Study on Antibacterial and Quorum-Sensing Inhibition Activities of Cinnamomum camphora Leaf Essential Oil
Abstract
:1. Introduction
2. Results
2.1. Analysis of the Components in C. camphora EO
2.2. Determination of Antimicrobial Activity and MIC of C. camphora EO
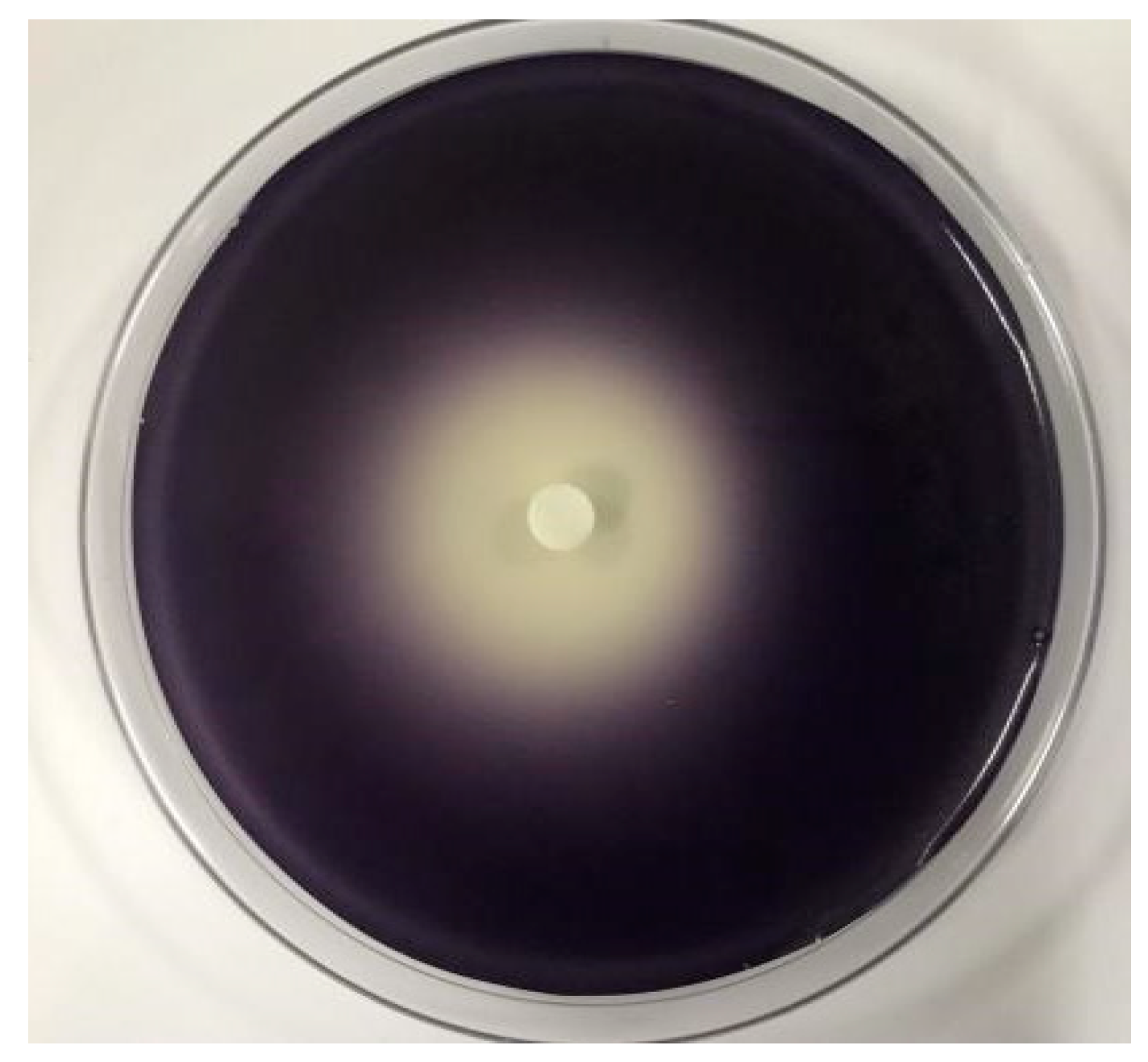
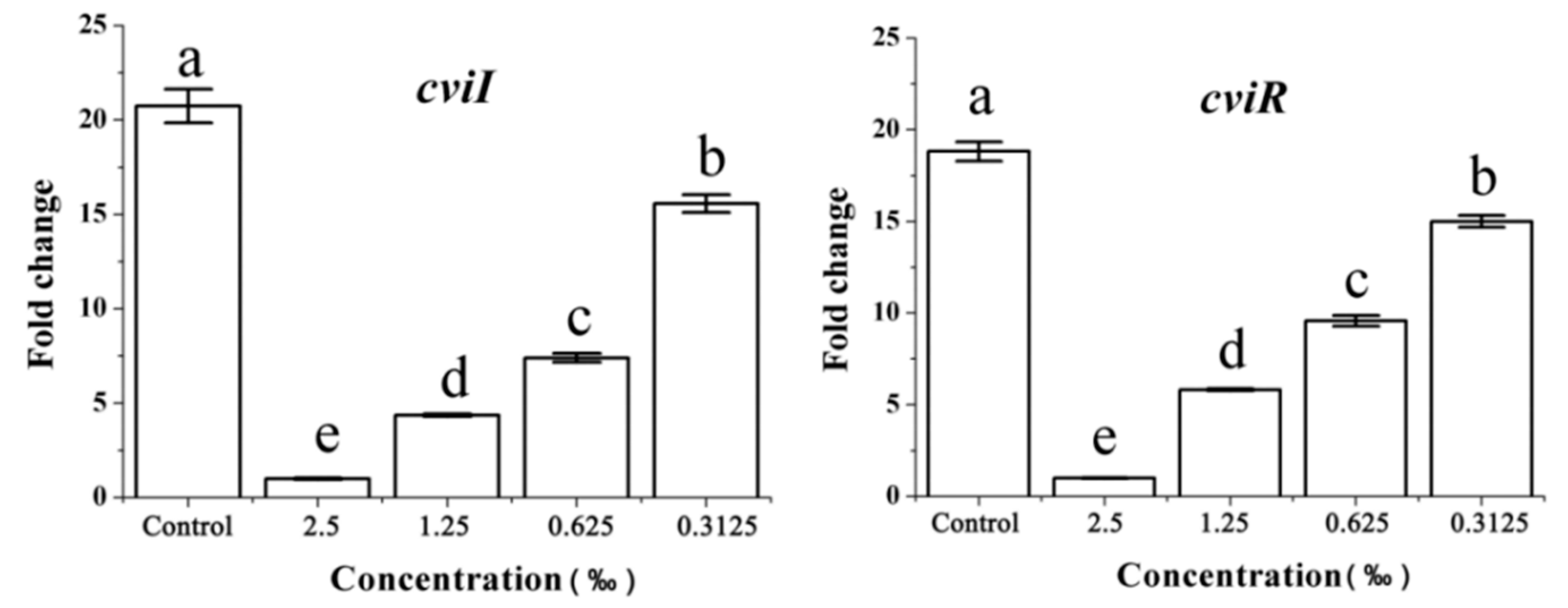
2.3. The Effect of C. camphora EO on Violacein of C. violaceum CV026
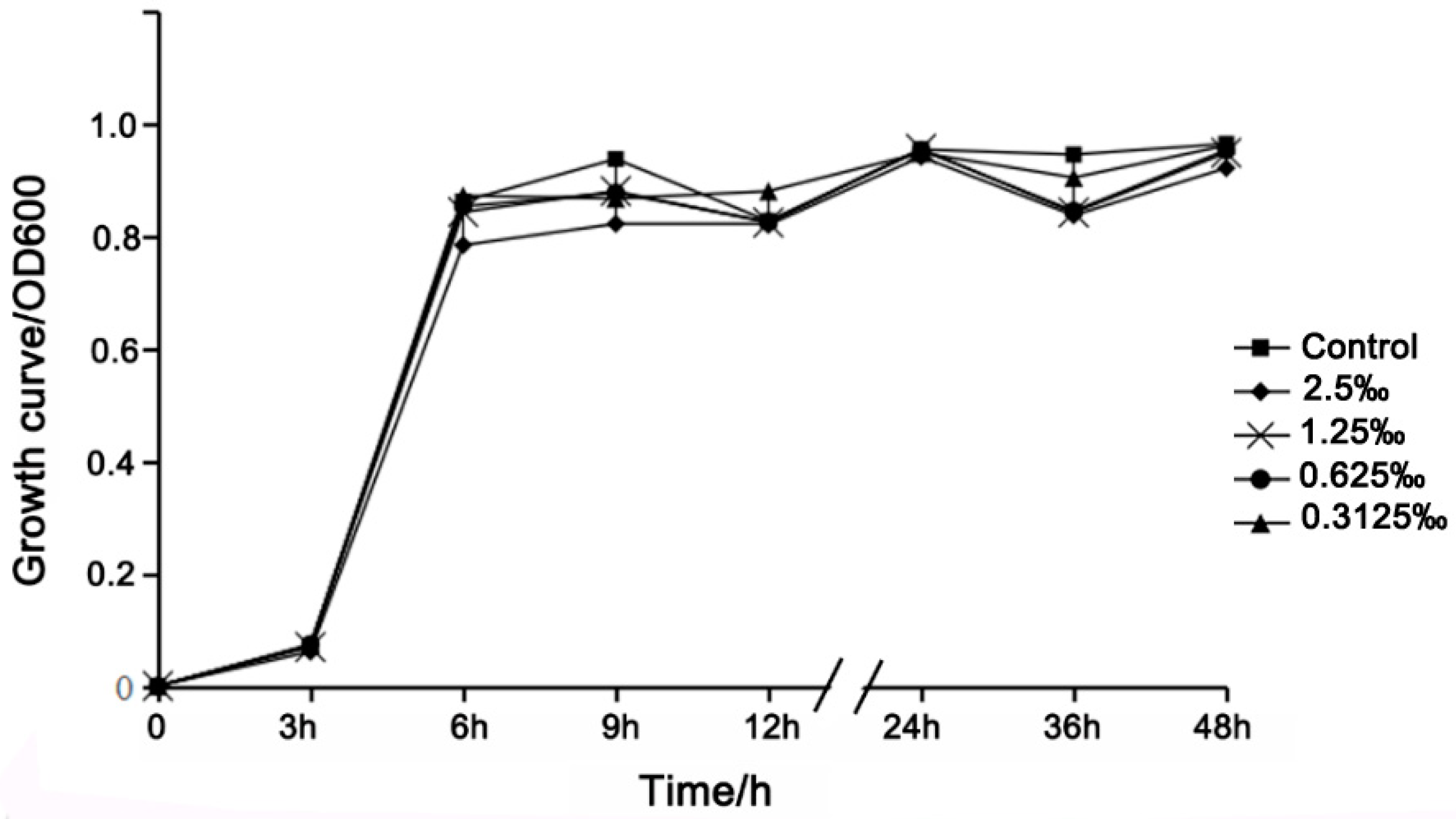
2.4. Growth Curve Analysis
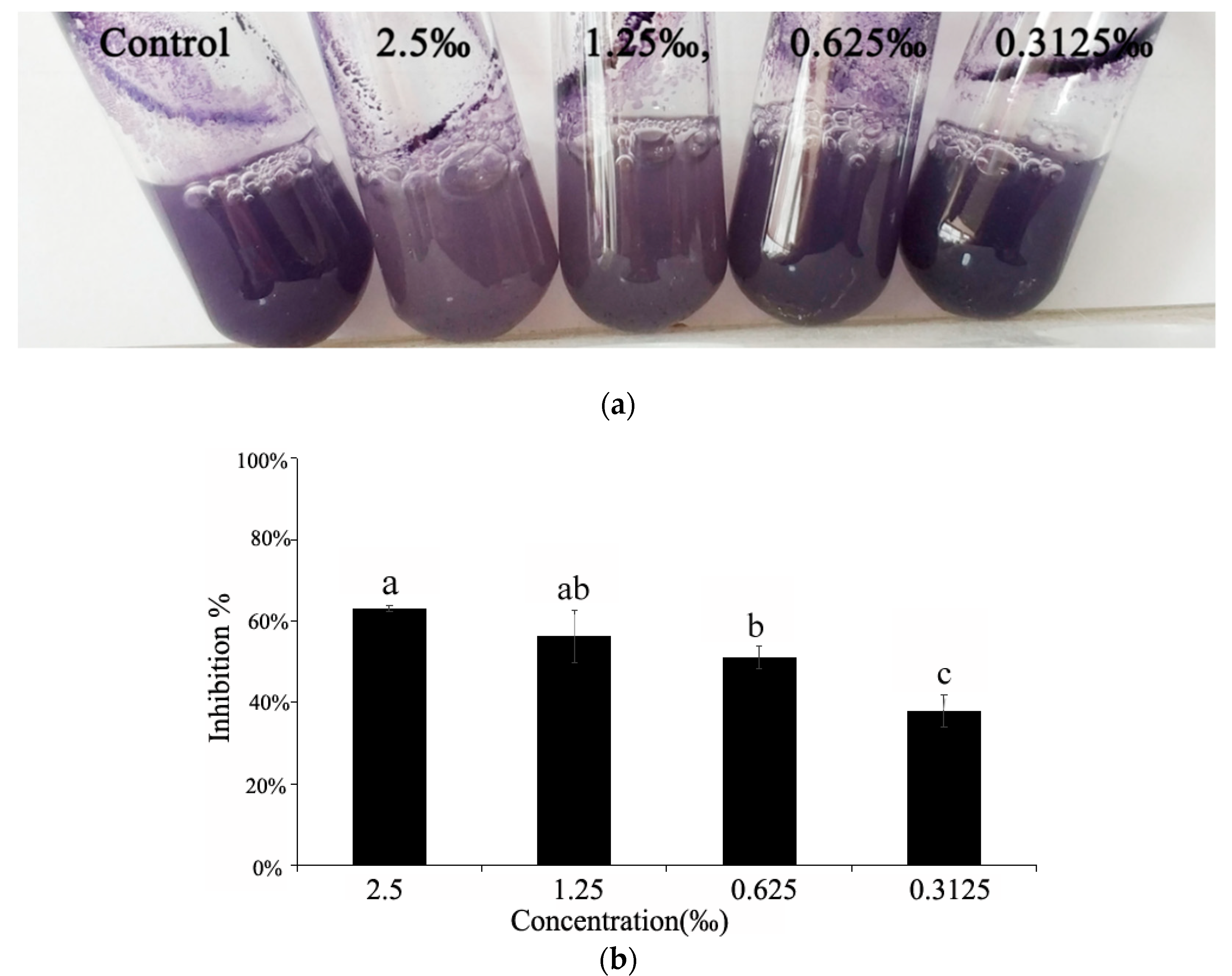
2.5. Violacein Detection in C. violaceum
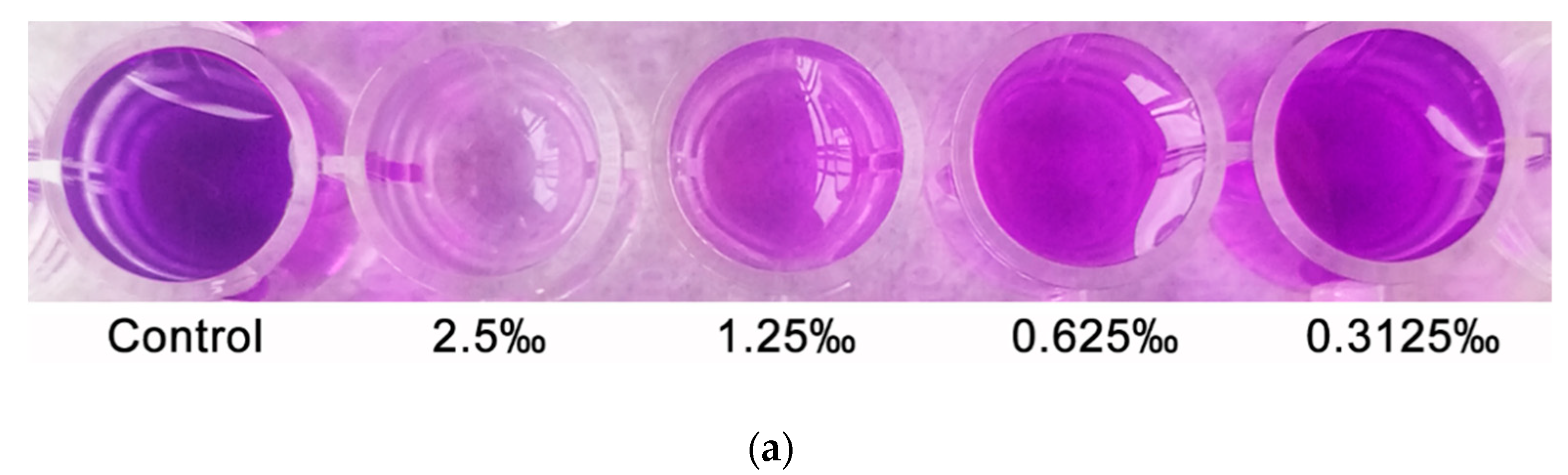
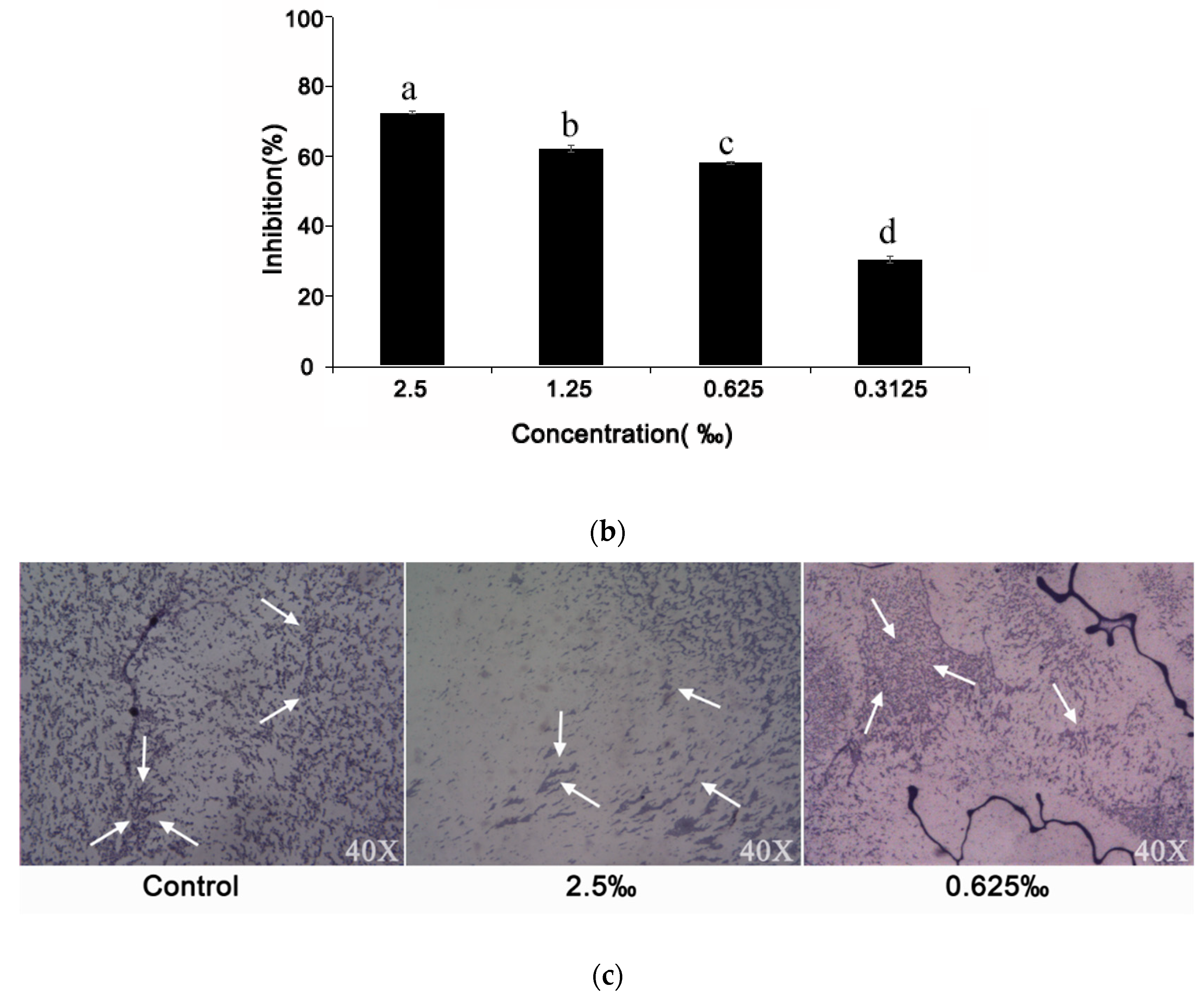
2.6. Effect of C. camphora EO on Biofilm Development
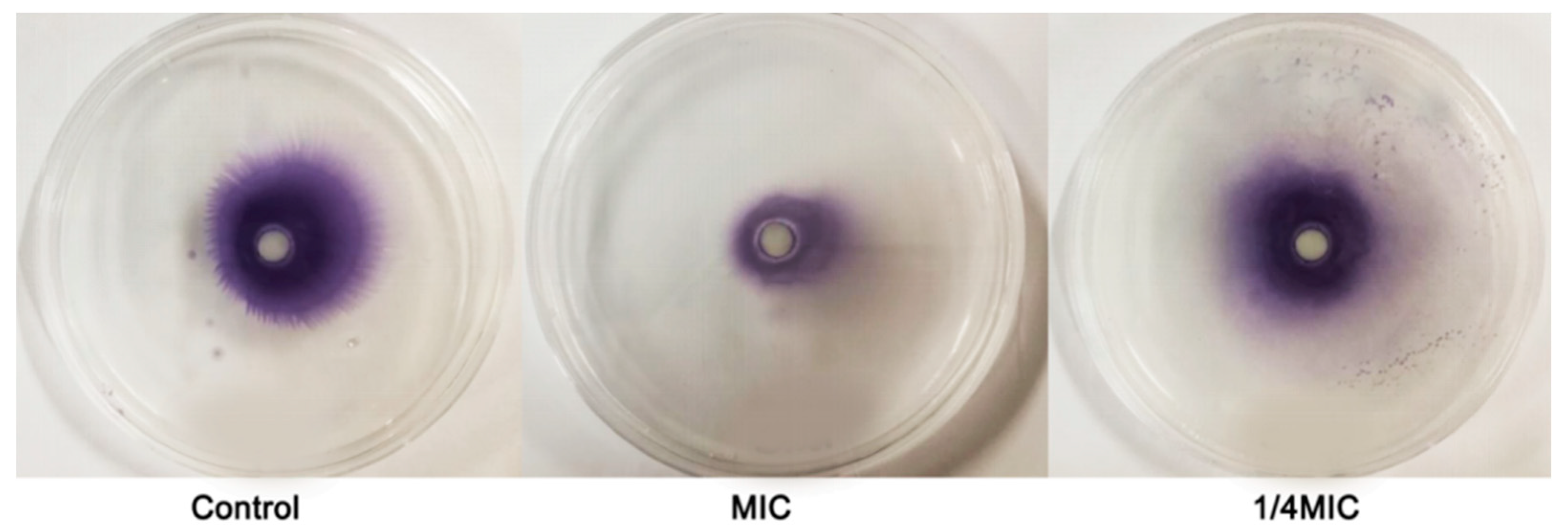
2.7. Swarming Motility
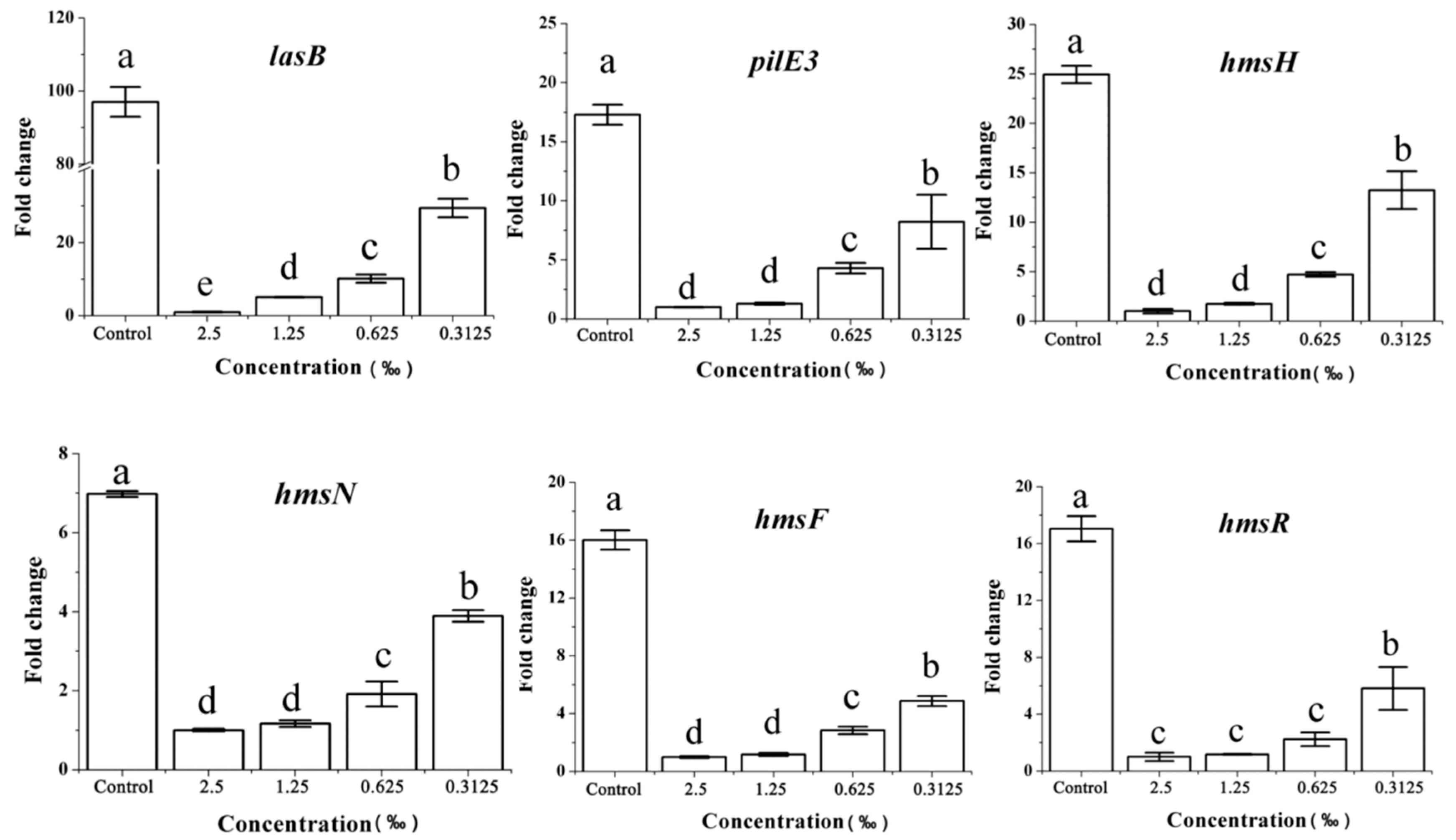
2.8. Expression of the QS-Related Gene in Response to C. camphora EO
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Equipment
Extraction of C. camphora EO by Steaming
4.3. Determination of Components of C. camphora EO by GC-MS
4.4. The Determination of Antimicrobial Activity and MIC of C. camphora EO
4.5. Quorum-Sensing Inhibition (QSI) Assays of C. camphora EO
4.6. Growth Curve Analysis
4.7. Violacein Detection in C. violaceum
4.8. Effect of C. camphora EO on Biofilm Development
4.9. Swarming Motility
4.10. Gene Expression Analysis
4.11. Statistics Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marinelli, L.; Stefano, A.D.; Cacciatore, I. Carvacrol and its derivatives as antibacterial agents. Phytochem. Rev. 2018, 17, 903–921. [Google Scholar] [CrossRef]
- Fuqua, W.C.; Winans, S.C.; Greenberg, E.P. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994, 176, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.T.; Lou, Z.; Yu, F.; Wang, P.; Wang, H. Anti-quorum sensing and anti-biofilm activity of Amomum tsaoko on foodborne pathogens. Saudi J. Biol. Sci. 2017, 24, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Adonizio, A.; Kong, K.F.; Mathee, K. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrob. Agents Chemother. 2008, 52, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Ta, C.A.; Freundorfer, M.; Mah, T.F.; Otárola-Rojas, M.; Garcia, M.; Sanchez-Vindas, P.; Poveda, L.; Maschek, J.A.; Baker, B.J.; Adonizio, A.L.; et al. Inhibition of bacterial quorum sensing and biofilm formation by extracts of neotropical rainforest plants. Planta Med. 2014, 80, 343–350. [Google Scholar] [CrossRef]
- Dastidar, S.G.; Kristiansen, J.E.; Molnar, J.; Amaral, L. Role of Phenothiazines and Structurally Similar Compounds of Plant Origin in the Fight against Infections by Drug Resistant Bacteria. Antibiotics 2013, 2, 58–72. [Google Scholar] [CrossRef] [Green Version]
- Noumi, E.; Merghni, A.; M Alreshidi, M.; Haddad, O.; Akmadar, G.; De Martino, L.; Mastouri, M.; Ceylan, O.; Snoussi, M.; Al-Sieni, A. Chromobacterium violaceum and Pseudomonas aeruginosa PAO1: Models for Evaluating Anti-Quorum Sensing Activity of Melaleuca alternifolia Essential Oil and Its Main Component Terpinen-4-ol. Molecules 2018, 23, 2672. [Google Scholar] [CrossRef]
- Durán, M.; Faljoni-Alario, A.; Durán, N. Chromobacterium violaceum and its important metabolites--review. Folia Microbiol. 2010, 55, 535–547. [Google Scholar] [CrossRef]
- Trinetta, V.; Morgan, M.T.; Coupland, J.N.; Yucel, U. Essential Oils Against Pathogen and Spoilage Microorganisms of Fruit Juices: Use of Versatile Antimicrobial Delivery Systems. J. Food Sci. 2017, 82, 471–476. [Google Scholar] [CrossRef]
- Husain, F.M.; Ahmad, I.; Khan, M.S.; Ahmad, E.; Tahseen, Q.; Khan, M.S.; Alshabib, N.A. Sub-MICs of Mentha piperita essential oil and menthol inhibits AHL mediated quorum sensing and biofilm of Gram-negative bacteria. Front. Microbiol. 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Vattem, D.A.; Mihalik, K.; Crixell, S.H.; Mclean, R.J.C. Dietary phytochemicals as quorum sensing inhibitors. Fitoterapia 2007, 78, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Yap, P.S.; Krishnan, T.; Chan, K.G.; Lim, S.H. Antibacterial Mode of Action of Cinnamomum verum Bark Essential Oil, Alone and in Combination with Piperacillin, Against a Multi-Drug-Resistant Escherichia coli Strain. J. Microbiol. Biotechnol. 2015, 25, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Packiavathy, I.A.S.V.; Priya, S.; Pandian, S.K.; Ravi, A.V. Inhibition of biofilm development of uropathogens by curcumin -An anti-quorum sensing agent from Curcuma longa. Food Chem. 2014, 148, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Krishnan, T.; Wang, H.; Chen, Y.; Yin, W.F.; Chong, Y.M.; Tan, L.Y.; Chong, T.M.; Chan, K.G. Non-antibiotic quorum sensing inhibitors acting against N-acyl homoserine lactone synthase as druggable target. Sci. Rep. 2014, 4, 7245. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Yan, W. Conservation and applications of camphor tree (Cinnamomum camphora) in China: Ethnobotany and genetic resources. Genet. Resour. Crop Evol. 2016, 63, 1049–1061. [Google Scholar]
- Duarte, A.; Luís, Â.; Oleastro, M.; Domingues, F.C. Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter spp. Food Control 2016, 61, 115–122. [Google Scholar] [CrossRef]
- Kim, M.K.; Zhao, A.; Wang, A.; Brown, Z.Z.; Muir, T.W.; Stone, H.A.; Bassler, B.L. Surface-attached molecules control Staphylococcus aureus quorum sensing and biofilm development. Nat. Microbiol. 2017, 2, 17080. [Google Scholar] [CrossRef]
- Verstraeten, N.; Braeken, K.; Debkumari, B.; Fauvart, M.; Fransaer, J.; Vermant, J.; Michiels, J. Living on a surface: Swarming and biofilm formation. Trends Microbiol. 2008, 16, 496–506. [Google Scholar] [CrossRef]
- Ghosh, R.; Tiwary, B.K.; Kumar, A.; Chakraborty, R. Guava Leaf Extract Inhibits Quorum-Sensing and Chromobacterium violaceum Induced Lysis of Human Hepatoma Cells: Whole Transcriptome Analysis Reveals Differential Gene Expression. PLoS ONE 2014, 9, e107703. [Google Scholar] [CrossRef]
- Zins, M.M.; Zimprich, C.A.; Petermann, S.R.; Rust, L. Expression and partial characterization of an elastase from Chromobacterium violaceum. Vet. Microbiol. 2001, 80, 63–74. [Google Scholar] [CrossRef]
- de Oca-Mejía, M.M.; Castillo-Juarez, I.; Martinez-Vazquez, M.; Soto-Hernandez, M.; Garcia-Contreras, R. Influence of quorum sensing in multiple phenotypes of the bacterial pathogen Chromobacterium violaceum. Pathog. Dis. 2015, 73, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Monte, J.; Abreu, A.; Borges, A.; Simões, L.; Simões, M. Antimicrobial activity of selected phytochemicals against Escherichia coli and Staphylococcus aureus and their biofilms. Pathogens 2014, 3, 473–498. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.-F.; Hu, F.; Thakur, K.; Li, X.-L.; Zhang, Y.-S.; Wei, Z.-J. Comparison of antibacterial effects and fumigant toxicity of essential oils extracted from different plants. Ind. Crop. Prod. 2018, 124, 192–200. [Google Scholar] [CrossRef]
- Klein, G.; Rüben, C.; Upmann, M. Antimicrobial activity of essential oil components against potential food spoilage microorganisms. Curr. Microbiol. 2013, 67, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-W.; Luo, H.-Z.; Jiang, H.; Jian, T.-K.; Chen, Z.-Q.; Jia, A.-Q. Hordenine: A novel quorum sensing inhibitor and antibiofilm agent against Pseudomonas aeruginosa. J. Agric. Food Chem. 2018, 66, 1620–1628. [Google Scholar] [CrossRef]
- da Silva Luz, I.; Gomes-Neto, N.J.; Magnani, M.; de Souza, E.L. Assessment of tolerance induction by Origanum vulgare L. essential oil or carvacrol in Pseudomonas aeruginosa cultivated in a meat-based broth and in a meat model. Food Sci. Technol. Int. 2015, 21, 571–580. [Google Scholar] [CrossRef]
- Gomes-Neto, N.J.; Luz, I.S.; Franco, O.L.; Magnani, M.; Souza, E.L. Tolerance evaluation in S almonella enterica serovar T yphimurium challenged with sublethal amounts of R osmarinus officinalis L. essential oil or 1, 8-cineole in meat model. Int. J. Food Sci. Technol. 2014, 49, 1912–1917. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, F.; Wang, J.; Zhong, N. Biofilm formation and control strategies of foodborne pathogens: Food safety perspectives. RSC Adv. 2017, 7, 36670–36683. [Google Scholar] [CrossRef]
- Brackman, G.; Nelis, H.J.; Coenye, T.; Tegos, A.; Mylonakis, E. Inhibition of Quorum Sensing as a Novel Antimicrobial Strategy. In Antimicrobial Drug Discovery: Emerging Strategies; Tegos, G., Mylonakis, E., Eds.; CABI: Wallingford, UK, 2012; pp. 115–134. [Google Scholar]
- Joshi, J.R.; Khazanov, N.; Senderowitz, H.; Burdman, S.; Lipsky, A.; Yedidia, I. Plant phenolic volatiles inhibit quorum sensing in pectobacteria and reduce their virulence by potential binding to ExpI and ExpR proteins. Sci. Rep. 2016, 6, 38126. [Google Scholar] [CrossRef]
- Manoharan, R.K.; Lee, J.H.; Kim, Y.G.; Kim, S.I.; Lee, J. Inhibitory effects of the essential oils α-longipinene and linalool on biofilm formation and hyphal growth of Candida albicans. Biofouling 2017, 33, 143–155. [Google Scholar] [CrossRef]
- Alves, S.; Duarte, A.; Sousa, S.; Domingues, F.C. Study of the major essential oil compounds of Coriandrum sativum against Acinetobacter baumannii and the effect of linalool on adhesion, biofilms and quorum sensing. Biofouling 2016, 32, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Ye, Z.M.; Huang, T.Y.; Chen, X.D.; Li, Y.Y.; Wu, S.H. Identification of volatiles in leaves of Alpinia zerumbet ‘Variegata’ using headspace solid-phase microextraction-gas chromatography-mass spectrometry. Nat. Prod. Commun. 2014, 9, 999. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, Z.; Wang, W.; Yang, C.; Liu, J.; Zhou, L.; Shen, Y.; Wang, Z.; Chen, J.; Wu, S. Composition Analysis of Essential Oil from Melaleuca bracteata Leaves Using Ultrasound-assisted Extraction and its Antioxidative and Antimicrobial Activities. BioResources 2018, 13, 8488–8504. [Google Scholar] [CrossRef]
- Lucero, M.; Estell, R.; Tellez, M.; Fredrickson, E. A retention index calculator simplifies identification of plant volatile organic compounds. Phytochem. Anal. 2010, 20, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Vandendool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Andotra, S.; Kalgotra, N.; Pandey, S.K. Syntheses, Characterization, Thermal, and Antimicrobial Studies of Lanthanum(III) Tolyl/Benzyldithiocarbonates. Bioinorg. Chem. Appl. 2014, 2014, 780631. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Huang, W.; Duan, Q.; Li, F.; Cheng, H. Sodium houttuyfonate affects production of N-acyl homoserine lactone and quorum sensing-regulated genes expression in Pseudomonas aeruginosa. Front. Microbiol. 2014, 5, 635. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, J.; Huang, F.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Hexanal as a QS inhibitor of extracellular enzyme activity of Erwinia carotovora and Pseudomonas fluorescens and its application in vegetables. Food Chem. 2018, 255, 1. [Google Scholar] [CrossRef]
- Chernin, L.S.; Winson, M.K.; Thompson, J.M.; Haran, S.; Bycroft, B.W.; Chet, I.; Williams, P.; Stewart, G.S. Chitinolytic activity in Chromobacterium violaceum: Substrate analysis and regulation by quorum sensing. J. Bacteriol. 1998, 180, 4435–4441. [Google Scholar]
- Khan, M.S.A.; Zahin, M.; Hasan, S.; Husain, F.M.; Ahmad, I. Inhibition of quorum sensing regulated bacterial functions by plant essential oils with special reference to clove oil. Lett. Appl. Microbiol. 2010, 49, 354–360. [Google Scholar] [CrossRef]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. Jove 2011, 47, e2437. [Google Scholar] [CrossRef] [PubMed]
- Packiavathy, I.A.S.V.; Agilandeswari, P.; Musthafa, K.S.; Pandian, S.K.; Ravi, A.V. Antibiofilm and quorum sensing inhibitory potential of Cuminum cyminum and its secondary metabolite methyl eugenol against Gram negative bacterial pathogens. Food Res. Int. 2012, 45, 85–92. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds essential oil of Cinnamomum camphora Leaf are available from the authors. |

| NO. | Compound Name | Molecular Formula | Molecular Weight | Relative Content | Retention Time/min | Retention Index |
|---|---|---|---|---|---|---|
| 1 | Linalool | C10H18O | 154.25 | 51.57% | 11.25 | 1021 |
| 2 | Cineole | C10H18O | 154.25 | 22.07% | 9.47 | 981 |
| 3 | Sabenene | C10H16 | 136.23 | 5.38% | 12.42 | 1046 |
| 4 | α-Terpineol | C10H18O | 154.25 | 3.81% | 7.78 | 939 |
| 5 | Caryophyllene | C15H24 | 204.35 | 2.77% | 13.54 | 1069 |
| 6 | Nerolidol | C15H26O | 222.37 | 1.97% | 6.61 | 911 |
| 7 | 1R-α-Pinene | C10H16 | 136.23 | 1.68% | 8.74 | 963 |
| 8 | camphor | C10H16O | 152.23 | 1.42% | 7.92 | 943 |
| 9 | β-Pinene | C10H16 | 136.23 | 1.12% | 9.43 | 980 |
| 10 | Terpineol | C10H18O | 154.25 | 0.89% | 13.20 | 1062 |
| 11 | Methyleugenol | C11H14O2 | 178.23 | 0.83% | 18.17 | 1167 |
| 12 | a-Humulene | C15H24 | 204.35 | 0.76% | 9.39 | 986 |
| 13 | Myrcene | C10H16 | 136.23 | 0.56% | 8.26 | 951 |
| 14 | Caryophyllene oxide | C15H24O | 220.35 | 0.45% | 10.18 | 998 |
| 15 | g-Terpinene | C10H16 | 136.23 | 0.40% | 15.81 | 1117 |
| 16 | α-Phellandrene | C10H16 | 136.23 | 0.35% | 18.91 | 1182 |
| 17 | Limonene | C10H16 | 136.23 | 0.32% | 17.71 | 1157 |
| 18 | Elixene | C15H24 | 204.35 | 0.26% | 9.86 | 990 |
| 19 | cis-4-Thujanol | C10H18O | 154.25 | 0.24% | 9.25 | 975 |
| 20 | Terpinolene | C10H16 | 136.23 | 0.22% | 19.09 | 1186 |
| 21 | Germacrene D | C15H24 | 204.35 | 0.19% | 17.71 | 1157 |
| 22 | α-Selinene | C15H24 | 204.35 | 0.16% | 6.38 | 905 |
| 23 | 2-isopropyltoluene | C10H14 | 134.22 | 0.11% | 19.89 | 1203 |
| Bacteria | Concentration/Antimicrobial Diameters (mm) | MIC | |||||
|---|---|---|---|---|---|---|---|
| 100% | 50% | 25% | 12.5% | Methanol | Kanamycin (250 µg/mL) | ||
| Escherichia coli ATCC25922 | 11.94 ± 0.235 ah | 11.81 ± 0.607 ah | 10.89 ± 0.545 bg | 8.44 ± 0.144 cg | 6.00 ± 0.00 | 15.72 ± 0.15 | 10‰ |
| Staphylococcus aureus ATCC25933 | 14.35 ± 0.489 af | 13.46 ± 0.364 bf | 11.74 ± 0.432 cf | 10.22 ± 0.020 df | 6.00 ± 0.00 | 20.78 ± 1.81 | 5‰ |
| Pseudomonas aeruginosa | 16.61 ± 0.44 ae | 15.19 ± 0.60 be | 13.59 ± 0.41 ce | 11.71 ± 1.17 de | 6.00 ± 0.00 | 21.02 ± 0.41 | 2.5‰ |
| Chromobacterium violaceum ATCC31532 | 13.93 ± 1.09 ag | 12.37 ± 0.57 bg | 11.89 ± 0.74 bcf | 10.52 ± 0.17 cf | 6.00 ± 0.00 | 19.89 ± 2.33 | 5‰ |
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| cviI | GAAACCGTCCTCGCATAAGG | ACAAGGTGGACTGGTACTGG |
| cviR | CCCAGCAATATGCCGCTATC | CATTGAGCTTGCGGATCACA |
| vioA | AAGAGCATGGCAAGGAATC | CTGGTTGGCGTCGTTATC |
| vioB | CTGGGCGTAATTGGGAATGG | CAAATACCTGGCCCATGTCG |
| vioC | GAACAAGTACGCCAACCT | GGAAGAAAGTCTGCTGGAA |
| vioD | GCCGCAACAAGTACATCT | GAAGGTGCTCATCGTGTC |
| vioE | ATAGGCCACCTTCTGCTTCC | GGCTACTGCTGGTTCGACTA |
| hmsH | CGCCGTATGTCTTCAGTT | CGCAGCCTATCGTAGATG |
| hmsF | GGCTGCTGATTCTCTGTTA | GTATAGACGCTGCGGTAG |
| hmsN | AAACCACACGCACCAGAAC | TGCATGAGCATGAAGACGAC |
| lasA | AGCCAGCCTTACGATTCCAT | GAGGAATAGCCGTTGTCGTG |
| lasB | AGAACGCCTTGTTGTACACG | GCAAGAACGACTTCCTGGTC |
| pilE3 | TACGCTGGTCGAGTTGATGA | GCGAATAGCACTGCTCCATC |
| rpoD (actin) | TCGGACATCAGCAAGGTT | GTGAAGGACAGCCAACAG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Li, D.; Huang, X.; Yang, H.; Qiu, Z.; Zou, L.; Liang, Q.; Shi, Y.; Wu, Y.; Wu, S.; et al. Study on Antibacterial and Quorum-Sensing Inhibition Activities of Cinnamomum camphora Leaf Essential Oil. Molecules 2019, 24, 3792. https://doi.org/10.3390/molecules24203792
Wang W, Li D, Huang X, Yang H, Qiu Z, Zou L, Liang Q, Shi Y, Wu Y, Wu S, et al. Study on Antibacterial and Quorum-Sensing Inhibition Activities of Cinnamomum camphora Leaf Essential Oil. Molecules. 2019; 24(20):3792. https://doi.org/10.3390/molecules24203792
Chicago/Turabian StyleWang, Wenting, Dongxiang Li, Xiaoqin Huang, Huixiang Yang, Ziwen Qiu, Liting Zou, Qin Liang, Yu Shi, Yingxiang Wu, Shaohua Wu, and et al. 2019. "Study on Antibacterial and Quorum-Sensing Inhibition Activities of Cinnamomum camphora Leaf Essential Oil" Molecules 24, no. 20: 3792. https://doi.org/10.3390/molecules24203792
APA StyleWang, W., Li, D., Huang, X., Yang, H., Qiu, Z., Zou, L., Liang, Q., Shi, Y., Wu, Y., Wu, S., Yang, C., & Li, Y. (2019). Study on Antibacterial and Quorum-Sensing Inhibition Activities of Cinnamomum camphora Leaf Essential Oil. Molecules, 24(20), 3792. https://doi.org/10.3390/molecules24203792




