Improving the Stability of Oil Body Emulsions from Diverse Plant Seeds Using Sodium Alginate
Abstract
1. Introduction
2. Results and Discussion
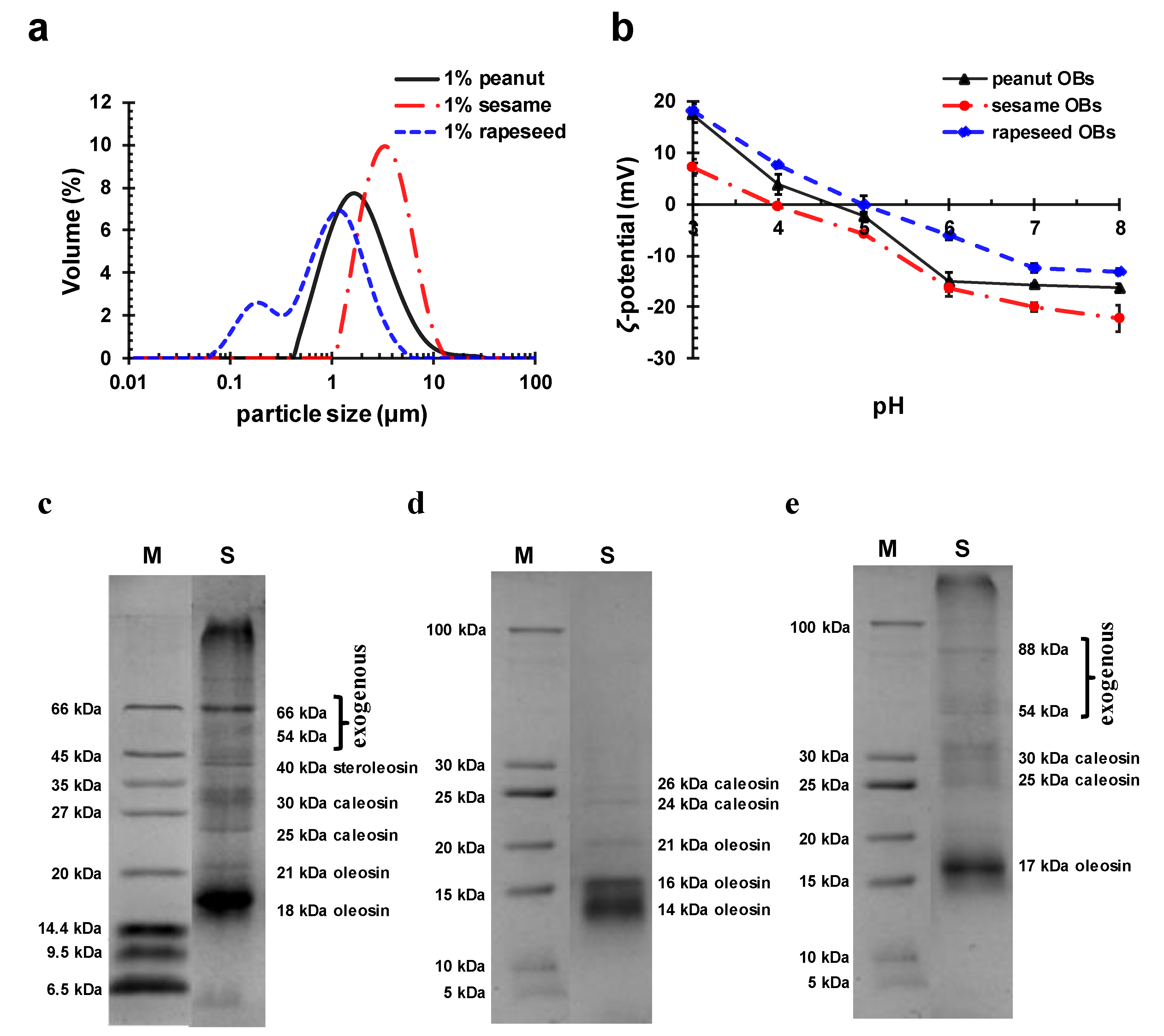
2.1. OB Characteristics
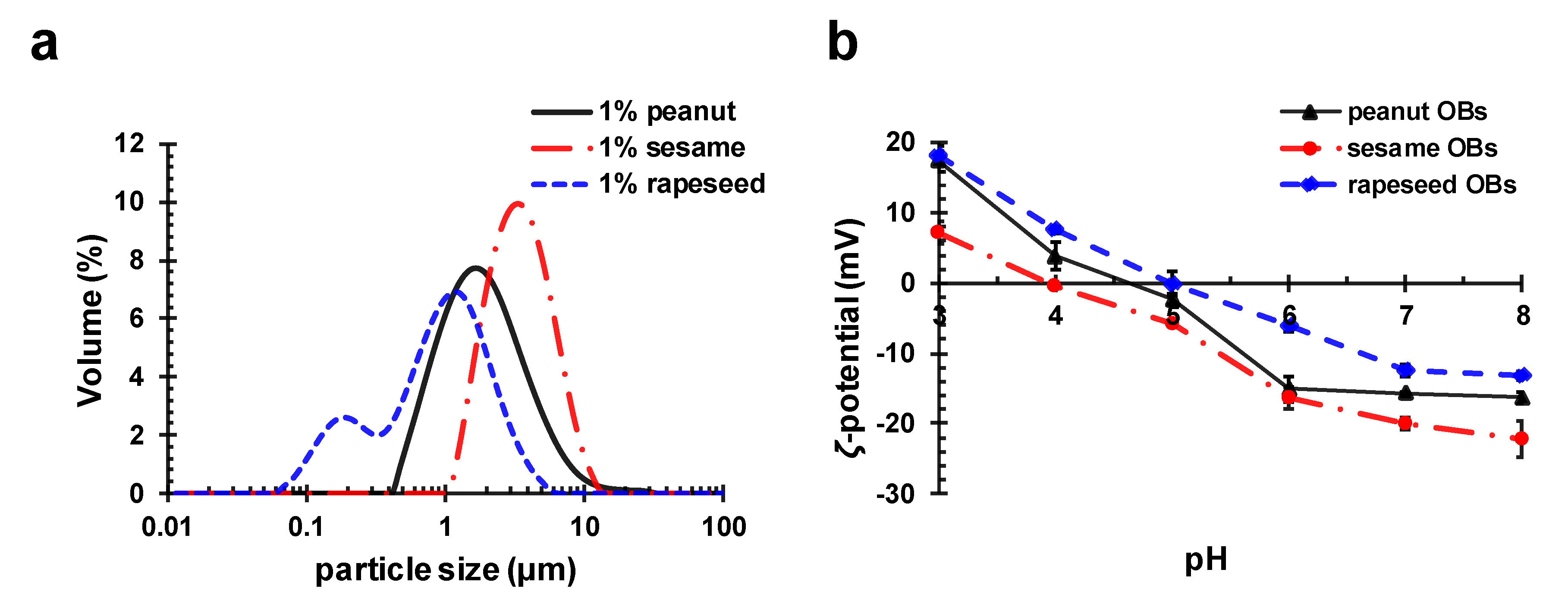
2.2. Influence of pH on the Creaming Stability of OB Emulsions
2.3. Influence of ALG on OB Emulsions
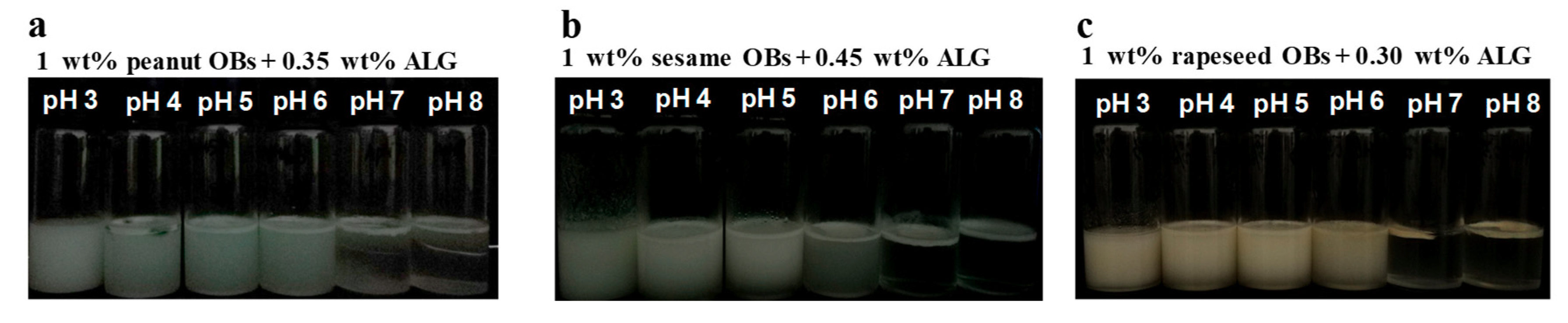
2.4. Influence of ALG on the Creaming Stability of OB Emulsions at pH 7
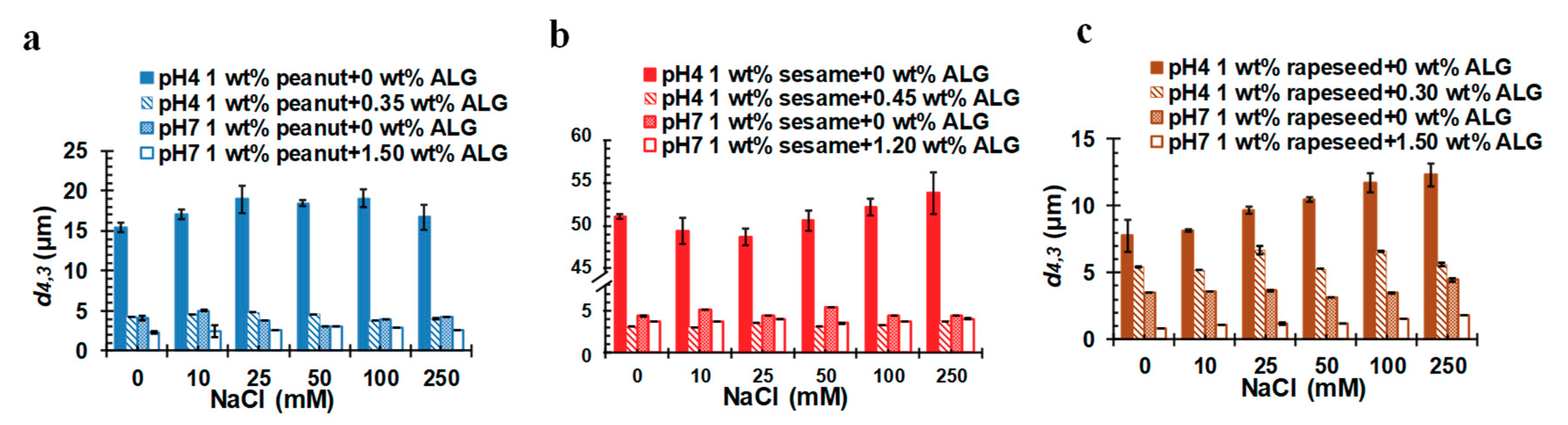
2.5. Influence of Salt on the Stability of OB Emulsions
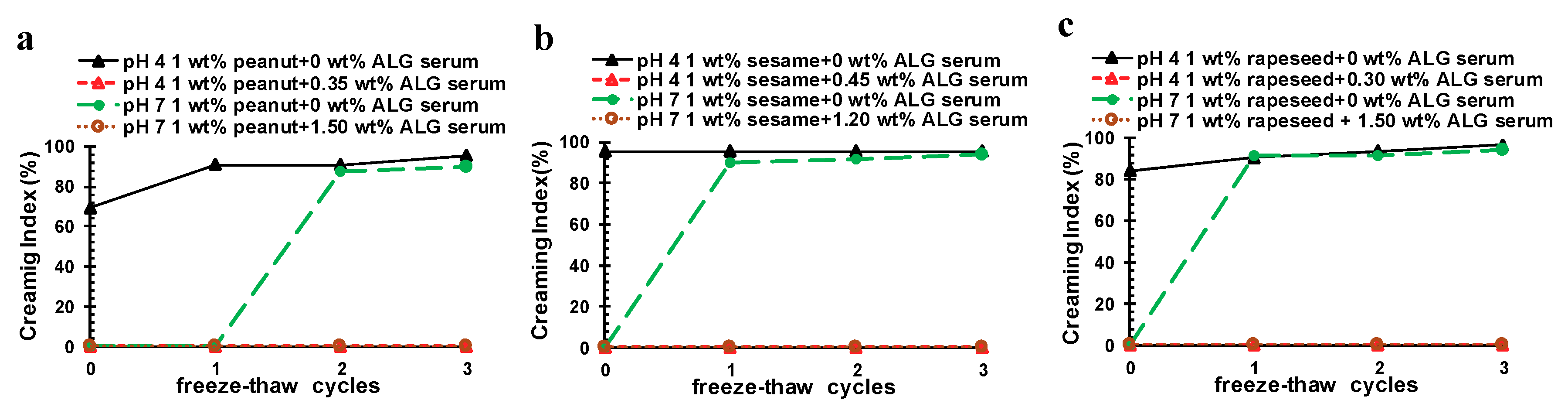
2.6. Influence of Freeze–Thaw Cycling and Thermal Treatment on the Stability of OB Emulsions
3. Materials and Methods
3.1. Materials
3.2. OB Extraction
3.3. Characterization of OB Chemical Compositions
3.4. Preparation of OB Emulsions with Different Concentrations of ALG
3.5. Particle Size, ζ-Potential, Microstructure, and Emulsion Stability Measurements
3.6. Viscosity Measurements of OB Emulsions
3.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Frandsen, G.I.; Mundy, J.; Tzen, J.T.C. Oil bodies and their associated proteins, oleosin and caleosin. Physiol. Plant. 2001, 112, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.J.; Liao, P.C.; Yang, H.H.; Tzen, J.T. Determination and analyses of the N-termini of oil-body proteins, steroleosin, caleosin and oleosin. Plant Physiol. Biochem. 2005, 43, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.G.; Sessions, R.B.; Clarke, A.R.; Tatham, A.S.; Shewry, P.R.; Napier, J.A. Characterization and modelling of the hydrophobic domain of a sunflower oleosin. Planta 2002, 214, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Nikiforidis, C.V.; Matsakidou, A.; Kiosseoglou, V. Composition, properties and potential food applications of natural emulsions and cream materials based on oil bodies. RSC Adv. 2014, 4, 25067–25078. [Google Scholar] [CrossRef]
- Qu, R.D.; Huang, A.H. Oleosin KD 18 on the surface of oil bodies in maize. Genomic and cDNA sequences and the deduced protein structure. J. Biol. Chem. 1990, 265, 2238–2243. [Google Scholar]
- Gohon, Y.; Vindigni, J.D.; Pallier, A.; Wien, F.; Celia, H.; Giuliani, A.; Tribet, C.; Chardot, T.; Briozzo, P. High water solubility and fold in amphipols of proteins with large hydrophobic regions: Oleosins and caleosin from seed lipid bodies. Biochim. Biophys. Acta BBA Biomembr. 2011, 1808, 706–716. [Google Scholar] [CrossRef]
- Jiang, P.L.; Tzen, J.T. Caleosin serves as the major structural protein as efficient as oleosin on the surface of seed oil bodies. Plant Signal. Behav. 2010, 5, 447–449. [Google Scholar] [CrossRef]
- Huang, A. Oleosins and oil bodies in seeds and other organs. Plant Physiol. 1996, 110, 1055–1061. [Google Scholar] [CrossRef]
- Tai, S.S.K.; Chen, M.C.M.; Peng, C.C.; Tzen, J.T.C. Gene family of oleosin isoforms and their structural stabilization in sesame seed oil bodies. Biosci. Biotechnol. Biochem. 2002, 66, 2146–2153. [Google Scholar] [CrossRef]
- Lin, L.J.; Tai, S.S.; Peng, C.C.; Tzen, J.T. Steroleosin, a sterol-binding dehydrogenase in seed oil bodies. Plant Physiol. 2002, 128, 1200–1211. [Google Scholar]
- Fisk, I.D.; White, D.A.; Carvalho, A.; Gray, D.A. Tocopherol—An intrinsic component of sunflower seed oil bodies. J. Am. Oil Chem. Soc. 2006, 83, 341–344. [Google Scholar] [CrossRef]
- White, D.A.; Fisk, I.D.; Makkhun, S.; Gray, D.A. In vitro assessment of the bioaccessibility of tocopherol and fatty acids from sunflower seed oil bodies. J. Agric. Food Chem. 2009, 57, 5720–5726. [Google Scholar] [CrossRef] [PubMed]
- White, D.; Fisk, I.; Gray, D.; White, D.; Gray, D. Characterisation of oat (Avena sativa L.) oil bodies and intrinsically associated E-vitamers. J. Cereal Sci. 2006, 43, 244–249. [Google Scholar] [CrossRef]
- Wu, N.N.; Huang, X.; Yang, X.Q.; Guo, J.; Yin, S.W.; He, X.T.; Wang, L.J.; Zhu, J.H.; Qi, J.R.; Zheng, E.L. In vitro assessment of the bioaccessibility of fatty acids and tocopherol from soybean oil body emulsions stabilized with ι-carrageenan. J. Agric. Food Chem. 2012, 60, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Tzen, J.T.; Lie, G.C.; Huang, A.H. Characterization of the charged components and their topology on the surface of plant seed oil bodies. J. Biol. Chem. 1992, 267, 15626–15634. [Google Scholar]
- Iwanaga, D.; Gray, D.; Decker, E.A.; Weiss, J.; McClements, D.J. Stabilization of soybean oil bodies using protective pectin coatings formed by electrostatic deposition. J. Agric. Food Chem. 2008, 56, 2240–2245. [Google Scholar] [CrossRef]
- Zielbauer, B.I.; Jackson, A.J.; Maurer, S.; Waschatko, G.; Ghebremedhin, M.; Rogers, S.E.; Heenan, R.K.; Porcar, L.; Vilgis, T.A. Soybean oleosomes studied by small angle neutron scattering (SANS). J. Colloid Interface Sci. 2018, 529, 197–204. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Kiosseoglou, V. Physicochemical stability of maize germ oil body emulsions as influenced by oil body surface−xanthan gum interactions. J. Agric. Food Chem. 2010, 58, 527–532. [Google Scholar] [CrossRef]
- Shakerardekani, A.; Karim, R.; Mirdamadiha, F. The effect of sorting on aflatoxin reduction of pistachio nuts. J. Food Agric. Environ. 2012, 10, 459–461. [Google Scholar] [CrossRef]
- Matsakidou, A.; Biliaderis, C.G.; Kiosseoglou, V. Preparation and characterization of composite sodium caseinate edible films incorporating naturally emulsified oil bodies. Food Hydrocoll. 2013, 30, 232–240. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Biliaderis, C.G.; Kiosseoglou, V. Rheological characteristics and physicochemical stability of dressing-type emulsions made of oil bodies–egg yolk blends. Food Chem. 2012, 134, 64–73. [Google Scholar] [CrossRef]
- Iwanaga, D.; Gray, D.A.; Fisk, I.D.; Decker, E.A.; Weiss, J.; McClements, D.J. Extraction and characterization of oil bodies from soy beans: A natural source of pre-emulsified soybean oil. J. Agric. Food Chem. 2007, 55, 8711–8716. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.N.; Yang, X.Q.; Teng, Z.; Yin, S.W.; Zhu, J.H.; Qi, J.R. Stabilization of soybean oil body emulsions using κ, ι, λ-carrageenan at different pH values. Food Res. Int. 2011, 44, 1059–1068. [Google Scholar] [CrossRef]
- Sukhotu, R.; Guo, S.; Xing, J.; Hu, Q.; Wang, R.; Shi, X.; Nishinari, K.; Fang, Y.; Guo, S. Changes in physiochemical properties and stability of peanut oil body emulsions by applying gum arabic. LWT Food Sci. Technol. 2016, 68, 432–438. [Google Scholar] [CrossRef]
- Nikiforidis, C.V.; Donsouzi, S.; Kiosseoglou, V. The interplay between diverse oil body extracts and exogenous biopolymers or surfactants. Food Res. Int. 2016, 83, 14–24. [Google Scholar] [CrossRef]
- Chen, B.; McClements, D.J.; Gray, D.A.; Decker, E.A. Stabilization of soybean oil bodies by enzyme (Laccase) cross-linking of adsorbed beet pectin coatings. J. Agric. Food Chem. 2010, 58, 9259–9265. [Google Scholar] [CrossRef]
- Chen, B.; McClements, D.J.; Decker, E.A. Role of continuous phase anionic polysaccharides on the oxidative stability of menhaden oil-in-water emulsions. J. Agric. Food Chem. 2010, 58, 3779–3784. [Google Scholar] [CrossRef]
- Hu, G.H. Functional Food Gum; Chemical Industry Press: Beijing, China, 2008. [Google Scholar]
- Su, C.; Feng, Y.; Ye, J.; Zhang, Y.; Gao, Z.; Zhao, M.; Yang, N.; Nishinari, K.; Fang, Y. Effect of sodium alginate on the stability of natural soybean oil body emulsions. RSC Adv. 2018, 8, 4731–4741. [Google Scholar] [CrossRef]
- Payne, G.; Lad, M.; Foster, T.; Khosla, A.; Gray, D.; Foster, T. Composition and properties of the surface of oil bodies recovered from Echium plantagineum. Colloids Surf. B Biointerfaces 2014, 116, 88–92. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Zhao, L.P.; Ying, Y.S.; Kong, X.Z.; Hua, Y.F.; Chen, Y.M. The characterization of soybean oil body integral oleosin isoforms and the effects of alkaline pH on them. Food Chem. 2015, 177, 288–294. [Google Scholar] [CrossRef]
- Deleu, M.; Vaca-Medina, G.; Fabre, J.F.; Roïz, J.; Valentin, R.; Mouloungui, Z. Interfacial properties of oleosins and phospholipids from rapeseed for the stability of oil bodies in aqueous medium. Colloids Surf. B Biointerfaces 2010, 80, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Matsumiya, K.; Nambu, Y.; Samoto, M.; Yanagisawa, M.; Matsumura, Y.; Matsuymiya, K. Interfacial and emulsifying properties of crude and purified soybean oil bodies. Food Struct. 2017, 12, 64–72. [Google Scholar] [CrossRef]
- Zhao, L.P.; Chen, Y.M.; Chen, Y.J.; Kong, X.Z.; Hua, Y.F. Effects of pH on protein components of extracted oil bodies from diverse plant seeds and endogenous protease-induced oleosin hydrolysis. Food Chem. 2016, 200, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liu, Y.; Zhao, M.; Ren, J.; Yang, B. Enzymatic hydrolysis and their effects on conformational and functional properties of peanut protein isolate. Food Chem. 2011, 127, 1438–1443. [Google Scholar] [CrossRef]
- Ying, Y.S.; Zhao, L.P.; Kong, L.Z.; Kong, X.Z.; Hua, Y.F.; Chen, Y.M. Solubilization of proteins in extracted oil bodies by SDS: A simple and efficient protein sample preparation method for Tricine-SDS-PAGE. Food Chem. 2015, 181, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Tzen, J.T.C.; Lai, Y.K.; Chan, K.L.; Huang, A.H.C. Oleosin Isoforms of high and low molecular weights are present in the oil bodies of diverse seed species. Plant Physiol. 1990, 94, 1282–1289. [Google Scholar] [CrossRef]
- De Chirico, S.; Di Bari, V.; Foster, T.; Gray, D. Enhancing the recovery of oilseed rape seed oil bodies (oleosomes) using bicarbonate-based soaking and grinding media. Food Chem. 2018, 241, 419–426. [Google Scholar] [CrossRef]
- Güzey, D.; McClements, D.J. Impact of electrostatic interactions on formation and stability of emulsions containing oil droplets coated by β-lactoglobulin−pectin complexes. J. Agric. Food Chem. 2007, 55, 475–485. [Google Scholar] [CrossRef]
- Maurer, S.; Waschatko, G.; Schach, D.; Zielbauer, B.I.; Dahl, J.; Weidner, T.; Bonn, M.; Vilgis, T.A. The role of intact oleosin for stabilization and function of oleosomes. J. Phys. Chem. B 2013, 117, 13872–13883. [Google Scholar] [CrossRef]
- Keddie, J.S.; Edwards, E.W.; Gibbons, T.; Shaw, C.H.; Murphy, D.J. Sequence of an oleocin cDNA from Brassica napus. Plant Mol. Biol. 1992, 19, 1079–1083. [Google Scholar] [CrossRef]
- Barre, A.; Simplicien, M.; Cassan, G.; Benoist, H.; Rougé, P. Oil bodies (oleosomes): Occurrence, structure, allergenicity. Rev. Fr. D’allergologie 2018, 58, 574–580. [Google Scholar] [CrossRef]
- Waschatko, G.; Schiedt, B.; Vilgis, T.A.; Junghans, A.; Junghans, S.A. Soybean oleosomes behavior at the air-water interface. J. Phys. Chem. B 2012, 116, 10832–10841. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.N.; Huang, X.; Yang, X.Q.; Guo, J.; Zheng, E.L.; Yin, S.W.; Zhu, J.H.; Qi, J.R.; He, X.T.; Zhang, J.B. Stabilization of soybean oil body emulsions using ι-carrageenan: Effects of salt, thermal treatment and freeze-thaw cycling. Food Hydrocoll. 2012, 28, 110–120. [Google Scholar] [CrossRef]
- Zhao, Z.G. Apply Colloid and Interface Chemistry; Chemical Industry Press: Beijing, China, 2008; p. 55. [Google Scholar]
- Güzey, D.; McClements, D.J. Formation, stability and properties of multilayer emulsions for application in the food industry. Adv. Colloid Interface Sci. 2006, 128, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.S.; Decker, E.A.; McClements, D.J. Application of multi-component biopolymer layers to improve the freeze–thaw stability of oil-in-water emulsions: β-Lactoglobulin–ι-carrageenan–gelatin. J. Food Eng. 2007, 80, 1246–1254. [Google Scholar] [CrossRef]
- Walstra, P. Principles of emulsion formation. Chem. Eng. Sci. 1993, 48, 333–349. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of International; Association of Official Analytical Chemists: Arlington, VA, USA, 2000. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |





| Fat (wt.%) | Protein (wt.%) | Moisture (wt.%) | Protein/Fat | ||
|---|---|---|---|---|---|
| Soybean [29] | 0.54 ± 0.01 | 29.40 ± 1.00 | 3.00 ± 0.20 | 53.60 ± 2.30 | 0.10 ± 0.01 |
| Peanut | 2.31 ± 0.12 | 72.91 ± 1.03 | 1.16 ± 0.01 | 22.72 ± 0.14 | 0.02 ± 0.01 |
| Sesame | 3.65 ± 0.01 | 80.56 ± 0.80 | 0.95 ± 0.01 | 17.66 ± 0.19 | 0.01 ± 0.01 |
| Rapeseed | 1.05 ± 0.01 | 55.34 ± 0.75 | 2.95 ± 0.04 | 37.38 ± 0.26 | 0.05 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, N.; Xu, Y.; Wang, Q.; Huang, P.; Nishinari, K.; Fang, Y. Improving the Stability of Oil Body Emulsions from Diverse Plant Seeds Using Sodium Alginate. Molecules 2019, 24, 3856. https://doi.org/10.3390/molecules24213856
Zhang Y, Yang N, Xu Y, Wang Q, Huang P, Nishinari K, Fang Y. Improving the Stability of Oil Body Emulsions from Diverse Plant Seeds Using Sodium Alginate. Molecules. 2019; 24(21):3856. https://doi.org/10.3390/molecules24213856
Chicago/Turabian StyleZhang, Yuemei, Nan Yang, Yao Xu, Qian Wang, Ping Huang, Katsuyoshi Nishinari, and Yapeng Fang. 2019. "Improving the Stability of Oil Body Emulsions from Diverse Plant Seeds Using Sodium Alginate" Molecules 24, no. 21: 3856. https://doi.org/10.3390/molecules24213856
APA StyleZhang, Y., Yang, N., Xu, Y., Wang, Q., Huang, P., Nishinari, K., & Fang, Y. (2019). Improving the Stability of Oil Body Emulsions from Diverse Plant Seeds Using Sodium Alginate. Molecules, 24(21), 3856. https://doi.org/10.3390/molecules24213856





