Microwave Assisted Reactions of Fluorescent Pyrrolodiazine Building Blocks
Abstract
1. Introduction
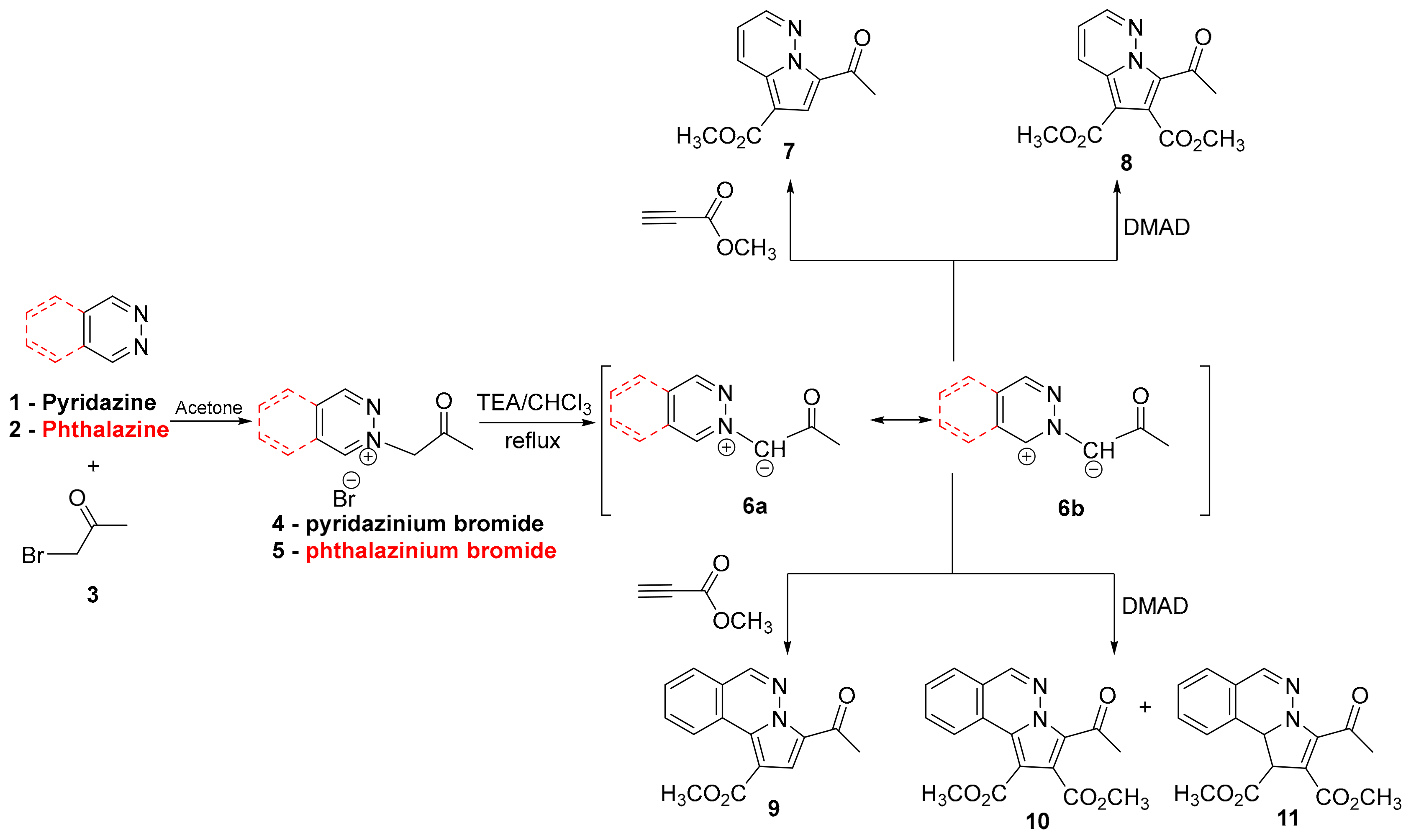
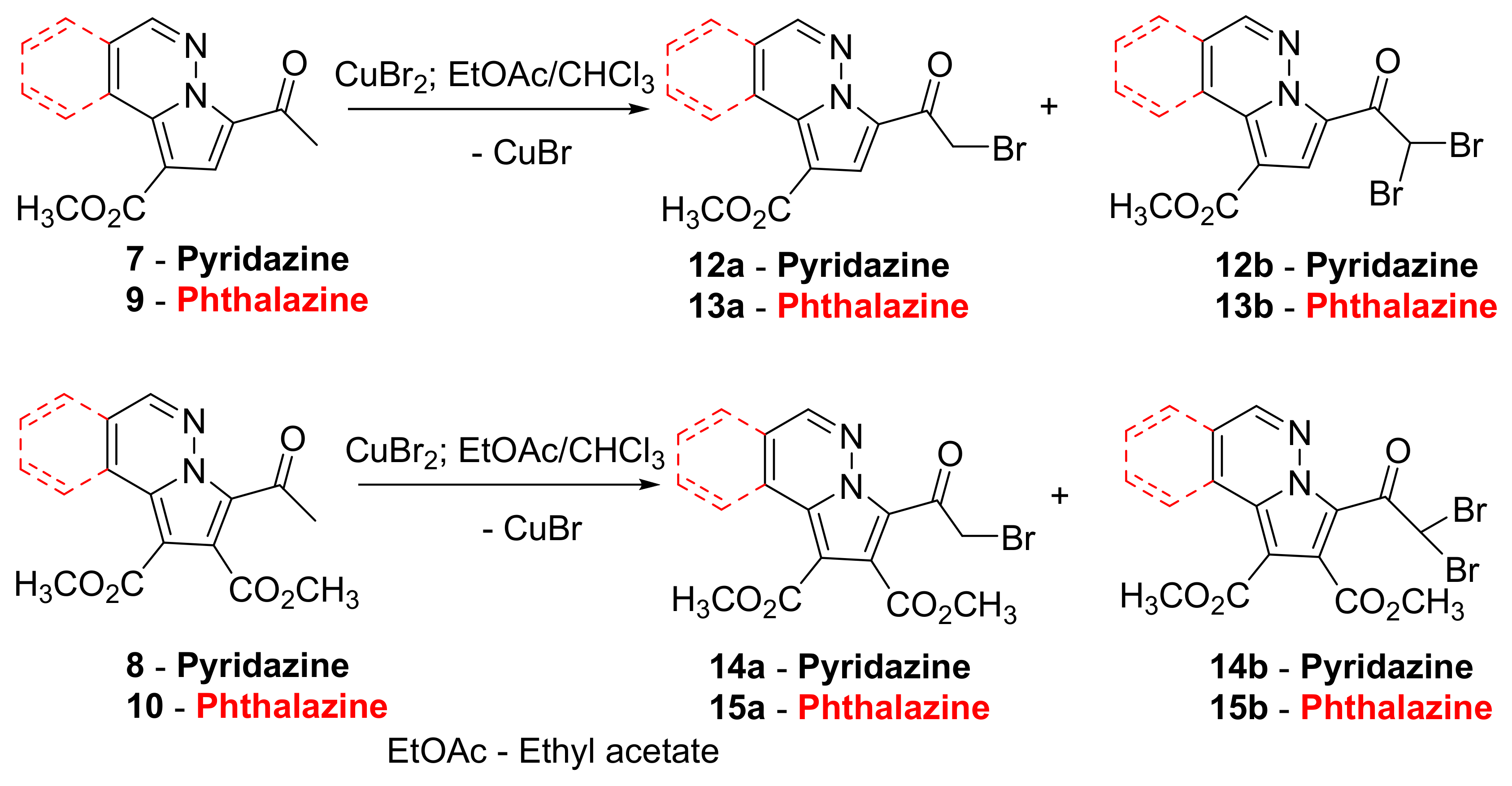
2. Results and Discussion
3. Experimental Section
3.1. General Procedure
3.1.1. General Procedure for Synthesis of Pyrrolodiazine Derivatives 7–11 under Conventional TH Conditions and MW Irradiation
3.1.2. General Procedure for Synthesis of α-brominated Pyrrolodiazines 12–15 under Conventional TH Conditions and MW Irradiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, Y.; Ma, B.; Wudl, F. Synthesis and optical properties of a series of pyrrolopyridazine derivatives: deep blue organic luminophors for electroluminescent devices. J. Mater. Chem. 1999, 9, 2183–2188. [Google Scholar] [CrossRef]
- Sandeep, C.; Basavaraj, P.; Venugopala, K.N.; Rashmi, S.K.; Rashmi, V.; Odhav, B. Efficient synthesis and characterization of ethyl 7-acetyl-2-substituted 3-(substitutedbenzoyl) indolizine-1-carboxylates for in vitro anticancer activity. Asian J. Chem. 2016, 28, 1043–1048. [Google Scholar] [CrossRef]
- Gundersen, L.L.; Charnock, C.; Negussie, A.H.; Rise, F.; Teklu, S. Synthesis of indolizine derivatives with selective antibacterial activity against Mycobacterium tuberculosis. Eur. J. Pharm. Sci. 2007, 30, 26–35. [Google Scholar] [CrossRef]
- Mantu, D.; Luca, M.C.; Moldoveanu, C.; Zbancioc, G.; Mangalagiu, I.I. Synthesis and antituberculosis activity of some new pyridazine derivatives. Part II. Eur. J. Med. Chem. 2010, 45, 5164–5168. [Google Scholar] [CrossRef]
- Zbancioc, A.M.; Zbancioc, G.; Tanase, C.; Miron, A.; Ursu, C.; Mangalagiu, I.I. Design, synthesis and in vitro anticancer activity of a new class of dual DNA intercalators. Lett. Drug Des. Discov. 2010, 7, 644–649. [Google Scholar] [CrossRef]
- Sonnet, P.; Dallemagne, P.; Guillon, J.; Enguehard, C.; Stiebing, S.; Tanguy, J.; Bureau, R.; Rault, S.; Auvray, P.; Moslemi, S.; et al. New aromatase inhibitors. Synthesis and biological activity of aryl–substituted pyrrolizine and indolizine derivatives. Bioorg. Med. Chem. 2000, 8, 945–955. [Google Scholar] [CrossRef]
- Narajji, C.; Karvekar, M.D.; Das, A.K. Synthesis and antioxidant activity of 3,3′–diselanediylbis (N, N–disubstituted indolizine–1–carboxamide) and derivatives. S. Afr. J. Chem. 2008, 61, 53–55, ISSN: 0379-4350. [Google Scholar]
- Huang, W.; Zuo, T.; Luo, X.; Jin, H.; Liu, Z.; Yang, Z.; Yu, X.; Zhang, L.; Zhang, L. Indolizine derivatives as HIV–1 VIF–ElonginC interaction inhibitors. Chem. Biol. Drug. Des. 2013, 81, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Mitsumori, T.; Bendikov, M.; Sedó, J.; Wudl, F. Synthesis and properties of novel highly fluorescent pyrrolopyridazine derivatives. Chem. Mater. 2003, 15, 3759–3768. [Google Scholar] [CrossRef]
- Mitsumori, T.; Bendikov, M.; Dautel, O.; Wudl, F.; Shioya, T.; Sato, H.; Sato, Y. Synthesis and properties of highly fluorescent indolizino[3,4,5-ab]isoindoles. J. Am. Chem. Soc. 2004, 126, 16793–16803. [Google Scholar] [CrossRef] [PubMed]
- Mitsumori, T.; Craig, I.M.; Martini, I.B.; Schwartz, B.J.; Wudl, F. Synthesis and color tuning properties of blue highly fluorescent vinyl polymers containing a pendant pyrrolopyridazine. Macromolecules 2005, 38, 4698–4704. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Askim, J.R.; Suslick, K.S. The optoelectronic nose: colorimetric and fluorometric sensor arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Qu, X. Cancer biomarker detection: recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef] [PubMed]
- Queralto, N.; Berliner, A.N.; Goldsmith, B.; Martino, R.; Rhodes, P.; Lim, S.H. Detecting cancer by breath volatile organic compound analysis: a review of array-based sensors. J. Breath Res. 2014, 8, 027112. [Google Scholar] [CrossRef]
- Tisler, M. Structure and reactivity correlation of bicyclic 10-π electron systems with bridgehead nitrogen. Pure Appl. Chem. 1980, 52, 1611–1621. [Google Scholar] [CrossRef][Green Version]
- Maftei, D.; Zbancioc, G.; Humelnicu, I.; Mangalagiu, I. Conformational effects on the lowest excited states of benzoyl-pyrrolopyridazine: insights from PCM time-dependent DFT. J. Phys. Chem. A 2013, 117, 3165–3175. [Google Scholar] [CrossRef] [PubMed]
- Rodriquez, M.; Taddei, M. Synthesis of heterocycles via microwave-assisted cycloadditions and cyclocondensations. In Microwave-Assisted Synthesis of Heterocycle; Van der Eycken, E., Kappe, C.O., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2006; pp. 213–266. ISBN 9783540309833. [Google Scholar]
- Perreux, L.; Loupy, A. Nonthermal effects of microwaves in organic synthesis. In Microwaves in Organic Synthesis, 2nd ed.; Loupy, A., Ed.; Wiley-VCH: Weinheim, Germany, 2006; pp. 134–218. ISBN 9783527314522. [Google Scholar]
- Zbancioc, G.; Mangalagiu, I.I. Pyrrolopyridazine derivatives as blue organic luminophores: synthesis and properties. Part 2. Tetrahedron 2010, 66, 278–282. [Google Scholar] [CrossRef]
- Zbancioc, G.; Huhn, T.; Groth, U.; Deleanu, C.; Mangalagiu, I.I. Pyrrolodiazine derivatives as blue organic luminophores: Synthesis and properties. Part 3. Tetrahedron 2010, 66, 4298–4306. [Google Scholar] [CrossRef][Green Version]
- Zbancioc, G.; Caprosu, M.; Moldoveanu, C.; Mangalagiu, I.I. Microwave assisted synthesis for dimers via [3 + 3] dipolar cycloadditions. Arkivoc 2005, 5, 174–187. [Google Scholar]
- Zbancioc, G.; Bejan, V.; Risca, M.; Moldoveanu, C.; Mangalagiu, I.I. Microwave assisted reactions of new azaheterocyles compounds. Molecules 2009, 14, 403–411. [Google Scholar] [CrossRef]
- Zbancioc, G.; Moldoveanu, C.; Zbancioc, A.M.; Mangalagiu, I.I. Microwave assisted synthesis of new pyrrolopyridazine derivatives with acetophenone skeleton. Part V. Curr. Microw. Chem. 2014, 1, 41–46. [Google Scholar] [CrossRef]
- Moldoveanu, C.; Mangalagiu, I.; Isac, D.L.; Airinei, A.; Zbancioc, G. A new pathway for the synthesis of a new class of blue fluorescent benzofuran derivatives. Molecules 2018, 23, 1968. [Google Scholar] [CrossRef] [PubMed]
- Al Matarneh, C.M.; Apostu, M.O.; Mangalagiu, I.I.; Danac, R. Reactions of ethyl cyanoformate with cycloimmonium salts: A direct pathway to fused or substituted azaheterocycles. Tetrahedron 2016, 72, 4230–4238. [Google Scholar] [CrossRef]
- Masaki, Y.; Otsuka, H.; Nakayama, Y.; Hioki, M. Studies on indolizines and azaindolizines. I. The 1,3-dipolar cycloaddition of acetylenic compounds to pyridazinium ylides. Chem. Pharm. Bull. 1973, 21, 2780–2783. [Google Scholar] [CrossRef][Green Version]
- Caira, M.R.; Georgescu, E.; Georgescu, F.; Albota, F.; Dumitrascu, F. Contributions to syntheses of pyrrolo[2,1-a]phthalazines. Monatsh. Chem. 2011, 142, 743–748. [Google Scholar] [CrossRef]
- Popa, M.M.; Barbu, L.; Draghici, C.; Dumitrascu, F. 3-Acetyl-pyrrolo[2,1-a]phthalazines by one-pot reaction. U.P.B. Sci. Bull. Series B 2011, 73, 109–114. [Google Scholar]
- Zbancioc, G.; Zbancioc, A.M.; Mangalagiu, I.I. Ultrasound and microwave assisted synthesis of dihydroxyacetophenone derivatives with or without 1, 2-diazine skeleton. Ultrason. Sonochem. 2014, 21, 802–811. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Compound | MW | Conventional TH | |||
|---|---|---|---|---|---|
| Reaction Time (min) | Yield, % | Reaction Time (min) | Yield, % | ||
| 7 | 10 | 90 | 360 | 77 | |
| 8 | 91 | 74 | |||
| 9 | 94 | 81 | |||
| 10 | 91 | 45 | |||
| 11 | 0 | 37 | |||
| Compound | Fluorescence (λmax, nm) (Quantum Yields (%)) | Absorption (λmax, nm) | ||
|---|---|---|---|---|
| Cyclohexane | Dichloromethane | Cyclohexane | Dichloromethane | |
| 7 | 426 (24) | 432 (21) | 360 | 358 |
| 8 | 422 (25) | 426 (26) | 356 | 354 |
| 9 | 428 (7) | 441 (5) | 327 | 325 |
| 10 | 439 (6) | 446 (4) | 324 | 322 |
| 11 | 491 | - | 374 | 372 |
| Compound | MW | Conventional TH | ||
|---|---|---|---|---|
| Reaction Time (min) | Yield, % | Reaction Time (min) | Yield, % | |
| 12a | 20 | 77 | 480 | 53 |
| 12b | 12 | 17 | ||
| 13a | 76 | 50 | ||
| 13b | 11 | 16 | ||
| 14a | 77 | 55 | ||
| 14b | 13 | 17 | ||
| 15a | 75 | 49 | ||
| 15b | 12 | 16 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moldoveanu, C.; Amariucai-Mantu, D.; Mangalagiu, V.; Antoci, V.; Maftei, D.; Mangalagiu, I.I.; Zbancioc, G. Microwave Assisted Reactions of Fluorescent Pyrrolodiazine Building Blocks. Molecules 2019, 24, 3760. https://doi.org/10.3390/molecules24203760
Moldoveanu C, Amariucai-Mantu D, Mangalagiu V, Antoci V, Maftei D, Mangalagiu II, Zbancioc G. Microwave Assisted Reactions of Fluorescent Pyrrolodiazine Building Blocks. Molecules. 2019; 24(20):3760. https://doi.org/10.3390/molecules24203760
Chicago/Turabian StyleMoldoveanu, Costel, Dorina Amariucai-Mantu, Violeta Mangalagiu, Vasilichia Antoci, Dan Maftei, Ionel I. Mangalagiu, and Gheorghita Zbancioc. 2019. "Microwave Assisted Reactions of Fluorescent Pyrrolodiazine Building Blocks" Molecules 24, no. 20: 3760. https://doi.org/10.3390/molecules24203760
APA StyleMoldoveanu, C., Amariucai-Mantu, D., Mangalagiu, V., Antoci, V., Maftei, D., Mangalagiu, I. I., & Zbancioc, G. (2019). Microwave Assisted Reactions of Fluorescent Pyrrolodiazine Building Blocks. Molecules, 24(20), 3760. https://doi.org/10.3390/molecules24203760










