β-Caryophyllene in the Essential Oil from Chrysanthemum Boreale Induces G1 Phase Cell Cycle Arrest in Human Lung Cancer Cells
Abstract
1. Introduction
2. Results
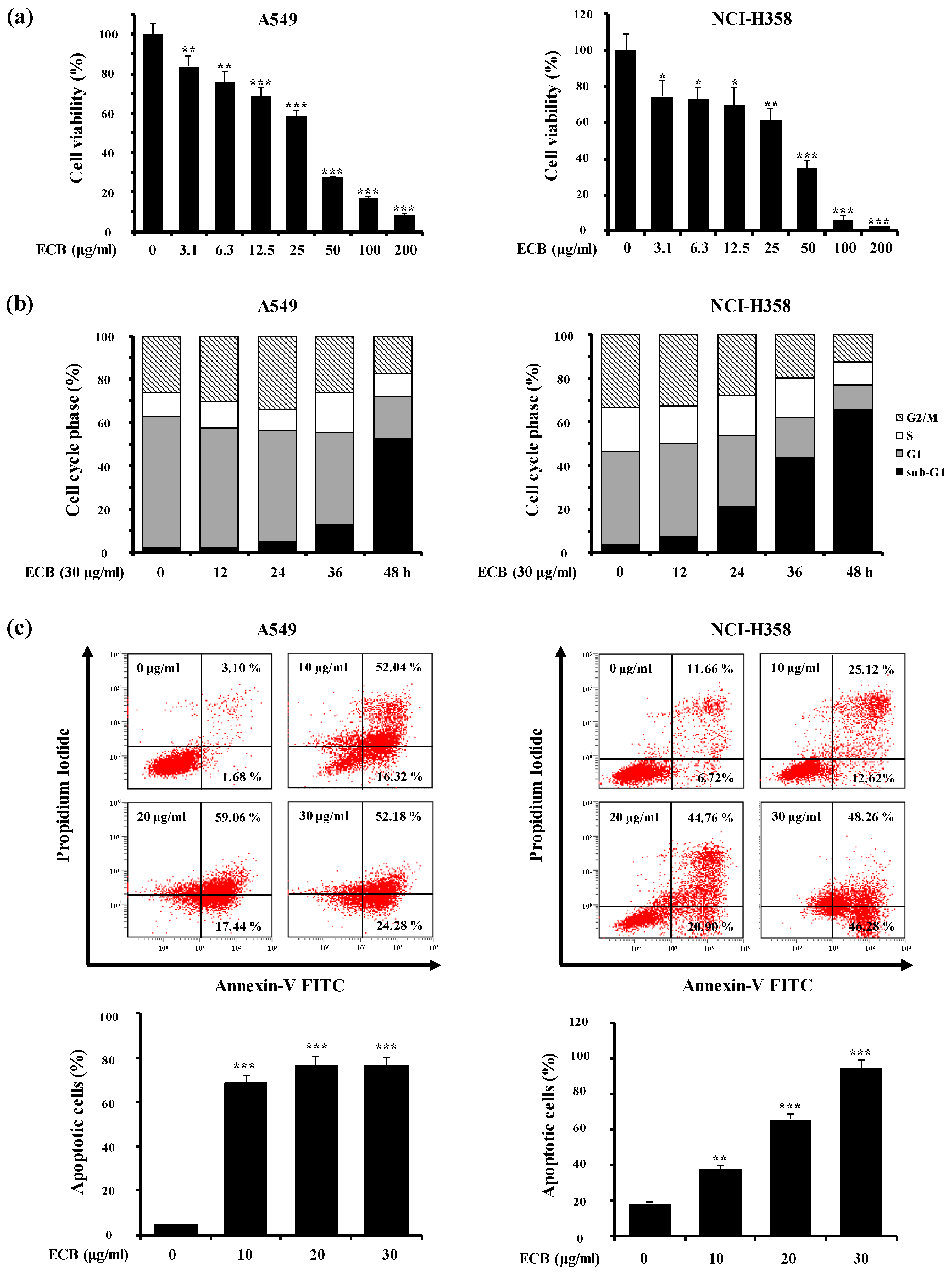
2.1. ECB Induces Apoptosis in Human Lung Cancer Cells
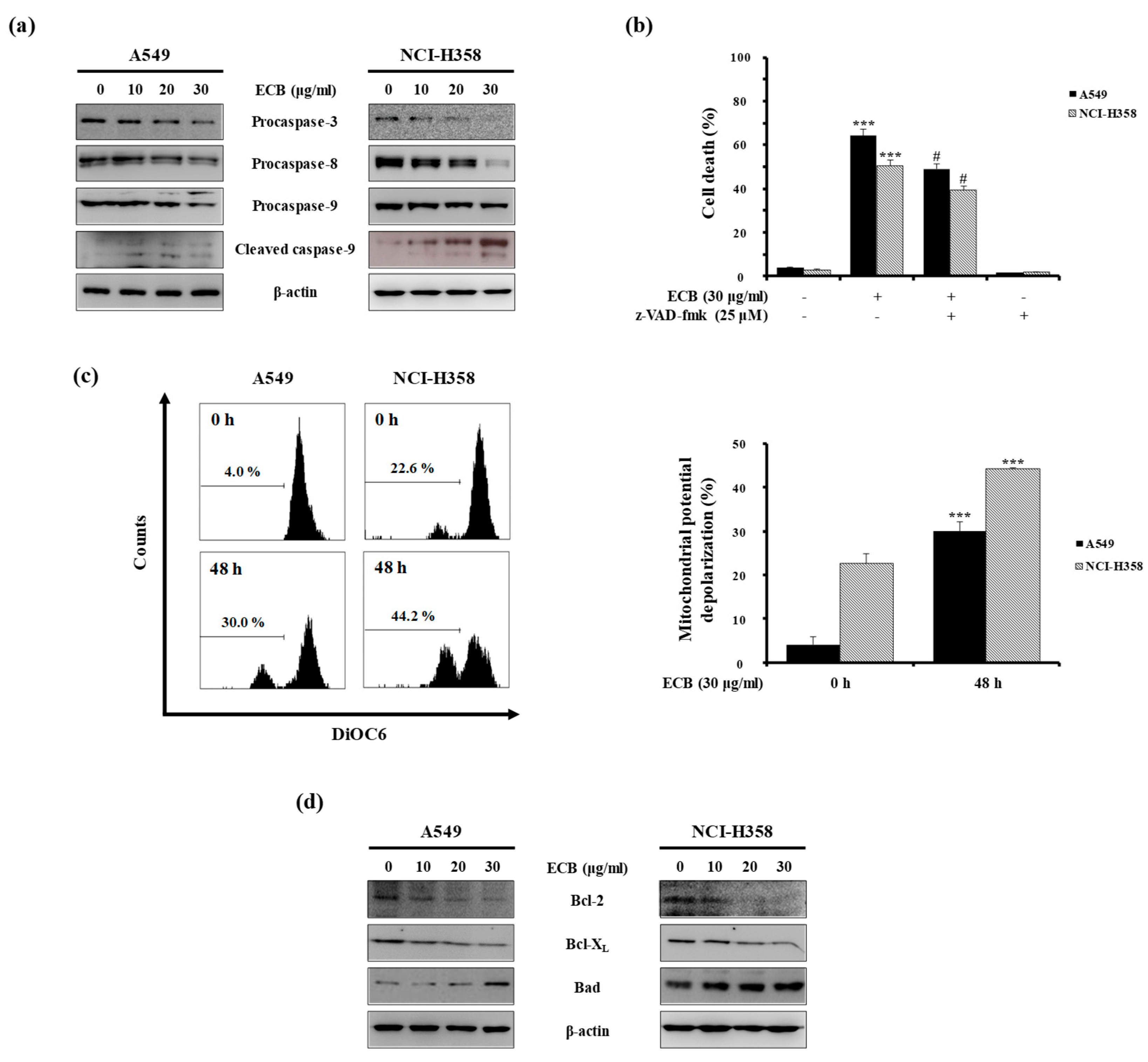
2.2. ECB-Induced Apoptosis is Mediated by Caspase Activation and Mitochondrial Pathway in Human Lung Cancer Cells
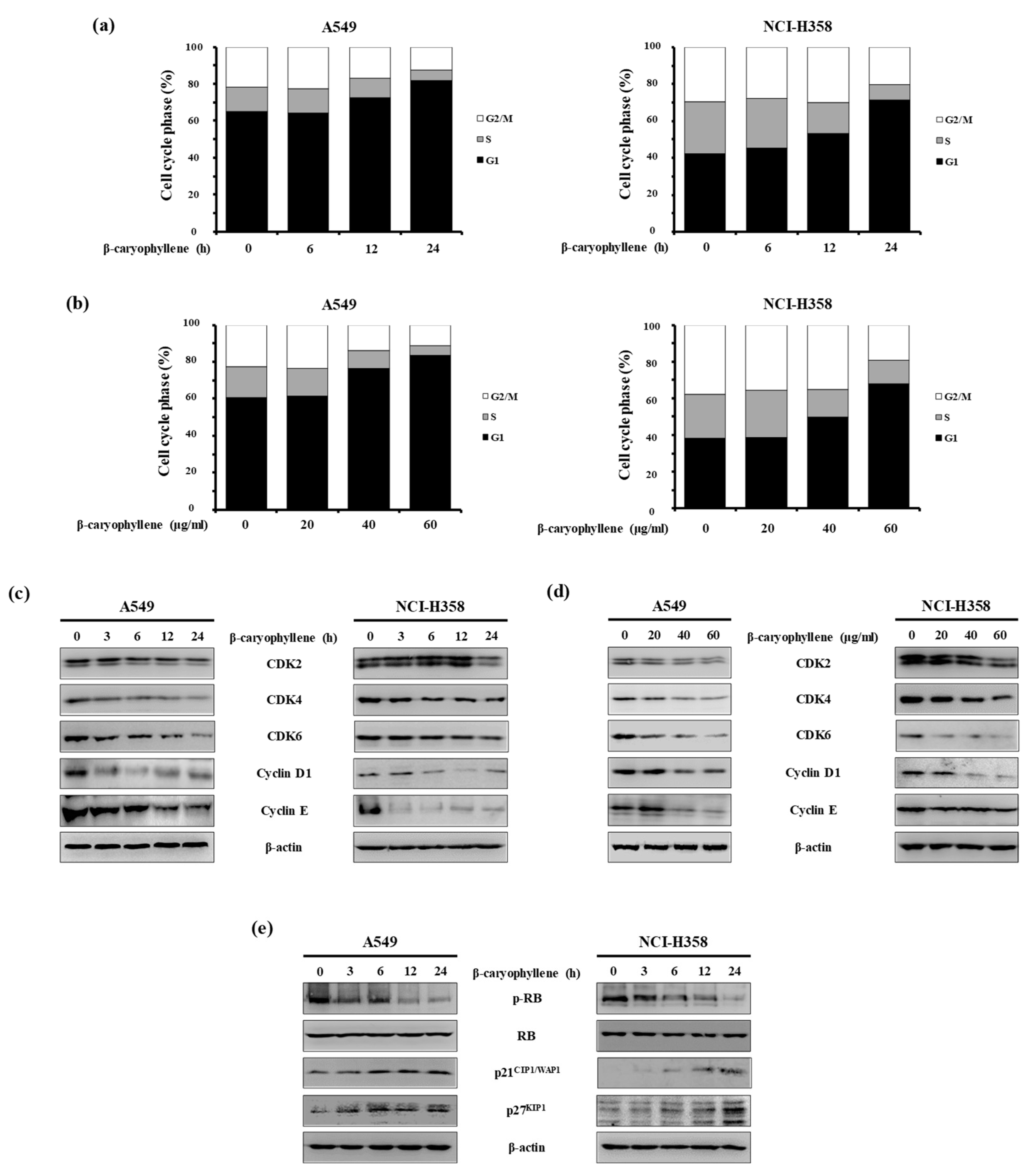
2.3. β-Caryophyllene Regulates G1 Cell Cycle Progression in Human Lung Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Essential Oil
4.3. GC/MS Analysis
4.4. Cell Culture
4.5. MTT Assay
4.6. PI Staining Analysis
4.7. PI and Annexin V Double Staining
4.8. Determination of Mitochondria Membrane Potential (MMP)
4.9. Western Blot Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Sher, T.; Dy, G.K.; Adjei, A.A. Small cell lung cancer. Mayo Clin. Proc. 2008, 83, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Hobbins, S.; West, D.; Peake, M.; Beckett, P.; Woolhouse, I. Patient characteristics, treatment and survival in pulmonary carcinoid tumours: An analysis from the UK National Lung Cancer Audit. BMJ Open 2016, 6, 012530. [Google Scholar] [CrossRef] [PubMed]
- Polański, J.; Jankowska-Polanska, B.; Rosińczuk, J.; Chabowski, M.; Szymańska-Chabowska, A. Quality of life of patients with lung cancer. OncoTargets Ther. 2016, 9, 1023–1028. [Google Scholar]
- Sun, G.; Fan, T.; Zhao, L.; Zhou, Y.; Zhong, R. The potential of combi-molecules with DNA-damaging function as anticancer agents. Future Med. Chem. 2017, 9, 403–435. [Google Scholar] [CrossRef]
- Guohui, S.; Lijiao, Z.; Rugang, Z. The Induction and Repair of DNA Interstrand Crosslinks and Implications in Cancer Chemotherapy. Anti-Cancer Agents Med. Chem. 2015, 16, 221–246. [Google Scholar] [CrossRef]
- Fan, T.; Sun, G.; Sun, X.; Zhao, L.; Zhong, R.; Peng, Y. Tumor Energy Metabolism and Potential of 3-Bromopyruvate as an Inhibitor of Aerobic Glycolysis: Implications in Tumor Treatment. Cancers 2019, 11, 317. [Google Scholar] [CrossRef]
- Greenwell, M.; Rahman, P. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar]
- Tewari, D.; Rawat, P.; Singh, P.K. Adverse drug reactions of anticancer drugs derived from natural sources. Food Chem. Toxicol. 2019, 123, 522–535. [Google Scholar] [CrossRef]
- Moon, K.S.; Ji, J.Y.; Cho, Y.J.; Lee, J.H.; Choi, M.S.; Kim, E.E. Therapeutic Effects of SB Natural Anticancer Drug in 50 Patients with Stage IV Pancreatic Cancer. J. Cancer Treat. Res. 2015, 3, 42. [Google Scholar] [CrossRef][Green Version]
- Hong, J.-M.; Kim, J.-H.; Kim, H.; Lee, W.J.; Hwang, Y.-I. SB365, Pulsatilla Saponin D Induces Caspase-Independent Cell Death and Augments the Anticancer Effect of Temozolomide in Glioblastoma Multiforme Cells. Molcules 2019, 24, 3230. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Yu, H.-H.; Cha, J.-D.; You, Y.-O.; Kim, K.-J.; Jeong, S.-I.; Kil, B.-S. Antibacterial Activity and Chemical Composition of Essential Oil of Chrysanthemum boreale. Planta Medica 2003, 69, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Yang, M.S.; Park, M.K.; Kim, S.C.; Yang, C.H.; Park, S.J.; Lee, J.R. A new cytotoxic guaianolide from Chrysanthemum boreale. Fitoterapia 2009, 80, 54–56. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A. Targeting the extrinsic apoptotic pathway in cancer: Lessons learned and future directions. J. Clin. Investig. 2015, 125, 487–489. [Google Scholar] [CrossRef]
- Zielinski, R.R.; Eigl, B.J.; Chi, K.N. Targeting the Apoptosis Pathway in Prostate Cancer. Cancer J. 2013, 19, 79–89. [Google Scholar] [CrossRef]
- Breckenridge, D.G.; Xue, D. Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Curr. Opin. Cell Biol. 2004, 16, 647–652. [Google Scholar] [CrossRef]
- Ahmad, N.; Adhami, V.M.; Afaq, F.; Feyes, D.K.; Mukhtar, H. Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin. Cancer Res. 2001, 7, 1466–1473. [Google Scholar]
- Downey, K.; Jafar, M.; Attygalle, A.D.; Hazell, S.; A Morgan, V.; Giles, S.L.; A Schmidt, M.; Ind, T.E.J.; Shepherd, J.H.; DeSouza, N.M. Influencing surgical management in patients with carcinoma of the cervix using a T2- and ZOOM-diffusion-weighted endovaginal MRI technique. Br. J. Cancer 2013, 109, 615–622. [Google Scholar] [CrossRef]
- Döll-Boscardin, P.M.; Sartoratto, A.; Maia, B.H.L.D.N.S.; De Paula, J.P.; Nakashima, T.; Farago, P.V.; Kanunfre, C.C. In Vitro Cytotoxic Potential of Essential Oils of Eucalyptus benthamii and Its Related Terpenes on Tumor Cell Lines. Evid.-Based Complement. Altern. Med. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Pyee, Y.; Chung, H.J.; Choi, T.J.; Park, H.J.; Hong, J.Y.; Kim, J.S.; Kang, S.S.; Lee, S.K. Suppression of inflammatory responses by handelin, a guaianolide dimer from chrysanthemum boreale, via downregulation of nf-kappab signaling and pro-inflammatory cytokine production. J. Nat. Prod. 2014, 77, 917–924. [Google Scholar] [CrossRef]
- Kim, B.-S.; Park, S.-J.; Kim, M.-K.; Kim, Y.-H.; Lee, S.-B.; Lee, K.-H.; Choi, N.-Y.; Lee, Y.-R.; Lee, Y.-E.; You, Y.-O. Inhibitory Effects of Chrysanthemum boreale Essential Oil on Biofilm Formation and Virulence Factor Expression of Streptococcus mutans. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–11. [Google Scholar]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The anticancer, antioxidant and antimicrobial properties of the sesquiterpene beta-caryophyllene from the essential oil of aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Ramachandhiran, D.; Sankaranarayanan, C.; Murali, R.; Babukumar, S.; Vinothkumar, V. Beta-caryophyllene promotes oxidative stress and apoptosis in kb cells through activation of mitochondrial-mediated pathway—An in-vitro and in-silico study. Arch. Physiol. Biochem. 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strzadala, L.; Szumny, A. Beta-caryophyllene and beta-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef]
- Patil, J.R.; Jayaprakasha, G.; Murthy, K.C.; Tichy, S.E.; Chetti, M.B.; Patil, B.S. Apoptosis-mediated proliferation inhibition of human colon cancer cells by volatile principles of Citrus aurantifolia. Food Chem. 2009, 114, 1351–1358. [Google Scholar] [CrossRef]
- Torres, A.; Vargas, Y.; Uribe, D.; Carrasco, C.; Torres, C.; Rocha, R.; Oyarzun, C.; San Martin, R.; Quezada, C. Pro-apoptotic and anti-angiogenic properties of the alpha /beta-thujone fraction from thuja occidentalis on glioblastoma cells. J. Neurooncol. 2016, 128, 9–19. [Google Scholar] [CrossRef]
- Pudelek, M.; Catapano, J.; Kochanowski, P.; Mrowiec, K.; Janik-Olchawa, N.; Czyz, J.; Ryszawy, D. Therapeutic potential of monoterpene alpha-thujone, the main compound of thuja occidentalis l. Essential oil, against malignant glioblastoma multiforme cells in vitro. Fitoterapia 2019, 134, 172–181. [Google Scholar] [CrossRef]
- Slameňová, D.; Horváthová, E. Cytotoxic, anti-carcinogenic and antioxidant properties of the most frequent plant volatiles. Neoplasma 2013, 60, 343–354. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, X.-W.; Du, F.-F.; Li, M.-J.; Xu, F.; Wang, F.-Q.; Liu, Y.; Li, C.; Sun, Y. Sensitive assay for measurement of volatile borneol, isoborneol, and the metabolite camphor in rat pharmacokinetic study of Borneolum (Bingpian) and Borneolum syntheticum (synthetic Bingpian). Acta Pharmacol. Sin. 2013, 34, 1337–1348. [Google Scholar] [CrossRef]
- Logue, S.E.; Martin, S.J. Caspase activation cascades in apoptosis. Biochem. Soc. Trans. 2008, 36, 1–9. [Google Scholar] [CrossRef]
- Matthews, G.M.; Newbold, A.; Johnstone, R.W. Intrinsic and Extrinsic Apoptotic Pathway Signaling as Determinants of Histone Deacetylase Inhibitor Antitumor Activity. Mol. Cell. Basis Metastasis Road Ther. 2012, 116, 165–197. [Google Scholar]
- Lee, K.-W.; Chung, K.-S.; Seo, J.-H.; Yim, S.-V.; Park, H.-J.; Choi, J.-H.; Lee, K.-T. Sulfuretin from heartwood of Rhus verniciflua triggers apoptosis through activation of Fas, Caspase-8, and the mitochondrial death pathway in HL-60 human leukemia cells. J. Cell. Biochem. 2012, 113, 2835–2844. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
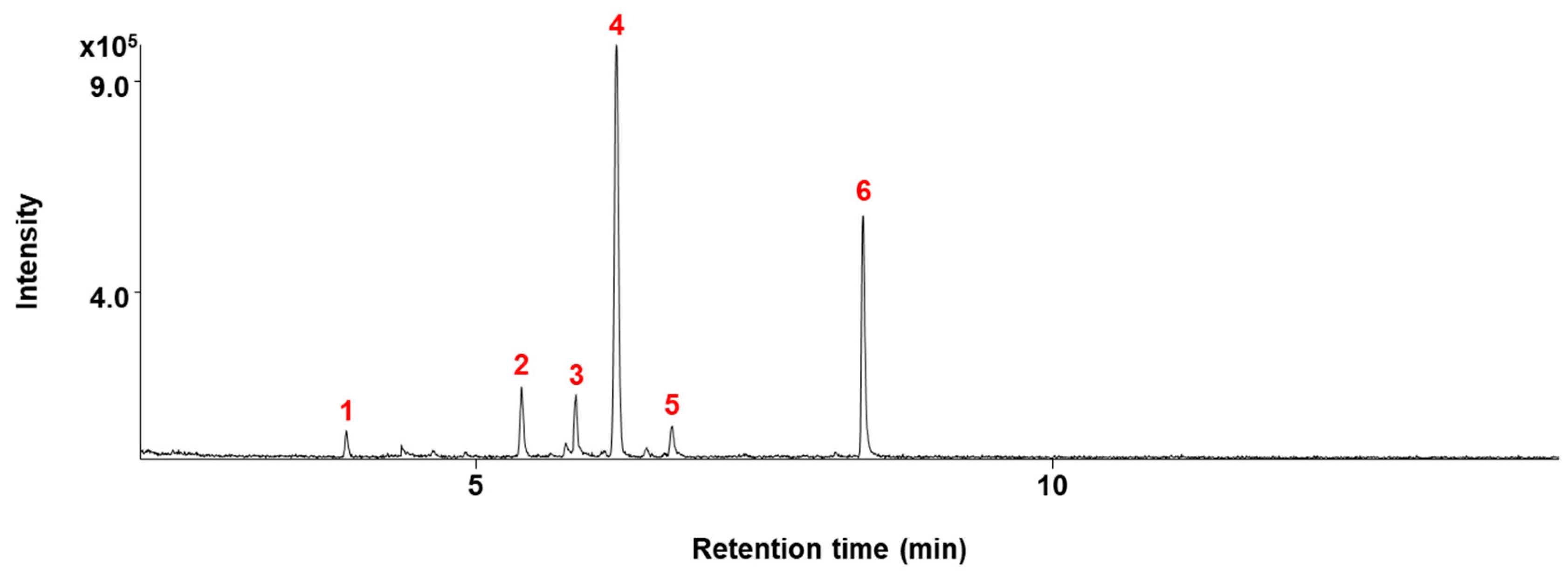
| Compounds Isolated from ECB | Chemical Structure | IC50 (μg/mL) | |
|---|---|---|---|
| A549 | NCI-H358 | ||
| 1,8-Cineole |  | >200 | >200 |
| Thujone | 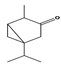 | >200 | >200 |
| β-Caryophyllene |  | 47.05 | 54.78 |
| Camphor | 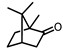 | >200 | >200 |
| Endo-borneol |  | >200 | >200 |
| 2-Isopropyl-5 methyl-3-cyclohexen-1-one | 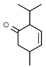 | >200 | >200 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, K.-S.; Hong, J.Y.; Lee, J.-H.; Lee, H.-J.; Park, J.Y.; Choi, J.-H.; Park, H.-J.; Hong, J.; Lee, K.-T. β-Caryophyllene in the Essential Oil from Chrysanthemum Boreale Induces G1 Phase Cell Cycle Arrest in Human Lung Cancer Cells. Molecules 2019, 24, 3754. https://doi.org/10.3390/molecules24203754
Chung K-S, Hong JY, Lee J-H, Lee H-J, Park JY, Choi J-H, Park H-J, Hong J, Lee K-T. β-Caryophyllene in the Essential Oil from Chrysanthemum Boreale Induces G1 Phase Cell Cycle Arrest in Human Lung Cancer Cells. Molecules. 2019; 24(20):3754. https://doi.org/10.3390/molecules24203754
Chicago/Turabian StyleChung, Kyung-Sook, Joo Young Hong, Jeong-Hun Lee, Hae-Jun Lee, Ji Yeon Park, Jung-Hye Choi, Hee-Juhn Park, Jongki Hong, and Kyung-Tae Lee. 2019. "β-Caryophyllene in the Essential Oil from Chrysanthemum Boreale Induces G1 Phase Cell Cycle Arrest in Human Lung Cancer Cells" Molecules 24, no. 20: 3754. https://doi.org/10.3390/molecules24203754
APA StyleChung, K.-S., Hong, J. Y., Lee, J.-H., Lee, H.-J., Park, J. Y., Choi, J.-H., Park, H.-J., Hong, J., & Lee, K.-T. (2019). β-Caryophyllene in the Essential Oil from Chrysanthemum Boreale Induces G1 Phase Cell Cycle Arrest in Human Lung Cancer Cells. Molecules, 24(20), 3754. https://doi.org/10.3390/molecules24203754






