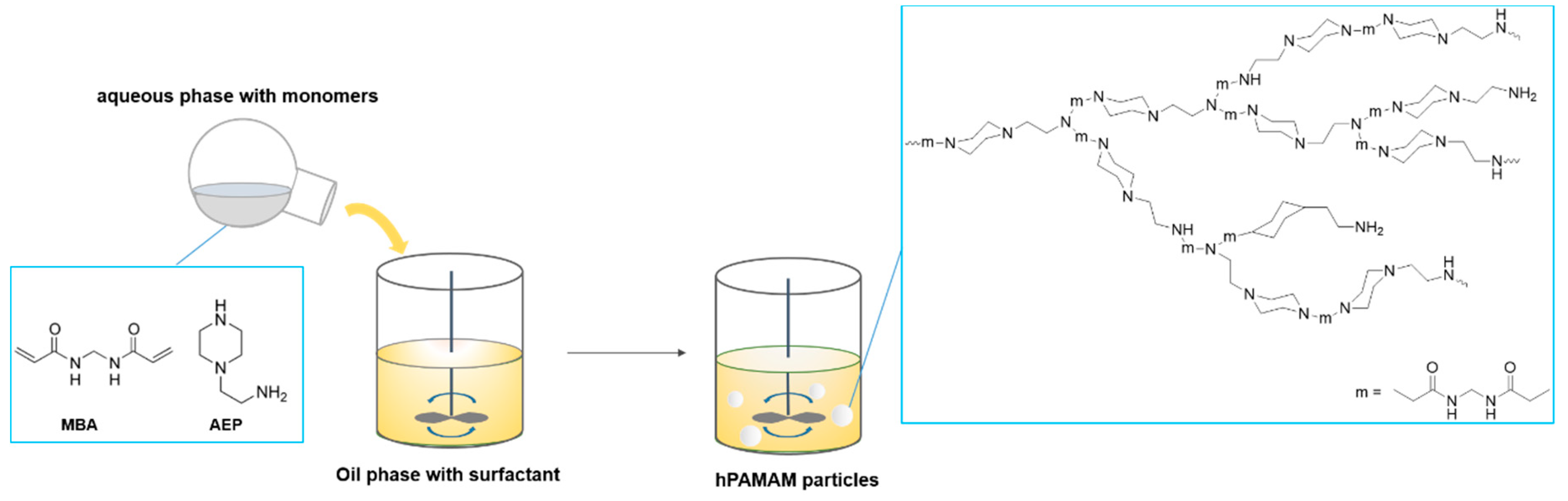
3.2.1. Synthesis of Poly(Amidoamine) Particles
3.2.2. Measurement of Copper Ion Absorption Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kapaj, S.; Peterson, H.; Liber, K.; Bhattacharya, P. Human health effects from chronic arsenic poisoning—A review. J. Environ. Sci. Heal.–Part A Toxic/Hazardous Subst. Environ. Eng. 2006, 41, 2399–2428. [Google Scholar] [CrossRef] [PubMed]
- Acharyya, S.K.; Chakraborty, P.; Lahiri, S.; Raymahashay, B.C.; Guha, S.; Bhowmik, A. Arsenic poisoning in the Ganges delta. Nature 1999, 401, 545–546. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-lado, L.; Sun, G.; Berg, M.; Zhang, Q.; Xue, H.; Zheng, Q.; Johnson, C.A. Groundwater Arsenic Contamination Throughout China. Science 2013, 341, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Gworek, B.; Bemowska-Kałabun, O.; Kijeńska, M.; Wrzosek-Jakubowska, J. Mercury in Marine and Oceanic Waters—A Review. Water. Air. Soil Pollut. 2016, 227, 371. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.A.; Van Sickle, J.; Herlihy, A.T.; Hughes, R.M. Mercury concentration in fish from streams and rivers throughout the western United States. Environ. Sci. Technol. 2007, 41, 58–65. [Google Scholar] [CrossRef]
- Kotte, M.R.; Kuvarega, A.T.; Cho, M.; Mamba, B.B.; Diallo, M.S. Mixed Matrix PVDF Membranes With in Situ Synthesized PAMAM Dendrimer-Like Particles: A New Class of Sorbents for Cu(II) Recovery from Aqueous Solutions by Ultrafiltration. Environ. Sci. Technol. 2015, 49, 9431–9442. [Google Scholar] [CrossRef] [PubMed]
- U.S. Agency for Toxic Substances and Disease Registry (ATSDR)—Toxicology Profile for Copper. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp132.pdf (accessed on 25 September 2019).
- Charerntanyarak, L. Heavy Metals Removal by Chemical Coagulation and Precipitation. Water. Sci. Technol. 1999, 39, 135–138. [Google Scholar] [CrossRef]
- Bakalár, T.; Búgel, M.; Gajdošová, L. Heavy metal removal using reverse osmosis. Acta Montan. Slovaca 2009, 14, 250–253. [Google Scholar]
- Dąbrowski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef]
- Alyüz, B.; Veli, S. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J. Hazard. Mater. 2009, 167, 482–488. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Y.; Hao, X.; Huang, W.; Feng, X. A novel potential-responsive ion exchange film system for heavy metal removal. J. Mater. Chem. A 2014, 2, 10263–10272. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Diallo, M.S.; Balogh, L.; Shafagati, A.; Johnson, J.H.; Goddard, W.A.; Tomalia, D.A. Poly(amidoamine) dendrimers: A new class of high capacity chelating agents for Cu(II) ions. Environ. Sci. Technol. 1999, 33, 820–824. [Google Scholar] [CrossRef]
- Diallo, M.S.; Christie, S.; Swaminathan, P.; Johnson, J.H.; Goddard, W.A. Dendrimer enhanced ultrafiltration. 1. Recovery of Cu(II) from aqueous solutions using PAMAM dendrimers with ethylene diamine core and terminal NH 2 groups. Environ. Sci. Technol. 2005, 39, 1366–1377. [Google Scholar] [CrossRef]
- Zhou, L.; Russell, D.H.; Zhao, M.; Crooks, R.M. Characterization of poly(amidoamine) dendrimers and their complexes with Cu2+ by matrix-assisted laser desorption ionization mass spectrometry. Macromolecules 2001, 34, 3567–3573. [Google Scholar] [CrossRef]
- Song, X.; Niu, Y.; Qiu, Z.; Zhang, Z.; Zhou, Y.; Zhao, J.; Chen, H. Adsorption of Hg(II) and Ag(I) from fuel ethanol by silica gel supported sulfur-containing PAMAM dendrimers: Kinetics, equilibrium and thermodynamics. Fuel 2017, 206, 80–88. [Google Scholar] [CrossRef]
- Niu, Y.; Yang, J.; Qu, R.; Gao, Y.; Du, N.; Chen, H.; Sun, C.; Wang, W. Synthesis of Silica-Gel-Supported Sulfur-Capped PAMAM Dendrimers for Efficient Hg(II) Adsorption: Experimental and DFT Study. Ind. Eng. Chem. Res. 2016, 55, 3679–3688. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, B.; He, S.; Man, R. Preparation of Graphene-Oxide/Polyamidoamine Dendrimers and Their Adsorption Properties toward Some Heavy Metal Ions. J. Chem. Eng. Data 2014, 59, 1719–1726. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Liu, Y.; Jiang, L.; Song, B.; Li, M.; Zeng, G.; Tan, X.; Cai, X.; Ding, Y. Adsorption of Cu(II), Pb(II), and Cd(II) ions from acidic aqueous solutions by diethylenetriaminepentaacetic acid-modified magnetic graphene oxide. J. Chem. Eng. Data 2017, 62, 407–416. [Google Scholar] [CrossRef]
- Hayati, B.; Maleki, A.; Najafi, F.; Daraei, H.; Gharibi, F.; McKay, G. Super high removal capacities of heavy metals (Pb2+ and Cu2+) using CNT dendrimer. J. Hazard. Mater. 2017, 336, 146–157. [Google Scholar] [CrossRef]
- Lee, S.; Eom, Y.; Park, J.; Lee, J.; Kim, S.Y. Micro-hydrogel Particles Consisting of Hyperbranched Polyamidoamine for the Removal of Heavy Metal Ions from Water. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Shin, J.; Yoo, J.; Seo, Y.S. Competitive adsorption of metals onto magnetic graphene oxide: Comparison with other carbonaceous adsorbents. Sci. World J. 2015, 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Liu, J.; Jiang, Y.; Teng, Q. Branched Polyethyleneimine-Functionalized Polystyrene Resin: Preparation and Adsorption of Cu2+. J. Chem. Eng. Data 2019, 64, 2618–2626. [Google Scholar] [CrossRef]
- Mohammadnezhad, G.; Moshiri, P.; Dinari, M.; Steiniger, F. In situ synthesis of nanocomposite materials based on modified-mesoporous silica MCM-41 and methyl methacrylate for copper (II) adsorption from aqueous solution. J. Iran. Chem. Soc. 2019, 16, 1491–1500. [Google Scholar] [CrossRef]
- Dinari, M.; Mohammadnezhad, G.; Soltani, R. Fabrication of poly(methyl methacrylate)/silica KIT-6 nanocomposites via in situ polymerization approach and their application for removal of Cu2+ from aqueous solution. RSC Adv. 2016, 6, 11419–11429. [Google Scholar] [CrossRef]
- Jiang, X.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R.; Shi, Z. Versatile core/shell-like alginate@polyethylenimine composites for efficient removal of multiple heavy metal ions (Pb2+, Cu2+, CrO42-): Batch and fixed-bed studies. Mater. Res. Bull. 2019, 118, 110526. [Google Scholar] [CrossRef]
- Igberase, E.; Osifo, P.; Ofomaja, A. The adsorption of copper (II) ions by polyaniline graft chitosan beads from aqueous solution: Equilibrium, kinetic and desorption studies. J. Environ. Chem. Eng. 2014, 2, 362–369. [Google Scholar] [CrossRef]
- Fan, C.; Li, K.; He, Y.; Wang, Y.; Qian, X.; Jia, J. Evaluation of magnetic chitosan beads for adsorption of heavy metal ions. Sci. Total Environ. 2018, 627, 1396–1403. [Google Scholar] [CrossRef]
- Deng, L.; Su, Y.; Su, H.; Wang, X.; Zhu, X. Sorption and desorption of lead (II) from wastewater by green algae Cladophora fascicularis. J. Hazard. Mater. 2007, 143, 220–225. [Google Scholar] [CrossRef]
- Gupta, V.K.; Rastogi, A. Sorption and desorption studies of chromium(VI) from nonviable cyanobacterium Nostoc muscorum biomass. J. Hazard. Mater. 2008, 154, 347–354. [Google Scholar] [CrossRef]
- Hammaini, A.; González, F.; Ballester, A.; Blázquez, M.L.; Muñoz, J.A. Biosorption of heavy metals by activated sludge and their desorption characteristics. J. Environ. Manage. 2007, 84, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.; Kwang, H.P. Adsorption and desorption characteristics of mercury(II) ions using aminated chitosan bead. Water Res. 2005, 39, 3938–3944. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Sorbent | Cu2+ Sorption Capacity (g/g) | Reference |
|---|---|---|
| Dowex M4195 | 0.054 | [22] |
| Graphene oxide/Fe3O4 | 0.023 | [23] |
| PEI-PS resin | 0.116 | [24] |
| m-MCM-41/PMMA | 0.042 | [25] |
| m-KIT-6 | 0.102 | [26] |
| Alginate@PEI-1.5 | 0.164 | [27] |
| Polyaniline grafted chitosan beads | 0.100 | [28] |
| Magnetic chitosan beads | 0.147 | [29] |
| PAMAM dendrimer | 0.329 | [15] |
| EDA-MBA hPAMAM particles | 0.170 | [22] |
| AEP-MBA hPAMAM particles | 0.223 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Eom, Y.; Lee, S.; Kim, S.Y. Copper Ions Removal from Water using A2B3 Type Hyperbranched Poly(amidoamine) Hydrogel Particles. Molecules 2019, 24, 3866. https://doi.org/10.3390/molecules24213866
Choi H, Eom Y, Lee S, Kim SY. Copper Ions Removal from Water using A2B3 Type Hyperbranched Poly(amidoamine) Hydrogel Particles. Molecules. 2019; 24(21):3866. https://doi.org/10.3390/molecules24213866
Chicago/Turabian StyleChoi, Hojung, Youngsik Eom, Sanghwa Lee, and Sang Youl Kim. 2019. "Copper Ions Removal from Water using A2B3 Type Hyperbranched Poly(amidoamine) Hydrogel Particles" Molecules 24, no. 21: 3866. https://doi.org/10.3390/molecules24213866
APA StyleChoi, H., Eom, Y., Lee, S., & Kim, S. Y. (2019). Copper Ions Removal from Water using A2B3 Type Hyperbranched Poly(amidoamine) Hydrogel Particles. Molecules, 24(21), 3866. https://doi.org/10.3390/molecules24213866





