Chemometrics Approaches in Forced Degradation Studies of Pharmaceutical Drugs
Abstract
1. Chemometrics
2. Degradation Products
2.1. The Generation of Degradation Products
2.2. Forced Degradation Studies
- To obtain the potential degradation potential of an API or drug product;
- To discover the degradation mechanism, such as hydrolysis, thermolysis, oxidation, photolysis, etc.;
- To elucidate the molecular structure of degradation product;
- To solve problems regarded to the API stability;
- To identify the conditions where the API or the drug product are more susceptible to degradation in order to ensure the quality of the final product, bringing to pharmaceutical industry enough knowledge for development, packaging, manufacture, manipulation, and storage;
- To obtain more stable formulations;
2.3. Strategies to Select the Degradation Conditions
2.4. Acceptable Limits of Impurities
- Reporting threshold: A limit of impurity that is not necessary to be reported.
- Identification threshold: A limit of impurity does not need to be structurally identified.
- Qualification threshold: The maximum amount of impurity that is not necessary to be qualified. Being “qualified” is the process of acquisition and evaluation of data that establishes biological security of an impurity or a degradation profile at the specified levels [40].
3. Applications of Chemometric Tools in Forced Degradation Studies
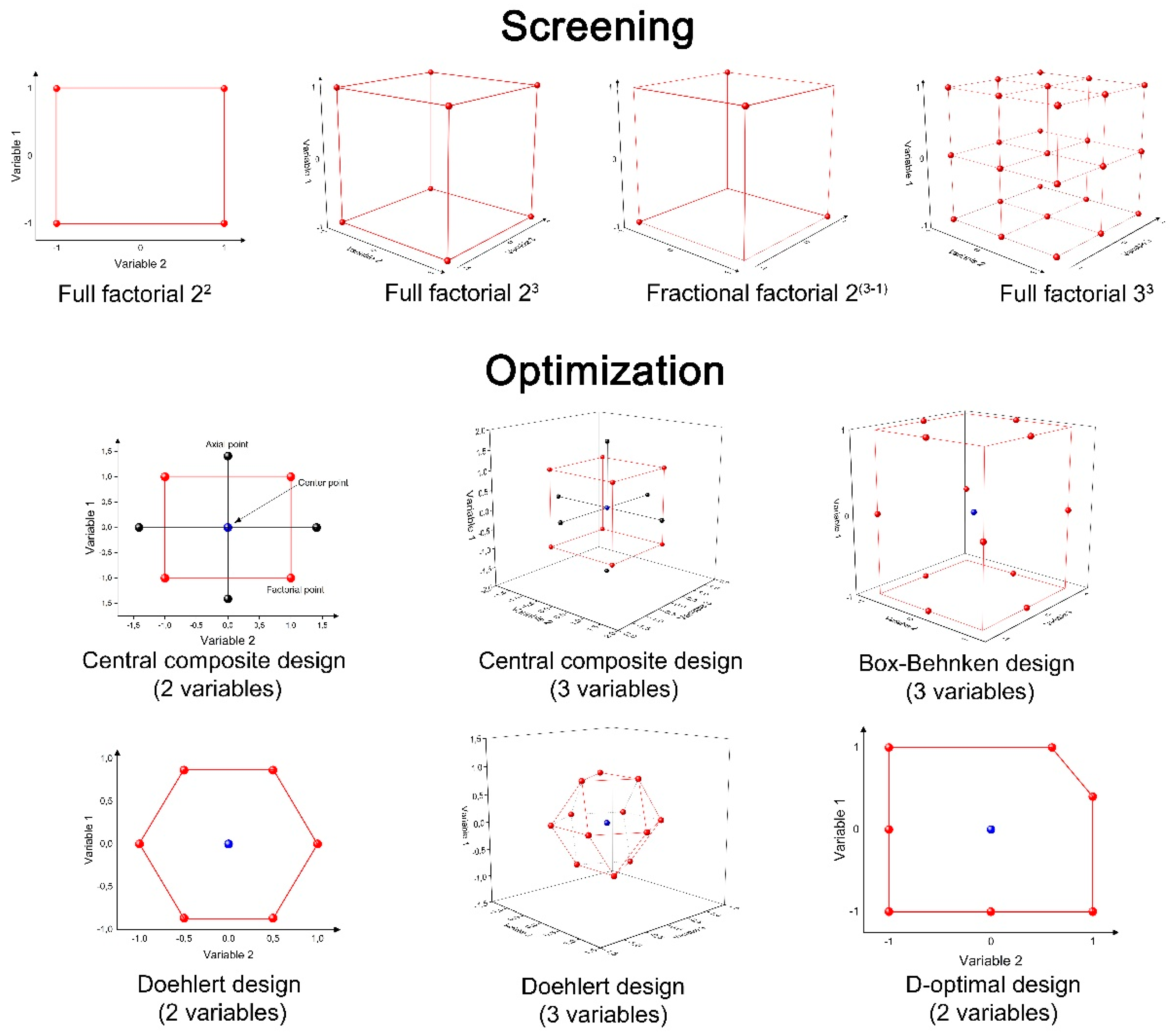
3.1. Design of Experiment (DoE)
- Determining how many experiments are necessary to achieve the goal;
- Reducing the number of experiments;
- Observing the synergic and antagonist interactions between variables;
- Allowing for the possibility to create mathematical models and surface response to describe the behavior of the variables and to predict the system’s response within an experimental domain;
- Decreasing the time, costs, and generation of lesser amounts of chemical waste, which contributes for the green chemistry principles [79].
3.2. About Fusion QbD®
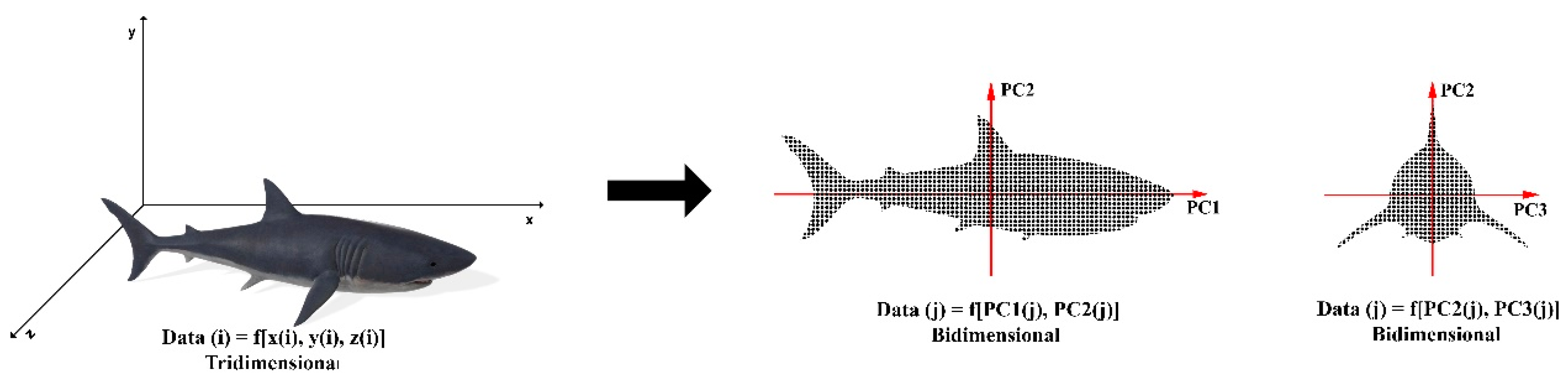
3.3. Principal Component Analysis (PCA)
3.4. Partial Least Squares (PLS)
3.5. Multivariate Curve Resolution (MCR)
3.6. Artificial Neural Network (ANN)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kiralj, R.; Ferreira, M.M.C. The past, present, and future of chemometrics worldwide: Some etymological, linguistic, and bibliometric investigations. J. Chemom. 2006, 20, 247–272. [Google Scholar] [CrossRef]
- Swarbrick, B.; Westad, F. An Overview of Chemometrics for the Engineering and Measurement Sciences. In Handbook of Measurement in Science and Engineering; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; p. 2309. [Google Scholar]
- Kumar, R.; Sharma, V. Chemometrics in forensic science. Trac Trends Anal. Chem. 2018, 105, 191–201. [Google Scholar] [CrossRef]
- Brown, S. The chemometrics revolution re-examined. J. Chemom. 2017, 31, e2864. [Google Scholar] [CrossRef]
- Hibbert David, B. Vocabulary of concepts and terms in chemometrics (IUPAC Recommendations 2016). Pure Appl. Chem. 2016, 88, 407. [Google Scholar] [CrossRef]
- Caballero, B.; Finglas, P.; Toldrá, F. Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Pomerantsev, A.L.; Rodionova, O.Y. Chemometric view on “comprehensive chemometrics”. Chemom. Intell. Lab. Syst. 2010, 103, 19–24. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Silva Junior, M.M.; Felix, C.S.A.; da Silva, D.L.F.; Santos, A.S.; Santos Neto, J.H.; de Souza, C.T.; Cruz Junior, R.A.; Souza, A.S. Multivariate optimization techniques in food analysis—A review. Food Chem. 2019, 273, 3–8. [Google Scholar] [CrossRef]
- De Luca, S.; De Filippis, M.; Bucci, R.; Magrì, A.D.; Magrì, A.L.; Marini, F. Characterization of the effects of different roasting conditions on coffee samples of different geographical origins by HPLC-DAD, NIR and chemometrics. Microchem. J. 2016, 129, 348–361. [Google Scholar] [CrossRef]
- Briandet, R.; Kemsley, E.K.; Wilson, R.H. Discrimination of Arabica and Robusta in Instant Coffee by Fourier Transform Infrared Spectroscopy and Chemometrics. J. Agric. Food Chem. 1996, 44, 170–174. [Google Scholar] [CrossRef]
- Santos, P.M.; Pereira-Filho, E.R.; Rodriguez-Saona, L.E. Rapid detection and quantification of milk adulteration using infrared microspectroscopy and chemometrics analysis. Food Chem. 2013, 138, 19–24. [Google Scholar] [CrossRef]
- Amorello, D.; Orecchio, S.; Pace, A.; Barreca, S. Discrimination of almonds (Prunus dulcis) geographical origin by minerals and fatty acids profiling. Nat. Prod. Res. 2016, 30, 2107–2110. [Google Scholar] [CrossRef]
- Wu, X.M.; Zuo, Z.T.; Zhang, Q.Z.; Wang, Y.Z. Classification of Paris species according to botanical and geographical origins based on spectroscopic, chromatographic, conventional chemometric analysis and data fusion strategy. Microchem. J. 2018, 143, 367–378. [Google Scholar] [CrossRef]
- Chen, H.; Lin, Z.; Tan, C. Fast discrimination of the geographical origins of notoginseng by near-infrared spectroscopy and chemometrics. J. Pharm. Biomed. Anal. 2018, 161, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Uríčková, V.; Sádecká, J. Determination of geographical origin of alcoholic beverages using ultraviolet, visible and infrared spectroscopy: A review. Spectrochim. Acta Part A 2015, 148, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Kanginejad, A.; Mani-Varnosfaderani, A. Chemometrics advances on the challenges of the gas chromatography–mass spectrometry metabolomics data: A review. J. Iran. Chem. Soc. 2018, 15, 2733–2745. [Google Scholar] [CrossRef]
- Liu, S.; Liang, Y.Z.; Liu, H.T. Chemometrics applied to quality control and metabolomics for traditional Chinese medicines. J. Chromatogr. B 2016, 1015–1016, 82–91. [Google Scholar] [CrossRef]
- Savorani, F.; Rasmussen, M.A.; Mikkelsen, M.S.; Engelsen, S.B. A primer to nutritional metabolomics by NMR spectroscopy and chemometrics. Food Res. Int. 2013, 54, 1131–1145. [Google Scholar] [CrossRef]
- Bhushan, N.; Hadpe, S.; Rathore, A.S. Chemometrics applications in biotech processes: Assessing process comparability. Biotechnol. Prog. 2012, 28, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.S.; Xu, Y.D.; Li, L.; Fang, K.T. Uniform experimental design in chemometrics. J. Chemom. 2018, 32, e3020. [Google Scholar] [CrossRef]
- Gadžurić, S.B.; Podunavac Kuzmanović, S.O.; Vraneš, M.B.; Petrin, M.; Bugarski, T.; Kovačević, S.Z. Multivariate Chemometrics with Regression and Classification Analyses in Heroin Profiling Based on the Chromatographic Data. Iran. J. Pharm. Res. IJPR 2016, 15, 725–734. [Google Scholar]
- Materazzi, S.; Gregori, A.; Ripani, L.; Apriceno, A.; Risoluti, R. Cocaine profiling: Implementation of a predictive model by ATR-FTIR coupled with chemometrics in forensic chemistry. Talanta 2017, 166, 328–335. [Google Scholar] [CrossRef]
- Muehlethaler, C.; Massonnet, G.; Esseiva, P. The application of chemometrics on Infrared and Raman spectra as a tool for the forensic analysis of paints. Forensic Sci. Int. 2011, 209, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Thanasoulias, N.C.; Parisis, N.A.; Evmiridis, N.P. Multivariate chemometrics for the forensic discrimination of blue ball-point pen inks based on their Vis spectra. Forensic Sci. Int. 2003, 138, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Brereton, R.G. Pattern recognition in chemometrics. Chemom. Intell. Lab. Syst. 2015, 149, 90–96. [Google Scholar] [CrossRef]
- Roggo, Y.; Degardin, K.; Margot, P. Identification of pharmaceutical tablets by Raman spectroscopy and chemometrics. Talanta 2010, 81, 988–995. [Google Scholar] [CrossRef]
- da Silva, V.H.; Soares-Sobrinho, J.L.; Pereira, C.F.; Rinnan, Å. Evaluation of chemometric approaches for polymorphs quantification in tablets using near-infrared hyperspectral images. Eur. J. Pharm. Biopharm. 2019, 134, 20–28. [Google Scholar] [CrossRef]
- Dinç, E.; Büker, E. Spectrochromatographic determination of dorzolamide hydrochloride and timolol maleate in an ophthalmic solution using three-way analysis methods. Talanta 2019, 191, 248–256. [Google Scholar] [CrossRef]
- Sakr, M.; Hanafi, R.; Fouad, M.; Al-Easa, H.; El-Moghazy, S. Design and optimization of a luminescent Samarium complex of isoprenaline: A chemometric approach based on Factorial design and Box-Behnken response surface methodology. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 208, 114–123. [Google Scholar] [CrossRef]
- Rodionova, O.Y.; Titova, A.V.; Demkin, N.A.; Balyklova, K.S.; Pomerantsev, A.L. Qualitative and quantitative analysis of counterfeit fluconazole capsules: A non-invasive approach using NIR spectroscopy and chemometrics. Talanta 2019, 195, 662–667. [Google Scholar] [CrossRef]
- Visco, G.; Avino, P. Employ of multivariate analysis and chemometrics in cultural heritage and environment fields. Environ. Sci. Pollut. Res. 2017, 24, 13863–13865. [Google Scholar] [CrossRef]
- Musumarra, G.; Fichera, M. Chemometrics and cultural heritage. Chemom. Intell. Lab. Syst. 1998, 44, 363–372. [Google Scholar] [CrossRef]
- Madariaga, J.M. Analytical chemistry in the field of cultural heritage. Anal. Methods 2015, 7, 4848–4876. [Google Scholar] [CrossRef]
- Barreca, S.; Mazzola, A.; Orecchio, S.; Tuzzolino, N. Polychlorinated Biphenyls in Sediments from Sicilian Coastal Area (Scoglitti) using Automated Soxhlet, GC-MS, and Principal Component Analysis. Polycycl. Aromat. Compd. 2014, 34, 237–262. [Google Scholar] [CrossRef]
- Granato, D.; Santos, J.S.; Escher, G.B.; Ferreira, B.L.; Maggio, R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018, 72, 83–90. [Google Scholar] [CrossRef]
- Panchuk, V.; Yaroshenko, I.; Legin, A.; Semenov, V.; Kirsanov, D. Application of chemometric methods to XRF-data—A tutorial review. Anal. Chim. Acta 2018, 1040, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Basniwal, P.K. Forced degradation and impurity profiling: Recent trends in analytical perspectives. J. Pharm. Biomed. Anal. 2013, 86, 11–35. [Google Scholar] [CrossRef]
- Rao, R.N.; Nagaraju, V. An overview of the recent trends in development of HPLC methods for determination of impurities in drugs. J. Pharm. Biomed. Anal. 2003, 33, 335–377. [Google Scholar]
- Holm, R.; Elder, D.P. Analytical advances in pharmaceutical impurity profiling. Eur. J. Pharm. Sci. 2016, 87, 118–135. [Google Scholar] [CrossRef]
- ICH. Impurities in New Drug Substances Q3a(R2); Published by food and Drug Administration: Silver Spring, MD, USA, 2008. Available online: https://www.fda.gov/media/71272/download (accessed on 10 August 2019).
- Melo, S.R.d.O.; Mello, M.H.d.; Silveira, D.; Simeoni, L.A. Advice on Degradation Products in Pharmaceuticals: A. PDA J. Pharm. Sci. Technol. 2014, 68, 221–238. [Google Scholar] [CrossRef]
- Pan, C.; Liu, F.; Motto, M. Identification of pharmaceutical impurities in formulated dosage forms. J. Pharm. Sci. 2011, 100, 1228–1259. [Google Scholar] [CrossRef]
- Sastry, R.P.; Venkatesan, C.; Sastry, B.; Mahesh, K. Identification and characterization of forced degradation products of pralatrexate injection by LC-PDA and LC–MS. J. Pharm. Biomed. Anal. 2016, 131, 400–409. [Google Scholar] [CrossRef]
- Skibiński, R.; Trawiński, J.; Komsta, Ł.; Bajda, K. Characterization of forced degradation products of clozapine by LC-DAD/ESI-Q-TOF. J. Pharm. Biomed. Anal. 2016, 131, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Attwood, D.; Florence, A.T.; Rothschild, Z. Princípios Físico-Químicos em Farmácia Volume 4; Edusp: São Paulo, Brazil, 2003. [Google Scholar]
- Gallardo, C.; Rojas, J.J.; Flórez, O.A. La temperatura cinética media en los estudios de estabilidad a largo plazo y almacenamiento de los medicamentos. Vitae 2004, 11, 67–72. [Google Scholar]
- Allen Jr, L.V.; Popovich, N.G.; Ansel, H.C. Formas Farmacêuticas e Sistemas de Liberação de Fármacos-9; Artmed Editora: Porto Alegre, Brazil, 2013. [Google Scholar]
- Blessy, M.; Patel, R.D.; Prajapati, P.N.; Agrawal, Y.K. Development of forced degradation and stability indicating studies of drugs—A review. J. Pharm. Anal. 2014, 4, 159–165. [Google Scholar] [CrossRef]
- Qiu, F.; Norwood, D.L. Identification of pharmaceutical impurities. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 877–935. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, S.; Anwar, Z.; Sheraz, M.A.; Sikorski, M. Photostability and photostabilization of drugs and drug products. Int. J. Photoenergy 2016, 2016. [Google Scholar] [CrossRef]
- Singh, S.; Handa, T.; Narayanam, M.; Sahu, A.; Junwal, M.; Shah, R.P. A critical review on the use of modern sophisticated hyphenated tools in the characterization of impurities and degradation products. J. Pharm. Biomed. Anal. 2012, 69, 148–173. [Google Scholar] [CrossRef]
- ICH. Stability Testing of New Drug Substances and Products Q1A (R2); Published by Food and Drug Administration: Silver Spring, MD, USA, 2003. Available online: https://www.fda.gov/media/71707/download (accessed on 11 August 2019).
- Singh, S.; Junwal, M.; Modhe, G.; Tiwari, H.; Kurmi, M.; Parashar, N.; Sidduri, P. Forced degradation studies to assess the stability of drugs and products. Trac Trends Anal. Chem. 2013, 49, 71–88. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, Y.-Y.; Chang, Y.; Chang, K.-W.; Hsia, Y.-C. Forced degradation behavior of epidepride and development of a stability-indicating method based on liquid chromatography–mass spectrometry. J. Food Drug Anal. 2014, 22, 248–256. [Google Scholar] [CrossRef]
- ANVISA Perguntas & Respostas. Assunto: RDC 53/2015 e Guia 4/2015. Available online: http://portal.anvisa.gov.br/documents/33836/418522/Perguntas+e+Respostas+-+RDC+53+2015+e+Guia+04+2015/6b3dec42-546c-4953-943f-4047b8b50f87 (accessed on 10 August 2019).
- Canavesi, R.; Aprile, S.; Varese, E.; Grosa, G. Development and validation of a stability-indicating LC-UV method for the determination of pantethine and its degradation product based on a forced degradation study. J. Pharm. Biomed. Anal. 2014, 97, 141–150. [Google Scholar] [CrossRef]
- Bhardwaj, S.P.; Singh, S. Study of forced degradation behavior of enalapril maleate by LC and LC-MS and development of a validated stability-indicating assay method. J. Pharm. Biomed. Anal. 2008, 46, 113–120. [Google Scholar] [CrossRef]
- Palaric, C.; Molinié, R.; Cailleu, D.; Fontaine, J.-X.; Mathiron, D.; Mesnard, F.; Gut, Y.; Renaud, T.; Petit, A.; Pilard, S. A Deeper Investigation of Drug Degradation Mixtures Using a Combination of MS and NMR Data: Application to Indapamide. Molecules 2019, 24, 1764. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Beg, S.; Samim, M.; Ahmad, F.J. Application of Chemometric Approach for Development and Validation of High Performance Liquid Chromatography Method for Estimation of Ropinirole Hydrochloride; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019. [Google Scholar]
- ICH. Photostability Testing of New Drug Substances and Products Q1B; Published by Food and Drug Administration: Silver Spring, MD, USA, 1996. Available online: https://www.fda.gov/media/71713/download (accessed on 15 August 2019).
- Bakshi, M.; Singh, S. Development of validated stability-indicating assay methods—Critical review. J. Pharm. Biomed. Anal. 2002, 28, 1011–1040. [Google Scholar] [CrossRef]
- Bansal, G.; Singh, M.; Jindal, K.C.; Singh, S. Ultraviolet-photodiode array and high-performance liquid chromatographic/mass spectrometric studies on forced degradation behavior of glibenclamide and development of a validated stability-indicating method. J. Aoac Int. 2008, 91, 709–719. [Google Scholar] [PubMed]
- World Health Organization. WHO Expert Committee on Specifications for Pharmaceutical Preparations: Thirty-Ninth Report; World Health Organization: Geneva, Switzerland, 2005; Volume 39. [Google Scholar]
- Sanitária, A.N.d.V. Resolução De Diretoria Colegiada—RDC Nº 53; Diário Oficial da União: Brasília, Brazil, 2015. [Google Scholar]
- Devrukhakar, P.S.; Shankar, M.S.; Shankar, G.; Srinivas, R. A stability-indicating LC–MS/MS method for zidovudine: Identification, characterization and toxicity prediction of two major acid degradation products. J. Pharm. Anal. 2017, 7, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Skibiński, R.; Trawiński, J.; Komsta, Ł.; Murzec, D. Characterization of forced degradation products of toloxatone by LC-ESI-MS/MS. Saudi Pharm. J. 2018, 26, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.N.; Shah, N.; Bhalani, V.; Mahajan, A. LC, MSn and LC–MS/MS studies for the characterization of degradation products of amlodipine. J. Pharm. Anal. 2015, 5, 33–42. [Google Scholar] [CrossRef]
- Rakibe, U.; Tiwari, R.; Mahajan, A.; Rane, V.; Wakte, P. LC and LC–MS/MS studies for the identification and characterization of degradation products of acebutolol. J. Pharm. Anal. 2018, 8, 357–365. [Google Scholar] [CrossRef]
- Martono, Y.; Rohman, A.; Martono, S.; Riyanto, S. Degradation study of stevioside using RP-HPLC and ESI-MS/MS. Malays. J. Fundam. Appl. Sci. 2018, 14, 138–141. [Google Scholar] [CrossRef][Green Version]
- Korany, M.A.; Haggag, R.S.; Ragab, M.A.A.; Elmallah, O.A. A validated stability indicating DAD–HPLC method for determination of pentoxifylline in presence of its pharmacopeial related substances. Bull. Fac. Pharm. 2013, 51, 211–219. [Google Scholar] [CrossRef]
- Saini, B.; Bansal, G. Isolation and characterization of a degradation product in leflunomide and a validated selective stability-indicating HPLC–UV method for their quantification. J. Pharm. Anal. 2015, 5, 207–212. [Google Scholar] [CrossRef]
- Abiramasundari, A.; Joshi, R.P.; Jalani, H.B.; Sharma, J.A.; Pandya, D.H.; Pandya, A.N.; Sudarsanam, V.; Vasu, K.K. Stability-indicating assay method for determination of actarit, its process related impurities and degradation products: Insight into stability profile and degradation pathways. J. Pharm. Anal. 2014, 4, 374–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Ghannam, S.M.; Al-Olayan, A.M. Stability-indicating HPLC method for the determination of nicardipine in capsules and spiked human plasma. Identification of degradation products using HPLC/MS. Arab. J. Chem. 2014, in press. [Google Scholar] [CrossRef]
- Alarfaj, N.A. Stability-indicating liquid chromatography for determination of clopidogrel bisulfate in tablets: Application to content uniformity testing. J. Saudi Chem. Soc. 2012, 16, 23–30. [Google Scholar] [CrossRef]
- Xia, M.; Hang, T.J.; Zhang, F.; Li, X.M.; Xu, X.Y. The stability of biapenem and structural identification of impurities in aqueous solution. J. Pharm. Biomed. Anal. 2009, 49, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.P.; Sahu, A.; Singh, S. Identification and characterization of degradation products of irbesartan using LC–MS/TOF, MSn, on-line H/D exchange and LC–NMR. J. Pharm. Biomed. Anal. 2010, 51, 1037–1046. [Google Scholar] [CrossRef]
- US Department of Health and Human Services. Guidance for Industry ANDAs: Impurities in Drug Substances; US Department of Health and Human Services, Food and Drug Administration: Washington, DC, USA, 1999.
- Robinson, T.J.; Borror, C.M.; Myers, R.H. Robust parameter design: A review. Qual. Reliab. Eng. Int. 2004, 20, 81–101. [Google Scholar] [CrossRef]
- Breitkreitz, M.C.; Souza, A.M.d.; Poppi, R.J. Experimento didático de quimiometria para planejamento de experimentos: Avaliação das condições experimentais na determinação espectrofotométrica de ferro II com o-fenantrolina. Química Nova 2014, 37, 564–573. [Google Scholar]
- Dejaegher, B.; Vander Heyden, Y. Experimental designs and their recent advances in set-up, data interpretation, and analytical applications. J. Pharm. Biomed. Anal. 2011, 56, 141–158. [Google Scholar] [CrossRef]
- Mäkelä, M. Experimental design and response surface methodology in energy applications: A tutorial review. Energy Convers. Manag. 2017, 151, 630–640. [Google Scholar] [CrossRef]
- Tye, H. Application of statistical ‘design of experiments’ methods in drug discovery. Drug Discov. Today 2004, 9, 485–491. [Google Scholar] [CrossRef]
- Altekar, M.; Homon, C.A.; Kashem, M.A.; Mason, S.W.; Nelson, R.M.; Patnaude, L.A.; Yingling, J.; Taylor, P.B. Assay Optimization: A Statistical Design of Experiments Approach. Clin. Lab. Med. 2007, 27, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.K.; Ramisetti, N.R.; Cecchi, T.; Swain, S.; Patro, C.S.; Panda, J. An overview of experimental designs in HPLC method development and validation. J. Pharm. Biomed. Anal. 2018, 147, 590–611. [Google Scholar] [CrossRef] [PubMed]
- Lafossas, C.; Benoit-Marquié, F.; Garrigues, J.C. Analysis of the retention of tetracyclines on reversed-phase columns: Chemometrics, design of experiments and quantitative structure-property relationship (QSPR) study for interpretation and optimization. Talanta 2019, 198, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Valente, J.F.A.; Sousa, A.; Queiroz, J.A.; Sousa, F. DoE to improve supercoiled p53-pDNA purification by O-phospho-l-tyrosine chromatography. J. Chromatogr. B 2019, 1105, 184–192. [Google Scholar] [CrossRef]
- Mahrouse, M.A.; Lamie, N.T. Experimental design methodology for optimization and robustness determination in ion pair RP-HPLC method development: Application for the simultaneous determination of metformin hydrochloride, alogliptin benzoate and repaglinide in tablets. Microchem. J. 2019, 147, 691–706. [Google Scholar] [CrossRef]
- Krishna, M.V.; Dash, R.N.; Jalachandra Reddy, B.; Venugopal, P.; Sandeep, P.; Madhavi, G. Quality by Design (QbD) approach to develop HPLC method for eberconazole nitrate: Application oxidative and photolytic degradation kinetics. J. Saudi Chem. Soc. 2016, 20, S313–S322. [Google Scholar] [CrossRef]
- Nadella, N.P.; Ratnakaram, V.N.; Srinivasu, N.; Technologies, R. Quality-by-design-based development and validation of a stability-indicating UPLC method for quantification of teriflunomide in the presence of degradation products and its application to in-vitro dissolution. J. Liquid Chromatogr. Relat. Technol. 2017, 40, 517–527. [Google Scholar] [CrossRef]
- Hadzieva Gigovska, M.; Petkovska, A.; Acevska, J.; Nakov, N.; Antovska, P.; Ugarkovic, S.; Dimitrovska, A. Comprehensive Assessment of Degradation Behavior of Simvastatin by UHPLC/MS Method, Employing Experimental Design Methodology. J. Int. J. Anal. Chem. 2018, 2018, 17. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Reddy, P.S.; Narayanan, K.L.; Bhosale, P.N. Development of RP-HPLC, Stability Indicating Method for Degradation Products of Linagliptin in Presence of Metformin HCl by Applying 2 Level Factorial Design; and Identification of Impurity-VII, VIII and IX and Synthesis of Impurity-VII. Sci. Pharm. 2017, 85, 25. [Google Scholar] [CrossRef]
- Wingert, N.R.; Ellwanger, J.B.; Bueno, L.M.; Gobetti, C.; Garcia, C.V.; Steppe, M.; Schapoval, E.E.S. Application of Quality by Design to optimize a stability-indicating LC method for the determination of ticagrelor and its impurities. Eur. J. Pharm. Sci. 2018, 118, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zhang, X.; Wang, H.; Jin, X. Using an innovative quality-by-design approach for the development of a stability-indicating UPLC/Q-TOF-ESI-MS/MS method for stressed degradation products of imatinib mesylate. RSC Adv. 2016, 6, 13050–13062. [Google Scholar] [CrossRef]
- Sharma, G.; Thakur, K.; Raza, K.; Katare, O.P. Stability kinetics of fusidic acid: Development and validation of stability indicating analytical method by employing Analytical Quality by Design approach in medicinal product(s). J. Chromatogr. B 2019, 1120, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, C. Application of quality by design concept to develop a dual gradient elution stability-indicating method for cloxacillin forced degradation studies using combined mixture-process variable models. J. Chromatogr. A 2017, 1514, 44–53. [Google Scholar] [CrossRef]
- Kalariya, P.D. Experimental Design Approach for Selective Separation of Vilazodone HCl and Its Degradants by LC-PDA and Characterization of Major Degradants by LC/QTOF–MS/MS. Chromatographia 2014, 77, 1299–1313. [Google Scholar] [CrossRef]
- Murthy, M.V.; Krishnaiah, C.; Srinivas, K.; Rao, K.S.; Kumar, N.R.; Mukkanti, K. Development and validation of RP-UPLC method for the determination of darifenacin hydrobromide, its related compounds and its degradation products using design of experiments. J. Pharm. Biomed. Anal. 2013, 72, 40–50. [Google Scholar] [CrossRef]
- Baghel, M.; Rajput, S.J. Stress degradation of edaravone: Separation, isolation and characterization of major degradation products. Biomed. Chromatogr. 2018, 32, e4146. [Google Scholar] [CrossRef]
- Yeram, P.; Hamrapurkar, P.; Mukhedkar, P. Implementation of Quality by Design approach to develop and validate stability indicating assay method for simultaneous estimation of sofosbuvir and ledipasvir in bulk drugs and tablet formulation. Int. J. Pharm. Sci. 2019, 10, 180–188. [Google Scholar]
- Sonawane, S.; Gide, P. Application of experimental design for the optimization of forced degradation and development of a validated stability-indicating LC method for luliconazole in bulk and cream formulation. Arab. J. Chem. 2016, 9, S1428–S1434. [Google Scholar] [CrossRef]
- Kurmi, M.; Kumar, S.; Singh, B.; Singh, S. Implementation of design of experiments for optimization of forced degradation conditions and development of a stability-indicating method for furosemide. J. Pharm. Biomed. Anal. 2014, 96, 135–143. [Google Scholar] [CrossRef]
- Fusion QbD Quality by Design Software Solutions. Available online: http://www.smatrix.com/ (accessed on 10 March 2019).
- Originlab. Available online: https://www.originlab.com/ (accessed on 10 March 2019).
- MATLAB for Artificial Intelligence. Available online: https://au.mathworks.com/ (accessed on 10 March 2019).
- Minitab 19. Available online: https://www.minitab.com/ (accessed on 10 March 2019).
- StatEase Statistics Made Easy. Available online: https://www.statease.com (accessed on 10 March 2019).
- Accelerate Innovation with Data Science. Available online: https://www.tibco.com/products/data-science (accessed on 10 March 2019).
- Dégardin, K.; Guillemain, A.; Guerreiro, N.V.; Roggo, Y. Near infrared spectroscopy for counterfeit detection using a large database of pharmaceutical tablets. J. Pharm. Biomed. Anal. 2016, 128, 89–97. [Google Scholar] [CrossRef] [PubMed]
- The basic building block of chemometrics. Analytical Chemistry. Available online: https://www.intechopen.com/books/analytical-chemistry/pca-the-basic-building-block-of-chemometrics (accessed on 15 August 2019).
- Godoy, J.L.; Vega, J.R.; Marchetti, J.L. Relationships between PCA and PLS-regression. Chemom. Intell. Lab. Syst. 2014, 130, 182–191. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Rutledge, D.N.; Jouan-Rimbaud Bouveresse, D. Independent Components Analysis with the JADE algorithm. Trac Trends Anal. Chem. 2013, 50, 22–32. [Google Scholar] [CrossRef]
- Tôrres, A.R.; Grangeiro, S.; Fragoso, W.D. Multivariate control charts for monitoring captopril stability. Microchem. J. 2015, 118, 259–265. [Google Scholar] [CrossRef]
- Tôrres, A.R.; Grangeiro, S.; Fragoso, W.D. Vibrational spectroscopy and multivariate control charts: A new strategy for monitoring the stability of captopril in the pharmaceutical industry. Microchem. J. 2017, 133, 279–285. [Google Scholar] [CrossRef]
- Bro, R. Multiway calibration. Multilinear PLS J. Chemom. 1996, 10, 47–61. [Google Scholar]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. A J. Chemom. Soc. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Krishnan, A.; Williams, L.J.; McIntosh, A.R.; Abdi, H. Partial Least Squares (PLS) methods for neuroimaging: A tutorial and review. NeuroImage 2011, 56, 455–475. [Google Scholar] [CrossRef]
- Medina, S.; Perestrelo, R.; Silva, P.; Pereira, J.A.M.; Câmara, J.S. Current trends and recent advances on food authenticity technologies and chemometric approaches. Trends Food Sci. Technol. 2019, 85, 163–176. [Google Scholar] [CrossRef]
- Rosipal, R.; Krämer, N. In Overview and Recent Advances in Partial Least Squares, International Statistical and Optimization Perspectives Workshop Subspace, Latent Structure and Feature Selection; Springer: New York, NY, USA, 2005; pp. 34–51. [Google Scholar]
- Biancolillo, A.; Marini, F. Chemometric Methods for Spectroscopy-Based Pharmaceutical Analysis. Front. Chem. 2018, 6, 576. [Google Scholar] [CrossRef] [PubMed]
- Sayed, R.A.; El-Masri, M.M.; Hassan, W.S.; El-Mammli, M.Y.; Shalaby, A. Validated Stability-Indicating Methods for Determination of Mometasone Furoate in Presence of its Alkaline Degradation Product. J. Chromatogr. Sci. 2017, 56, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Attia, K.A.-S.M.; Abdel-Aziz, O.; Magdy, N.; Mohamed, G.F. Development and validation of different chemometric-assisted spectrophotometric methods for determination of cefoxitin-sodium in presence of its alkali-induced degradation product. Future J. Pharm. Sci. 2018, 4, 241–247. [Google Scholar] [CrossRef]
- Attia, K.A.M.; Nassar, M.W.I.; El-Zeiny, M.B.; Serag, A. Stability indicating methods for the analysis of cefprozil in the presence of its alkaline induced degradation product. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 159, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Alamein, A.M.A.A.; Hussien, L.A.E.A.; Mohamed, E.H. Univariate spectrophotometry and multivariate calibration: Stability-indicating analytical tools for the quantification of pimozide in bulk and pharmaceutical dosage form. Bull. Fac. Pharm. 2015, 53, 173–183. [Google Scholar] [CrossRef]
- Hegazy, M.A.E.-M.; Eissa, M.S.; Abd El-Sattar, O.I.; Abd El-Kawy, M. Two and three way spectrophotometric-assisted multivariate determination of linezolid in the presence of its alkaline and oxidative degradation products and application to pharmaceutical formulation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.A.-M.; Eissa, M.S.; Abd El-Sattar, O.I.; Abd El-Kawy, M.M. Determination of a novel ACE inhibitor in the presence of alkaline and oxidative degradation products using smart spectrophotometric and chemometric methods. J. Pharm. Anal. 2014, 4, 132–143. [Google Scholar] [CrossRef][Green Version]
- Souza, J.A.L.; Albuquerque, M.M.; Grangeiro, S.; Pimentel, M.F.; de Santana, D.P.; Simões, S.S. Quantification of captopril disulphide as a degradation product in captopril tablets using near infrared spectroscopy and chemometrics. Vib. Spectrosc. 2012, 62, 35–41. [Google Scholar] [CrossRef]
- Abou Al Alamein, A.M. Validated stability-indicating methods for the determination of zafirlukast in the presence of its alkaline hydrolysis degradation product. Bull. Fac. Pharm. 2012, 50, 111–119. [Google Scholar] [CrossRef][Green Version]
- Naguib, I.A. Stability indicating analysis of bisacodyl by partial least squares regression, spectral residual augmented classical least squares and support vector regression chemometric models: A comparative study. Bull. Fac. Pharm. 2011, 49, 91–100. [Google Scholar] [CrossRef]
- Abdelwahab, N.S.J.A.M. Determination of atenolol, chlorthalidone and their degradation products by TLC-densitometric and chemometric methods with application of model updating. Anal. Methods 2010, 2, 1994–2001. [Google Scholar] [CrossRef]
- Wagieh, N.E.; Hegazy, M.A.; Abdelkawy, M.; Abdelaleem, E.A. Quantitative determination of oxybutynin hydrochloride by spectrophotometry, chemometry and HPTLC in presence of its degradation product and additives in different pharmaceutical dosage forms. Talanta 2010, 80, 2007–2015. [Google Scholar] [CrossRef] [PubMed]
- Moneeb, M.S. Chemometric determination of rabeprazole sodium in presence of its acid induced degradation products using spectrophotometry, polarography and anodic voltammetry at a glassy carbon electrode. Pak. J. Pharm. Sci. 2008, 21, 214–224. [Google Scholar] [PubMed]
- Fayed, A.S.; Shehata, M.; Ibrahim, A.; Hassan, N.; Weshahy, S.A. Validated stability-indicating methods for determination of cilostazol in the presence of its degradation products according to the ICH guidelines. J. Pharm. Biomed. Anal. 2007, 45, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Ragno, G.; Ioele, G.; De Luca, M.; Garofalo, A.; Grande, F.; Risoli, A. A critical study on the application of the zero-crossing derivative spectrophotometry to the photodegradation monitoring of lacidipine. J. Pharm. Biomed. Anal. 2006, 42, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.A.; Ashour, A.; Hassan, N.Y.; Fayed, A.S.; El-Zeany, B.A. Liquid chromatography and chemometric methods for determination of rofecoxib in presence of its photodegradate and alkaline degradation products. Anal. Chim. Acta 2004, 519, 23–30. [Google Scholar] [CrossRef]
- Jaumot, J.; de Juan, A.; Tauler, R. MCR-ALS GUI 2.0: New features and applications. Chemom. Intell. Lab. Syst. 2015, 140, 1–12. [Google Scholar] [CrossRef]
- Ruckebusch, C.; Blanchet, L. Multivariate curve resolution: A review of advanced and tailored applications and challenges. Anal. Chim. Acta 2013, 765, 28–36. [Google Scholar] [CrossRef]
- Firmani, P.; Hugelier, S.; Marini, F.; Ruckebusch, C. MCR-ALS of hyperspectral images with spatio-spectral fuzzy clustering constraint. Chemom. Intell. Lab. Syst. 2018, 179, 85–91. [Google Scholar] [CrossRef]
- Devos, O.; Schröder, H.; Sliwa, M.; Placial, J.P.; Neymeyr, K.; Métivier, R.; Ruckebusch, C. Photochemical multivariate curve resolution models for the investigation of photochromic systems under continuous irradiation. Anal. Chim. Acta 2019, 1053, 32–42. [Google Scholar] [CrossRef]
- Alcaraz, M.R.; Aguirre, A.; Goicoechea, H.C.; Culzoni, M.J.; Collins, S.E. Resolution of intermediate surface species by combining modulated infrared spectroscopy and chemometrics. Anal. Chim. Acta 2019, 1049, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.W.; Oram, K.G.; Rutan, S.C.; Stoll, D.R. Rational Design of Mixtures for Chromatographic Peak Tracking Applications via Multivariate Selectivity. Anal. Chim. Acta 2019, 2, 100010. [Google Scholar] [CrossRef]
- Marín-García, M.; Ioele, G.; Franquet-Griell, H.; Lacorte, S.; Ragno, G.; Tauler, R. Investigation of the photodegradation profile of tamoxifen using spectroscopic and chromatographic analysis and multivariate curve resolution. Chemom. Intell. Lab. Syst. 2018, 174, 128–141. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Q.; Cong, P.; Zhu, Z. Determination of the paracetamol degradation process with online UV spectroscopic and multivariate curve resolution-alternating least squares methods: Comparative validation by HPLC. Anal. Methods 2013, 5, 5286–5293. [Google Scholar] [CrossRef]
- Gómez-Canela, C.; Bolivar-Subirats, G.; Tauler, R.; Lacorte, S. Powerful combination of analytical and chemometric methods for the photodegradation of 5-Fluorouracil. J. Pharm. Biomed. Anal. 2017, 137, 33–41. [Google Scholar] [CrossRef]
- Bērziņš, K.; Kons, A.; Grante, I.; Dzabijeva, D.; Nakurte, I.; Actiņš, A. Multi-technique approach for qualitative and quantitative characterization of furazidin degradation kinetics under alkaline conditions. J. Pharm. Biomed. Anal. 2016, 129, 433–440. [Google Scholar] [CrossRef]
- De Luca, M.; Ioele, G.; Mas, S.; Tauler, R.; Ragno, G. A study of pH-dependent photodegradation of amiloride by a multivariate curve resolution approach to combined kinetic and acid–base titration UV data. Analyst 2012, 137, 5428–5435. [Google Scholar] [CrossRef]
- Mas, S.; Tauler, R.; de Juan, A. Chromatographic and spectroscopic data fusion analysis for interpretation of photodegradation processes. J. Chromatogr. A 2011, 1218, 9260–9268. [Google Scholar] [CrossRef]
- De Luca, M.; Mas, S.; Ioele, G.; Oliverio, F.; Ragno, G.; Tauler, R. Kinetic studies of nitrofurazone photodegradation by multivariate curve resolution applied to UV-spectral data. Int. J. Pharm. 2010, 386, 99–107. [Google Scholar] [CrossRef]
- Javidnia, K.; Hemmateenejad, B.; Miri, R.; Saeidi-Boroujeni, M. Application of a self-modeling curve resolution method for studying the photodegradation kinetics of nitrendipine and felodipine. J. Pharm. Biomed. Anal. 2008, 46, 597–602. [Google Scholar] [CrossRef]
- Shamsipur, M.; Hemmateenejad, B.; Akhond, M.; Javidnia, K.; Miri, R. A study of the photo-degradation kinetics of nifedipine by multivariate curve resolution analysis. J. Pharm. Biomed. Anal. 2003, 31, 1013–1019. [Google Scholar] [CrossRef]
- Arabzadeh, V.; Sohrabi, M.R.; Goudarzi, N.; Davallo, M. Using artificial neural network and multivariate calibration methods for simultaneous spectrophotometric analysis of Emtricitabine and Tenofovir alafenamide fumarate in pharmaceutical formulation of HIV drug. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 215, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Marini, F.; Bucci, R.; Magrì, A.L.; Magrì, A.D. Artificial neural networks in chemometrics: History, examples and perspectives. Microchem. J. 2008, 88, 178–185. [Google Scholar] [CrossRef]
- Golubović, J.B.; Protić, A.D.; Zečević, M.L.; Otašević, B.M. Quantitative structure retention relationship modeling in liquid chromatography method for separation of candesartan cilexetil and its degradation products. Chemom. Intell. Lab. Syst. 2015, 140, 92–101. [Google Scholar] [CrossRef]
| API: Year | Acid | Base | Neutral | Thermolysis | Oxidation | Photolysis |
|---|---|---|---|---|---|---|
| Zidovudine: 2017 [65] | 2 M HCl | 2 M NaOH | - | Acid/base at 80 °C for 72 h | 10% H2O2 at room temperature for 10 h | 1.2 × 106 lx × h of fluorescent light and 200 W h/m2 UV light |
| Toloxatone: 2018 [66] | 1 M HCl | 0.01 M NaOH | H2O | All hydrolysis at 80 °C for 2 h | 0.01% H2O2 at room temperature for 2 h | 2700 kJ/m2/h of UV-VIS and UVC 7.5 W/m2 |
| Amlodipine: 2015 [67] | 1 M HCl at 80 °C for 30 min | 1 M NaOH at 80 °C for 1 h | H2O at 80 °C for 2 h | 50 °C for 48 h | 15% H2O2 at room temperature for 48 h | 1.2 × 106 lx × h of fluorescent light and 200 Wh/m2 UV-A light for 14 days |
| Acebutolol: 2018 [68] | 1 M HCl | 2 M HCl | H2O | All hydrolysis at 80 °C | 3% H2O2 at 80 °C | Not less than 1.2 × 106 lx × h and ultraviolet energy of not less than 200 W h/m2 |
| Stevioside: 2018 [69] | 0.1 M HCl/0.1 M H3PO4 | 0.1 M NaOH | H2O | All hydrolysis at 80 °C for 8 h | 10% H2O2 at 25 °C for 72 h | UV254nm lamp for 48 h |
| Pentoxifylline: 2013 [70] | 2 M HCl at 70 °C for 4 h | 2 M NaOH at 70 °C for 4 h | H2O at 70 °C for 4 h | Dry heat under at 105 °C for 4 h | 30% H2O2 at 70 °C for 4 h | Sunlight for 8 h |
| Leflunomide: 2015 [71] | 0.1–5 M at 85 °C for 8 h | 0.1 M NaOH at 85 °C for 8 h | H2O at 85 °C for 8 h | 50 °C for 30 days | 30% H2O2 at room temperature for 24 h | UV and white light for 14 days |
| Actarit: 2014 [72] | 0.1 M HCl at 70 °C for 24 h | 0.1 M NaOH at 70 °C for 24 h | H2O at 70 °C for 14 days | Dry heat at 70 °C for 14 days | 3% H2O2 for 14 days | UV light |
| Nicardipine: 2014 [73] | 1 M HCl at 60 °C for 1 h | 0.1–0.5 M NaOH at 50–80 °C for 1 h | - | - | 5% H2O2 at 30–50 °C for 1 h | UV254–365nm light at room temperature |
| Clopidogrel bisulfate: 2010 [74] | 1 M HCl | 1 M NaOH | - | All hydrolysis at 80 °C for 1 h | 5% H2O2 | - |
| Biapenem: 2009 [75] | pH from 2.5 to 7.5 at 80 °C for 40 min | From room temperature to 100 °C in pH 3.5 | - | - | ||
| Irbesartan: 2010 [76] | 1 M HCl at 80 °C for 24 h | 2 M NaOH at 80 °C for 48 h | H2O at 80 °C for 48 h | 50 °C | 30% H2O2 at room temperature for 2 days | 8500 lx fluorescent and 0.05 W/m2 UV light |
| Maximum Daily Dose | Threshold | |
|---|---|---|
| Reporting Threshold | ≤1 g | 0.1% |
| >1 g | 0.05% | |
| Identification Threshold | <1 mg | 1.0% or 5 µg TDI, whichever is lower |
| 1 mg–10 mg | 0.5% or 20 µg TDI, whichever is lower | |
| >10 mg–2 g | 0.2% or 2 mg TDI, whichever is lower | |
| >2 g | 0.10% | |
| Qualification Threshold | <10 mg | 1.0% or 50 µg TDI, whichever is lower |
| 10 mg–100 mg | 0.5% or 200 µg TDI, whichever is lower | |
| >100 mg–2 g | 0.2% or 3 mg TDI, whichever is lower | |
| >2 g | 0.15% |
| Variable | Level (−1) | Level (0) | Level (+1) |
|---|---|---|---|
| TBHAH (mM) | 5 | 7.5 | 10 |
| pH | 2.6 | 2.9 | 3.2 |
| Organic phase (v/v) | 20 | 25 | 30 |
| Experiment | x1 | x2 | x3 | Experiment | x1 | x2 | x3 | Experiment | x1 | x2 | x3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 10 | −1 | −1 | 0 | 19 | −1 | −1 | 1 |
| 2 | 0 | −1 | −1 | 11 | 0 | −1 | 0 | 20 | 0 | −1 | 1 |
| 3 | 1 | −1 | −1 | 12 | 1 | −1 | 0 | 21 | 1 | −1 | 1 |
| 4 | −1 | 0 | −1 | 13 | −1 | 0 | 0 | 22 | −1 | 0 | 1 |
| 5 | 0 | 0 | −1 | 14 | 0 | 0 | 0 | 23 | 0 | 0 | 1 |
| 6 | 1 | 0 | −1 | 15 | 1 | 0 | 0 | 24 | 1 | 0 | 1 |
| 7 | −1 | 1 | −1 | −1 | 1 | 0 | 25 | −1 | 1 | 1 | |
| 8 | 0 | 1 | −1 | 17 | 0 | 1 | 0 | 26 | 0 | 1 | 1 |
| 9 | 1 | 1 | −1 | 18 | 1 | 1 | 0 | 27 | 1 | 1 | 1 |
| API | Design | Ref |
|---|---|---|
| Teriflunomide | Full factorial 33 | [90] |
| Simvastatin | Plackett Burman/Box-Behnken | [91] |
| Linagliptin | Full factorial | [92] |
| Ticagrelor | Fractional Factorial Resolution V/Central composite | [93] |
| Imatinib mesylate | Box Behnken | [94] |
| Fusidic acid | Taguchi/Central Composite | [95] |
| Cloxacillin | Plackett Burman | [96] |
| Vilazodone hydrochloride | Central composite experimental | [97] |
| Darifenacin hydrobromide | Central composite | [98] |
| Edaravone | Placket Burman/Box Behnken | [99] |
| Sofosbuvir and Ledipasvir | Box Behnken | [100] |
| Variable | High Level (+1) | Low Level (−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acid | Basic | Oxid. | Dry Heat | Wet Heat | Acid | Basic | Oxid. | Dry Heat | Wet Heat | |
| Conc. (x1)/mol×L−1 | 1 | 0.1 | 30% | - | - | 0.1 | 0.01 | 3% | - | - |
| Time (x2)/min | 75 | 30 | 24 h | 360 | 120 | 15 | 10 | 2h | 30 | 30 |
| Temperature (x3)/°C | 100 | 100 | - | 200 | 100 | 60 | 60 | - | 50 | 60 |
| 23 Full Factorial Design | 22 Full Factorial Design | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Exp. | X1 | X2 | X3 | Acid Condition | Basic Condition | Exp. | X1 | X2 | Oxidative Condition |
| 1 | −1 | −1 | −1 | 0% | 0% | 1 | −1 | −1 | 0% |
| 2 | +1 | −1 | −1 | 4% | 3% | 2 | −1 | +1 | 48% |
| 3 | −1 | +1 | −1 | 10% | 8% | 3 | +1 | −1 | 51% |
| 4 | +1 | +1 | −1 | 23% | 11% | 4 | +1 | +1 | 100% |
| 5 | −1 | −1 | +1 | 8% | 19% | ||||
| 6 | +1 | −1 | +1 | 32% | 26% | ||||
| 7 | −1 | +1 | +1 | 21% | 38% | ||||
| 8 | +1 | +1 | +1 | 41% | 43% | ||||
| Author | API | Forced Degradation Condition | Chemometric Tool | Year | Ref. |
|---|---|---|---|---|---|
| Attia et al. | Cefprozil | Basic hydrolysis | PLS; SRACLS | 2016 | [124] |
| Alamein et al. | Pimozide | Acid and basic hydrolysis | CLS; PCR; PLS | 2015 | [125] |
| Hegazy et al. | Linezolid | Acid and basic hydrolysis; oxidative | PLS; PCR; Parafac; N-PLS | 2014 | [126] |
| Hegazy et al. | Imidapril hydrochloride | Basic hydrolysis; oxidative | PCR; PLS | 2014 | [127] |
| Souza et al. | Captopril | Thermolysis | PLS | 2012 | [128] |
| Abou Al Alamein | Zafirlukast | Basic hydrolysis | PLS | 2012 | [129] |
| Naguib | Bisacodyl | Acid hydrolysis | PLSR; SRACLS | 2011 | [130] |
| Abdelwahab | Atenolol; Chlorthalidone | Acid and basic hydrolysis | PCR; PLS | 2010 | [131] |
| Wagieh et al. | Oxybutynin hydrochloride | Basic hydrolysis | PCR; PLS | 2010 | [132] |
| Moneeb | Rabeprazole sodium | Acid hydrolysis | CLS; PCR; PLS | 2008 | [133] |
| S Fayed et al. | Cilostazol | Acid hydrolysis | PLS; CRACLS | 2007 | [134] |
| Ragno et al. | Lacidipine | Photodegradation | PLS; PCR; MLRA | 2006 | [135] |
| Shehata et al. | Rofecoxib | Basic hydrolysis; photodegradation | PLS; CRACLS | 2004 | [136] |
| Author | API | Forced Degradation Condition | Chemometric Tool | Year | Ref. |
|---|---|---|---|---|---|
| Gómez-Canela | 5-Fluorouracil | Photodegradation | MCR-ALS | 2017 | [145] |
| Bērziņš et al. | Furazidin | Basic hydrolysis | HS-MCR-ALS | 2016 | [146] |
| Luca et al. | Amiloride | Photodegradation | MCR-ALS | 2012 | [147] |
| Sílvia Mas et al. | ketoprofen | Photodegradation | MCR-ALS; HSMCR | 2011 | [148] |
| Luca et al. | Nitrofurazone | Photodegradation | HS-MCR-ALS | 2010 | [149] |
| Javidnia et al. | Nitrendipine and felodipine | Photodegradation | MCR | 2008 | [150] |
| Shamsipur et al. | Nifedipine | Photodegradation | MCR | 2003 | [151] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roberto de Alvarenga Junior, B.; Lajarim Carneiro, R. Chemometrics Approaches in Forced Degradation Studies of Pharmaceutical Drugs. Molecules 2019, 24, 3804. https://doi.org/10.3390/molecules24203804
Roberto de Alvarenga Junior B, Lajarim Carneiro R. Chemometrics Approaches in Forced Degradation Studies of Pharmaceutical Drugs. Molecules. 2019; 24(20):3804. https://doi.org/10.3390/molecules24203804
Chicago/Turabian StyleRoberto de Alvarenga Junior, Benedito, and Renato Lajarim Carneiro. 2019. "Chemometrics Approaches in Forced Degradation Studies of Pharmaceutical Drugs" Molecules 24, no. 20: 3804. https://doi.org/10.3390/molecules24203804
APA StyleRoberto de Alvarenga Junior, B., & Lajarim Carneiro, R. (2019). Chemometrics Approaches in Forced Degradation Studies of Pharmaceutical Drugs. Molecules, 24(20), 3804. https://doi.org/10.3390/molecules24203804





