HU-671, a Novel Oleoyl Serine Derivative, Exhibits Enhanced Efficacy in Reversing Ovariectomy-Induced Osteoporosis and Bone Marrow Adiposity
Abstract
1. Introduction
2. Results
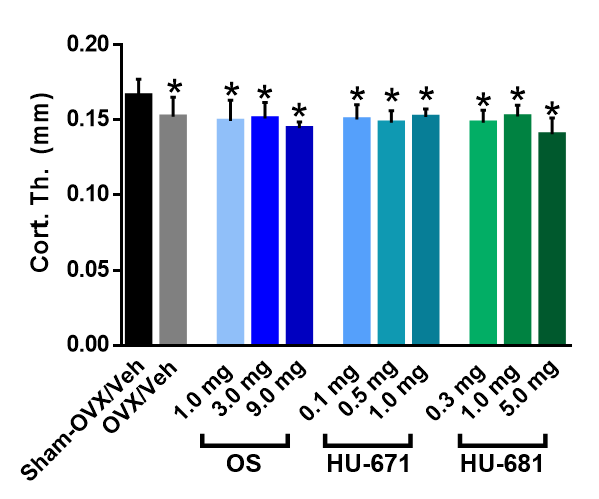
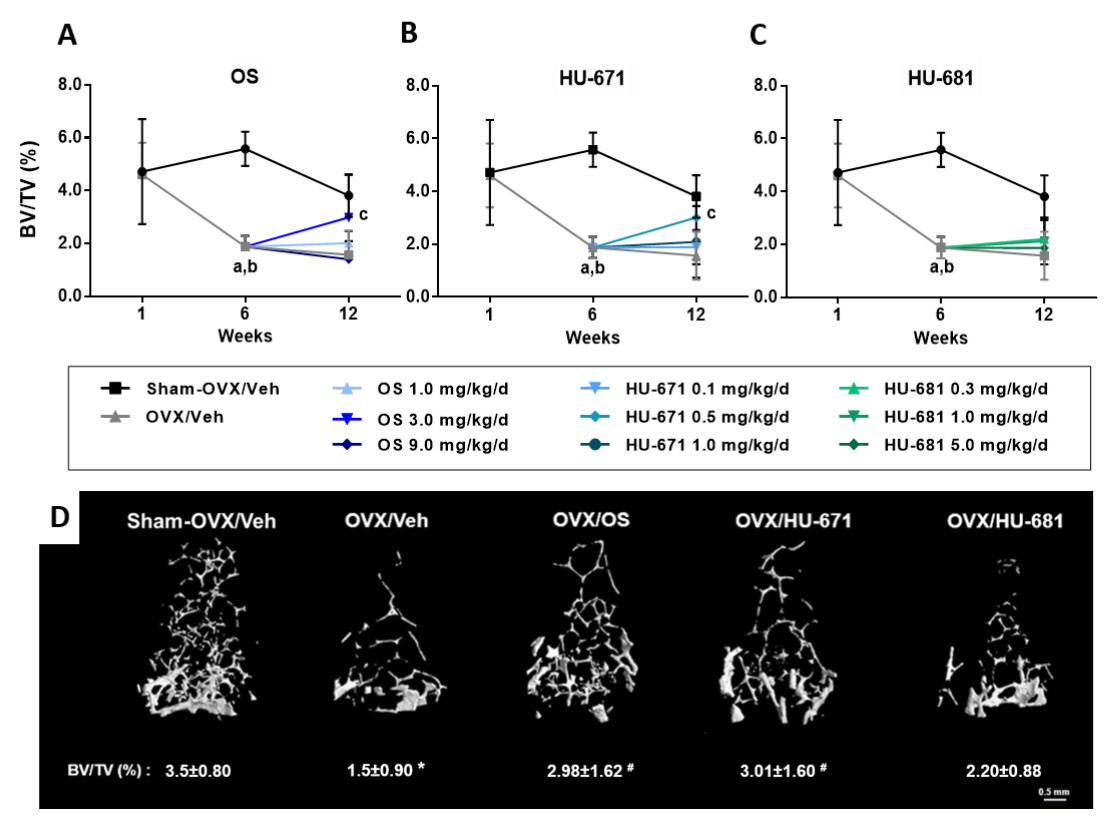
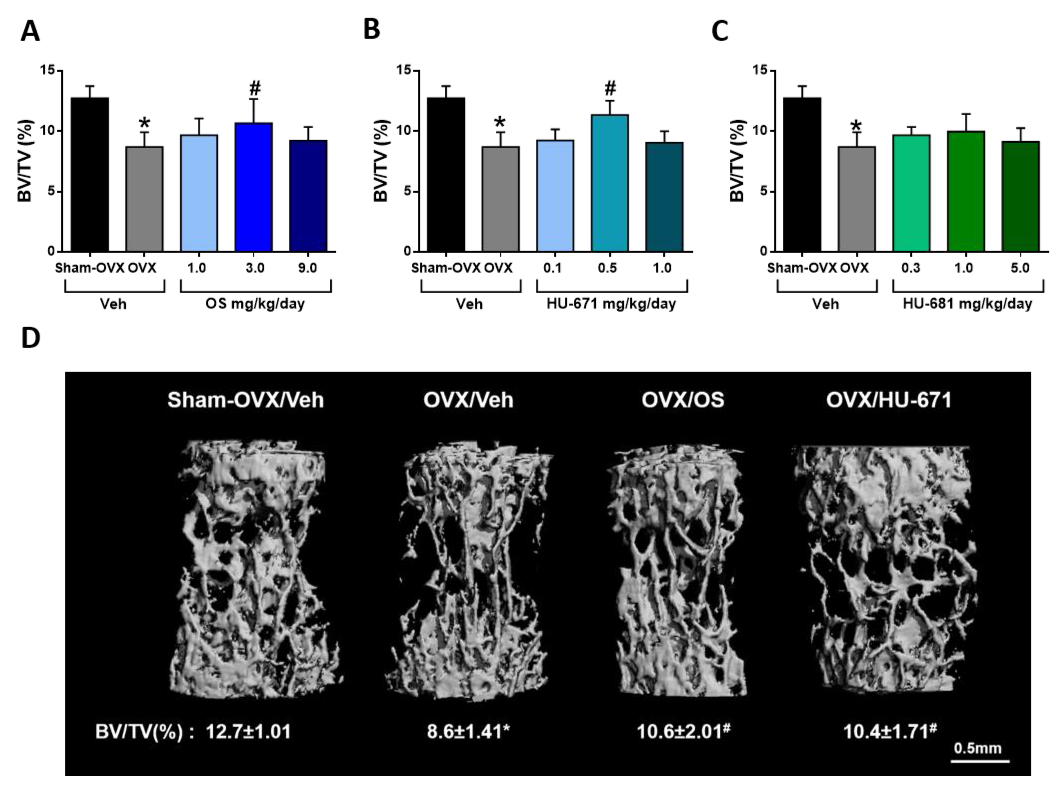
2.1. HU-671 Rescues Ovariectomy-Induced Bone Loss
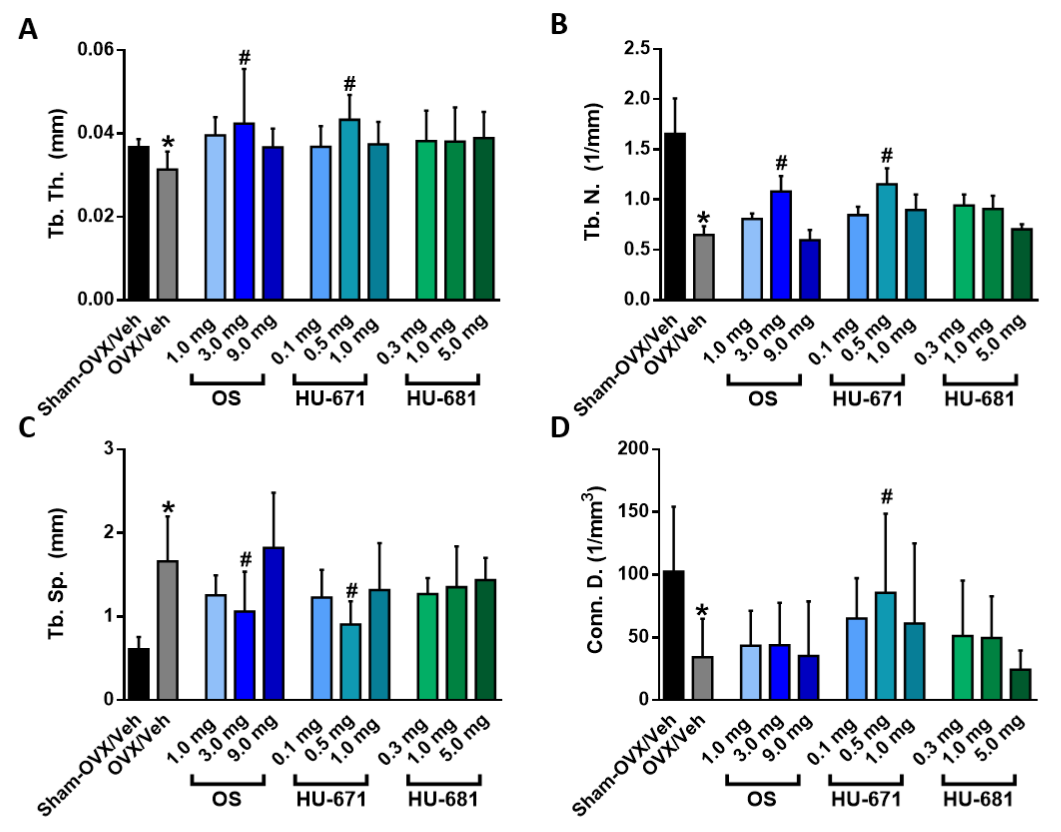
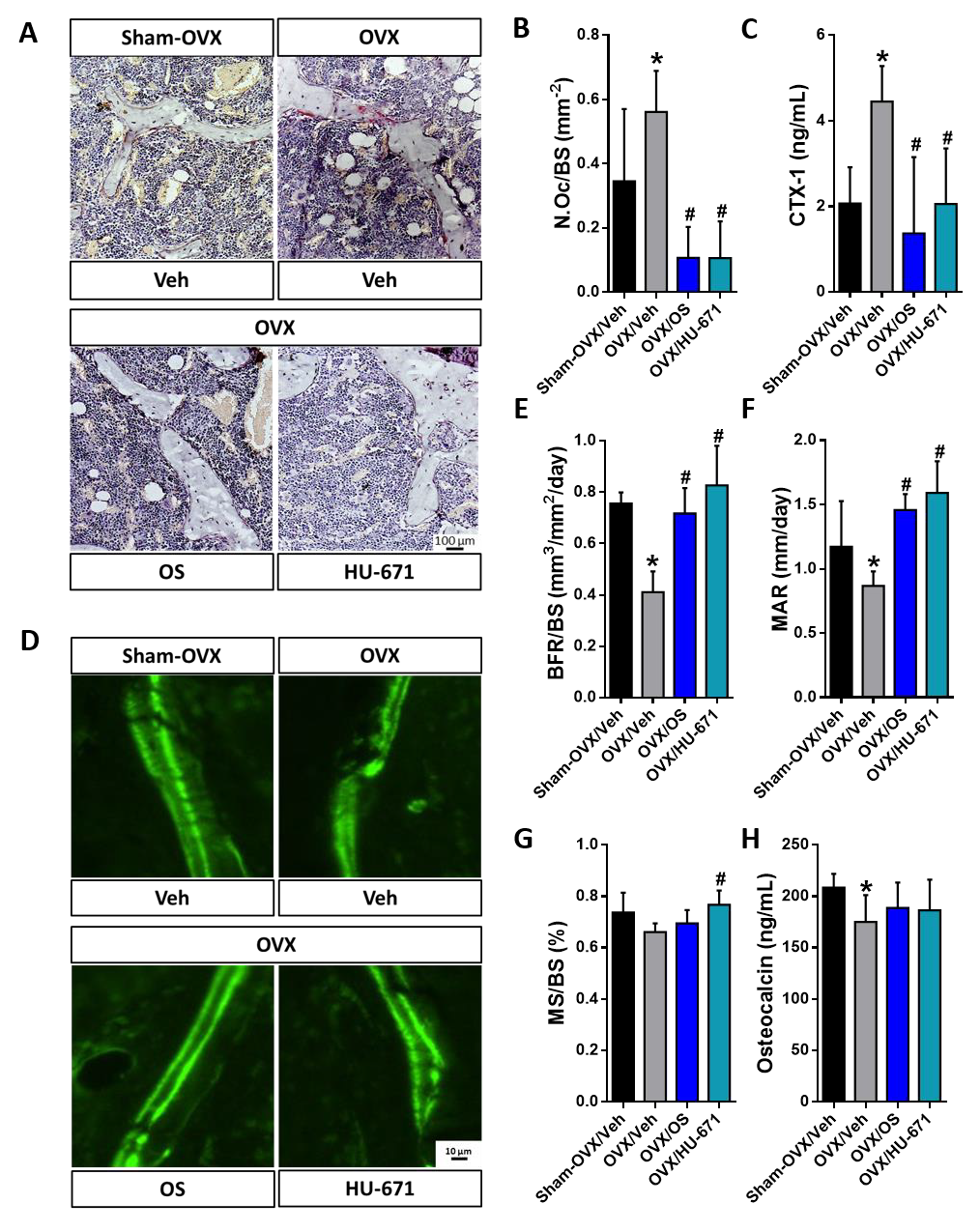
2.2. HU-671 Mitigates Bone Resorption and Enhances Bone Formation
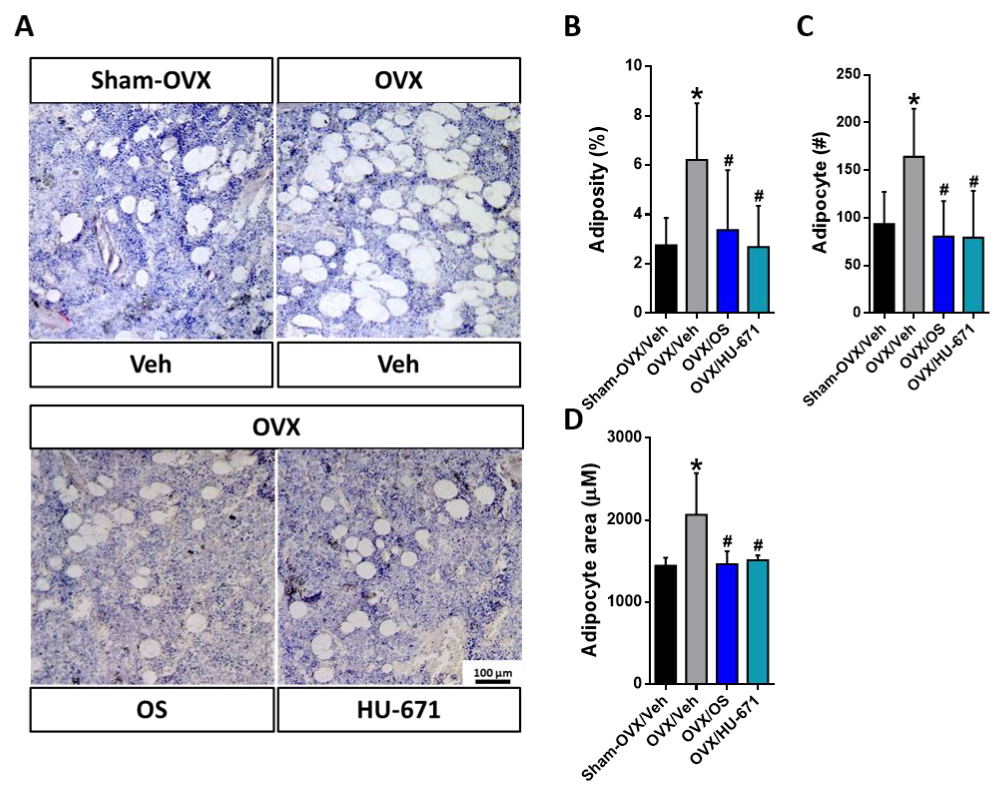
2.3. HU-671 Reduces the OVX-Induced Increase in Bone Marrow Adiposity
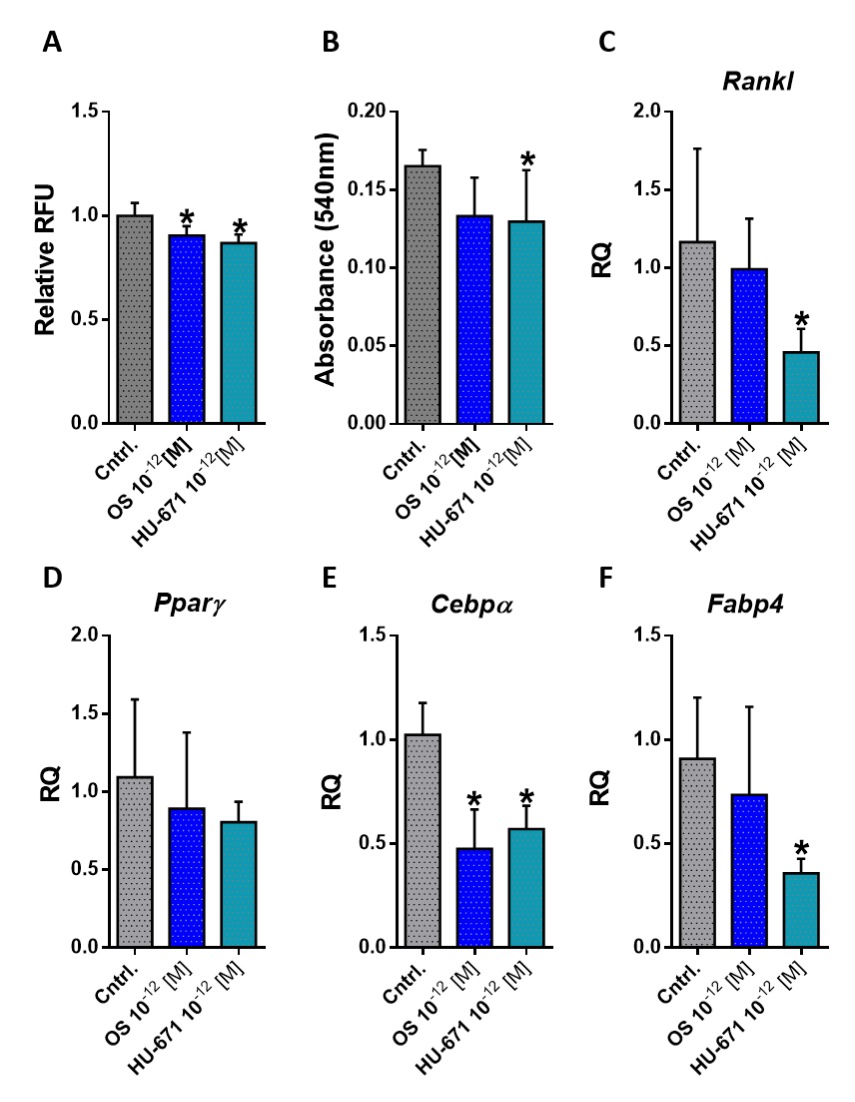
2.4. HU-671 Has Enhanced Inhibitory Effects on Osteoblast-To-Adipocyte Trans-Differentiation
3. Discussion
4. Materials and Methods
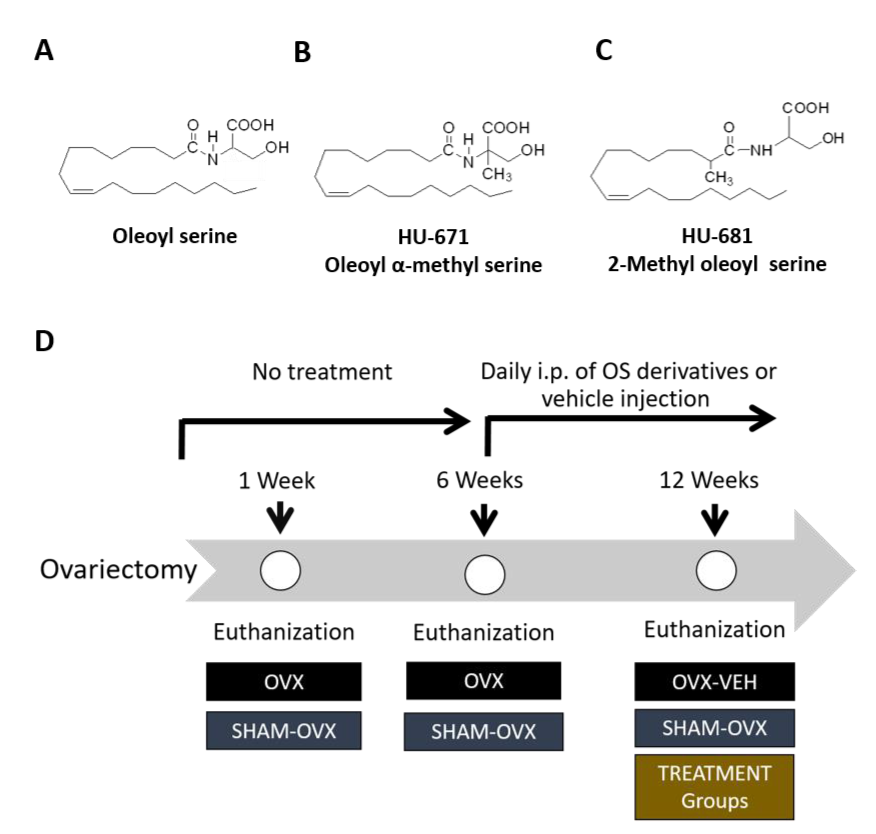
4.1. Methylated OS Derivatives
4.2. Animals and Experimental Protocol
4.3. The Effects of Methylated OS Derivatives on Bone Structure and Remodeling
4.4. Cell Culture
4.5. AdipoRed Labelling
4.6. Oil Red O Staining
4.7. Serum Markers of Bone Remodeling
4.8. Real-Time PCR
4.9. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Downey, P.A.; Siegel, M.I. Bone biology and the clinical implications for osteoporosis. Phys. Ther. 2006, 86, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.D.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. Biomed. Res. Int. 2015, 2015, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and molecular regulation of bone remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Datta, H.K.; Ng, W.F.; Walker, J.A.; Tuck, S.P.; Varanasi, S.S. The cell biology of bone metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef]
- Amling, M.; Takeda, S.; Karsenty, G. A neuro (endo)crine regulation of bone remodeling. BioEssays 2000, 22, 970–975. [Google Scholar] [CrossRef]
- Bajayo, A.; Bar, A.; Denes, A.; Bachar, M.; Kram, V.; Attar-Namdar, M.; Zallone, A.; Kovacs, K.J.; Yirmiya, R.; Bab, I. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc. Natl. Acad. Sci. USA 2012, 109, 15455–15460. [Google Scholar] [CrossRef]
- Seeman, E. Reduced bone formation and increased bone resorption: Rational targets for the treatment of osteoporosis. Osteoporos. Int. 2003, 14, 2–8. [Google Scholar] [CrossRef]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef]
- Boskey, A.L.; Coleman, R. Aging and Bone. J. Dent. Res. 2010, 89, 1333–1348. [Google Scholar] [CrossRef]
- Gabet, Y.; Bab, I. Microarchitectural changes in the aging skeleton. Curr. Osteoporos. Rep. 2011, 9, 177–183. [Google Scholar] [CrossRef]
- Bab, I.; Smoum, R.; Bradshaw, H.; Mechoulam, R. Skeletal lipidomics: Regulation of bone metabolism by fatty acid amide family. Br. J. Pharmacol. 2011, 163, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Melck, D.; Bisogno, T.; Di Marzo, V. Endocannabinoids and fatty acid amides in cancer, inflammation and related disorders. Chem. Phys. Lipids 2000, 108, 191–209. [Google Scholar] [CrossRef]
- Connor, M.; Vaughan, C.W.; Vandenberg, R.J. N-acyl amino acids and N-acyl neurotransmitter conjugates: Neuromodulators and probes for new drug targets. Br. J. Pharmacol. 2010, 160, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- De Diego, I.; Peleg, S.; Fuchs, B. The role of lipids in aging-related metabolic changes. Chem. Phys. Lipids 2019. [Google Scholar] [CrossRef]
- Hanuš, L.; Shohami, E.; Bab, I.; Mechoulam, R. N-Acyl amino acids and their impact on biological processes. BioFactors 2014, 40, 381–388. [Google Scholar] [CrossRef]
- Tam, J.; Ofek, O.; Fride, E.; Ledent, C.; Gabet, Y.; Müller, R.; Zimmer, A.; Mackie, K.; Mechoulam, R.; Shohami, E.; et al. Involvement of neuronal cannabinoid receptor CB1 in regulation of bone mass and bone remodeling. Mol. Pharmacol. 2006, 70, 786–792. [Google Scholar] [CrossRef]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E.; et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701. [Google Scholar] [CrossRef]
- Bab, I.; Ofek, O.; Tam, J.; Rehnelt, J.; Zimmer, A. Endocannabinoids and the regulation of bone metabolism. J. Neuroendocrinol. 2008, 20 (Suppl. S1), 69–74. [Google Scholar] [CrossRef]
- Deis, S.; Srivastava, R.K.; de Azua, I.R.; Bindila, L.; Baraghithy, S.; Lutz, B.; Bab, I.; Tam, J. Age-related regulation of bone formation by the sympathetic cannabinoid CB1 receptor. Bone 2018, 108, 34–42. [Google Scholar] [CrossRef]
- Zimmer, A. A collaboration investigating endocannabinoid signalling in brain and bone. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 229–235. [Google Scholar] [CrossRef]
- Tam, J.; Trembovler, V.; Di Marzo, V.; Petrosino, S.; Leo, G.; Alexandrovich, A.; Regev, E.; Casap, N.; Shteyer, A.; Ledent, C.; et al. The cannabinoid CB1 receptor regulates bone formation by modulating adrenergic signaling. FASEB J. 2008, 22, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanuš, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Carnovali, M.; Ottria, R.; Pasqualetti, S.; Banfi, G.; Ciuffreda, P.; Mariotti, M. Effects of bioactive fatty acid amide derivatives in zebrafish scale model of bone metabolism and disease. Pharmacol. Res. 2016, 104, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Smoum, R.; Bar, A.; Tan, B.; Milman, G.; Attar-Namdar, M.; Ofek, O.; Stuart, J.M.; Bajayo, A.; Tam, J.; Kram, V.; et al. Oleoyl serine, an endogenous N-acyl amide, modulates bone remodeling and mass. Proc. Natl. Acad. Sci. USA 2010, 107, 17710–17715. [Google Scholar] [CrossRef]
- Baraghithy, S.; Smoum, R.; Drori, A.; Hadar, R.; Gammal, A.; Hirsch, S.; Attar-Namdar, M.; Nemirovski, A.; Gabet, Y.; Langer, Y.; et al. Magel2 Modulates Bone Remodeling and Mass in Prader-Willi Syndrome by Affecting Oleoyl Serine Levels and Activity. J. Bone Miner. Res. 2019, 34, 93–105. [Google Scholar] [CrossRef]
- Abadji, V.; Lin, S.; Taha, G.; Griffin, G.; Stevenson, L.A.; Pertwee, R.G.; Makriyannis, A. (R)-Methanandamide: A Chiral Novel Anandamide Possessing Higher Potency and Metabolic Stability. J. Med. Chem. 1994, 37, 1889–1893. [Google Scholar] [CrossRef]
- Martin, R.B.; Zissimos, S.L. Relationships between marrow fat and bone turnover in ovariectomized and intact rats. Bone 1991, 12, 123–131. [Google Scholar] [CrossRef]
- Rosen, C.J.; Bouxsein, M.L. Mechanisms of disease: Is osteoporosis the obesity of bone? Nat. Clin. Pract. Rheumatol. 2006, 2, 35. [Google Scholar] [CrossRef]
- Gao, B.; Yang, L.; Luo, Z.-J. Transdifferentiation between bone and fat on bone metabolism. Int. J. Clin. Exp. Pathol. 2014, 7, 1834–1841. [Google Scholar]
- Tam, J.; Hinden, L.; Drori, A.; Udi, S.; Azar, S.; Baraghithy, S. The therapeutic potential of targeting the peripheral endocannabinoid/CB1 receptor system. Eur. J. Intern. Med. 2018, 49, 23–29. [Google Scholar] [CrossRef]
- Agas, D.; Marchetti, L.; Hurley, M.M.; Sabbieti, M.G. Prostaglandin F2α: A bone remodeling mediator. J. Cell. Physiol. 2013, 228, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Smoum, R.; Baraghithy, S.; Chourasia, M.; Breuer, A.; Mussai, N.; Attar-Namdar, M.; Kogan, N.M.; Raphael, B.; Bolognini, D.; Cascio, M.G.; et al. CB2 cannabinoid receptor agonist enantiomers HU-433 and HU-308: An inverse relationship between binding affinity and biological potency. Proc. Natl. Acad. Sci. USA 2015, 112, 8774–8779. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Crozier, G.; Bisogno, T.; Cavaliere, P.; Innis, S.; Di Marzo, V. Anandamide and diet: Inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets. Proc. Natl. Acad. Sci. USA 2001, 98, 6402–6406. [Google Scholar] [CrossRef] [PubMed]
- Ntambi, J.M.; Miyazaki, M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog. Lipid Res. 2004, 43, 91–104. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 2003, 2599–2608. [Google Scholar] [CrossRef]
- Fernández-Real, J.M.; Bulló, M.; Moreno-Navarrete, J.M.; Ricart, W.; Ros, E.; Estruch, R.; Salas-Salvadó, J. A mediterranean diet enriched with olive oil is associated with higher serum total osteocalcin levels in elderly men at high cardiovascular risk. J. Clin. Endocrinol. Metab. 2012, 97, 3792–3798. [Google Scholar] [CrossRef]
- Garciá-Martínez, O.; Rivas, A.; Ramos-Torrecillas, J.; De Luna-Bertos, E.; Ruiz, C. The effect of olive oil on osteoporosis prevention. Int. J. Food Sci. Nutr. 2014, 65, 834–840. [Google Scholar] [CrossRef]
- Keiler, A.M.; Zierau, O.; Bernhardt, R.; Scharnweber, D.; Lemonakis, N.; Termetzi, A.; Skaltsounis, L.; Vollmer, G.; Halabalaki, M. Impact of a functionalized olive oil extract on the uterus and the bone in a model of postmenopausal osteoporosis. Eur. J. Nutr. 2014, 53, 1073–1081. [Google Scholar] [CrossRef]
- Bradshaw, H.B.; Leishman, E. Levels of bioactive lipids in cooking oils: Olive oil is the richest source of oleoyl serine. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 247–252. [Google Scholar] [CrossRef][Green Version]
- Guan, Z.; Li, S.; Smith, D.C.; Shaw, W.A.; Raetz, C.R.H. Identification of N-acylphosphatidylserine molecules in eukaryotic cells. Biochemistry 2007, 46, 14500–14513. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bari, M.; Di Rienzo, M.; Finazzi-Agrò, A.; Rossi, A. Progesterone activates fatty acid amide hydrolase (FAAH) promoter in human T lymphocytes through the transcription factor Ikaros: Evidence for a synergistic effect of leptin. J. Biol. Chem. 2003, 278, 32726–32732. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, P.; Pucci, M.; Di Siena, S.; Di Giacomo, D.; Pirazzi, V.; Geremia, R.; MacCarrone, M. The faah gene is the first direct target of estrogen in the testis: Role of histone demethylase LSD1. Cell. Mol. Life Sci. 2012, 69, 4177–4190. [Google Scholar] [CrossRef] [PubMed]
- Gallily, R.; Yekhtin, Z.; Hanuš, L.O. Overcoming the Bell-Shaped Dose-Response of Cannabidiol by Using Extract Enriched in Cannabidiol. Pharmacol. Amp. Pharm. 2015, 6, 75–85. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Rodrigues, N.P.; Silva, A.L.; Bernardo, S.A.; Hallak, J.E.C.; Guimarães, F.S.; Crippa, J.A.S. Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front. Pharmacol. 2017, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Luce, V.; Fernandez Solari, J.; Rettori, V.; De Laurentiis, A. The inhibitory effect of anandamide on oxytocin and vasopressin secretion from neurohypophysis is mediated by nitric oxide. Regul. Pept. 2013, 188, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Darmani, N.A. The potent emetogenic effects of the endocannabinoid, 2-AG (2-arachidonoylglycerol) are blocked by delta(9)-tetrahydrocannabinol and other cannnabinoids. J. Pharmacol. Exp. Ther. 2002, 300, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Gabet, Y.; Kohavi, D.; Muller, R.; Chorev, M.; Bab, I. Intermittently administered parathyroid hormone 1-34 reverses bone loss and structural impairment in orchiectomized adult rats. Osteoporos Int. 2005, 16, 1436–1443. [Google Scholar] [CrossRef]
- Li, X.; Ominsky, M.S.; Warmington, K.S.; Niu, Q.T.; Asuncion, F.J.; Barrero, M.; Dwyer, D.; Grisanti, M.; Stolina, M.; Kostenuik, P.J.; et al. Increased bone formation and bone mass induced by sclerostin antibody is not affected by pretreatment or cotreatment with alendronate in osteopenic, ovariectomized rats. Endocrinology 2011, 152, 3312–3322. [Google Scholar] [CrossRef][Green Version]
- Alexander, J.M.; Bab, I.; Fish, S.; Muller, R.; Uchiyama, T.; Gronowicz, G.; Nahounou, M.; Zhao, Q.; White, D.W.; Chorev, M.; et al. Human parathyroid hormone 1-34 reverses bone loss in ovariectomized mice. J. Bone Min. Res. 2001, 16, 1665–1673. [Google Scholar] [CrossRef]
- Justesen, J.; Stenderup, K.; Ebbesen, E.N.; Mosekilde, L.; Steiniche, T.; Kassem, M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2001, 2, 165–171. [Google Scholar] [CrossRef]
- Kawai, M.; de Paula, F.J.; Rosen, C.J. New insights into osteoporosis: The bone-fat connection. J. Intern. Med. 2012, 272, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, P.K.; Horowitz, M.C.; MacDougald, O.A.; Scheller, E.L.; Rodeheffer, M.S.; Rosen, C.J.; Klibanski, A. Marrow fat and bone--new perspectives. J. Clin. Endocrinol. Metab. 2013, 98, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Duque, G.; Rivas, D.; Li, W.; Li, A.; Henderson, J.E.; Ferland, G.; Gaudreau, P. Age-related bone loss in the LOU/c rat model of healthy ageing. Exp. Gerontol. 2009, 44, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Cheng, Z.; Busse, C.; Pham, A.; Nakamura, M.C.; Lane, N.E. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: A longitudinal study of gene expression in bone tissue from glucocorticoid- treated mice. Arthritis Rheum. 2008, 58, 1674–1686. [Google Scholar] [CrossRef] [PubMed]
- Li, G.W.; Xu, Z.; Chen, Q.W.; Chang, S.X.; Tian, Y.N.; Fan, J.Z. The temporal characterization of marrow lipids and adipocytes in a rabbit model of glucocorticoid-induced osteoporosis. Skelet. Radiol. 2013, 42, 1235–1244. [Google Scholar] [CrossRef]
- Botolin, S.; McCabe, L.R. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology 2007, 148, 198–205. [Google Scholar] [CrossRef]
- Di Iorgi, N.; Rosol, M.; Mittelman, S.D.; Gilsanz, V. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J. Clin. Endocrinol. Metab. 2008, 93, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Wren, T.A.L.; Chung, S.A.; Dorey, F.J.; Bluml, S.; Adams, G.B.; Gilsanz, V. Bone marrow fat is inversely related to cortical bone in young and old subjects. J. Clin. Endocrinol. Metab. 2011, 96, 782–786. [Google Scholar] [CrossRef]
- Schwartz, A.V.; Sigurdsson, S.; Hue, T.F.; Lang, T.F.; Harris, T.B.; Rosen, C.J.; Vittinghoff, E.; Siggeirsdottir, K.; Sigurdsson, G.; Oskarsdottir, D.; et al. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J. Clin. Endocrinol. Metab. 2013, 98, 2294–2300. [Google Scholar] [CrossRef]
- Duque, G.; Li, W.; Vidal, C.; Bermeo, S.; Rivas, D.; Henderson, J. Pharmacological inhibition of PPARγ increases osteoblastogenesis and bone mass in male C57BL/6 mice. J. Bone Miner. Res. 2013, 28, 639–648. [Google Scholar] [CrossRef]
- Duque, G.; Li, W.; Adams, M.; Xu, S.; Phipps, R. Effects of risedronate on bone marrow adipocytes in postmenopausal women. Osteoporos. Int. 2011, 22, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Burman, P.; Ritzen, E.M.; Lindgren, A.C. Endocrine dysfunction in Prader-Willi syndrome: A review with special reference to GH. Endocr. Rev. 2001, 22, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Fournier, C.; Perrier, A.; Thomas, M.; Laroche, N.; Dumas, V.; Rattner, A.; Vico, L.; Guignandon, A. Reduction by strontium of the bone marrow adiposity in mice and repression of the adipogenic commitment of multipotent C3H10T1/2 cells. Bone 2012, 50, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Wang, L.; Wang, X.-K.; Lai, P.-L.; Huang, M.-J.; Jin, D.; Zhong, Z.-M.; Chen, J.-T.; Bai, X.-C. Risedronate inhibits bone marrow mesenchymal stem cell adipogenesis and switches RANKL/OPG ratio to impair osteoclast differentiation. J. Surg. Res. 2013, 180, e21–e29. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. Overexpression of Runx2 and MKP-1 stimulates transdifferentiation of 3T3-L1 preadipocytes into bone-forming osteoblasts in vitro. Calcif. Tissue Int. 2011, 88, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, M.; Ono, N.; Bringhurst, F.R.; Kronenberg, H.M.; Guo, J. Loss of wnt/β-catenin signaling causes cell fate shift of preosteoblasts from osteoblasts to adipocytes. J. Bone Miner. Res. 2012, 27, 2344–2358. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Astudillo, P.; Ríos, S.; Pino, A.M. Involvement of adipogenic potential of human bone marrow mesenchymal stem cells (MSCs) in osteoporosis. Curr. Stem Cell Res. Ther. 2008, 3, 208–218. [Google Scholar] [CrossRef]
- Benisch, P.; Schilling, T.; Klein-Hitpass, L.; Frey, S.P.; Seefried, L.; Raaijmakers, N.; Krug, M.; Regensburger, M.; Zeck, S.; Schinke, T.; et al. The Transcriptional Profile of Mesenchymal Stem Cell Populations in Primary Osteoporosis Is Distinct and Shows Overexpression of Osteogenic Inhibitors. PLoS ONE 2012, 7, e45142. [Google Scholar] [CrossRef]
- Berendsen, A.D.; Olsen, B.R. Osteoblast-adipocyte lineage plasticity in tissue development, maintenance and pathology. Cell. Mol. Life Sci. 2014, 71, 493–497. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Min. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Kato, M.; Patel, M.S.; Levasseur, R.; Lobov, I.; Chang, B.H.; Glass, D.A., 2nd; Hartmann, C.; Li, L.; Hwang, T.H.; Brayton, C.F.; et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J. Cell Biol. 2002, 157, 303–314. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| FORWARD | REVERSE | |
|---|---|---|
| Mus musculus Gapdh | ACCAGGGAGGGCTGCAGTCC | TCAGTTCGGAGCCCACACGC |
| Mus musculus Cebpα | CAAGAACAGCAACGAGTACCG | GTCACTGGTCAACTCCAGCAC |
| Mus musculus Fabp4 | AAGGTGAAGAGCATCATAACCCT | TCACGCCTTTCATAACACATTCC |
| Mus musculus Pparγ | TCGCTGATGCACTGCCTATG | GAGAGGTCCACAGAGCTGATT |
| Mus musculus Rankl | TCCAGCTATGATGGAAGGCT | GTACCAAGAGGACAGAGTG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baraghithy, S.; Smoum, R.; Attar-Namdar, M.; Mechoulam, R.; Bab, I.; Tam, J. HU-671, a Novel Oleoyl Serine Derivative, Exhibits Enhanced Efficacy in Reversing Ovariectomy-Induced Osteoporosis and Bone Marrow Adiposity. Molecules 2019, 24, 3719. https://doi.org/10.3390/molecules24203719
Baraghithy S, Smoum R, Attar-Namdar M, Mechoulam R, Bab I, Tam J. HU-671, a Novel Oleoyl Serine Derivative, Exhibits Enhanced Efficacy in Reversing Ovariectomy-Induced Osteoporosis and Bone Marrow Adiposity. Molecules. 2019; 24(20):3719. https://doi.org/10.3390/molecules24203719
Chicago/Turabian StyleBaraghithy, Saja, Reem Smoum, Malka Attar-Namdar, Raphael Mechoulam, Itai Bab, and Joseph Tam. 2019. "HU-671, a Novel Oleoyl Serine Derivative, Exhibits Enhanced Efficacy in Reversing Ovariectomy-Induced Osteoporosis and Bone Marrow Adiposity" Molecules 24, no. 20: 3719. https://doi.org/10.3390/molecules24203719
APA StyleBaraghithy, S., Smoum, R., Attar-Namdar, M., Mechoulam, R., Bab, I., & Tam, J. (2019). HU-671, a Novel Oleoyl Serine Derivative, Exhibits Enhanced Efficacy in Reversing Ovariectomy-Induced Osteoporosis and Bone Marrow Adiposity. Molecules, 24(20), 3719. https://doi.org/10.3390/molecules24203719







