6-Shogaol Inhibits Advanced Glycation End-Products-Induced IL-6 and ICAM-1 Expression by Regulating Oxidative Responses in Human Gingival Fibroblasts
Abstract
1. Introduction
2. Results
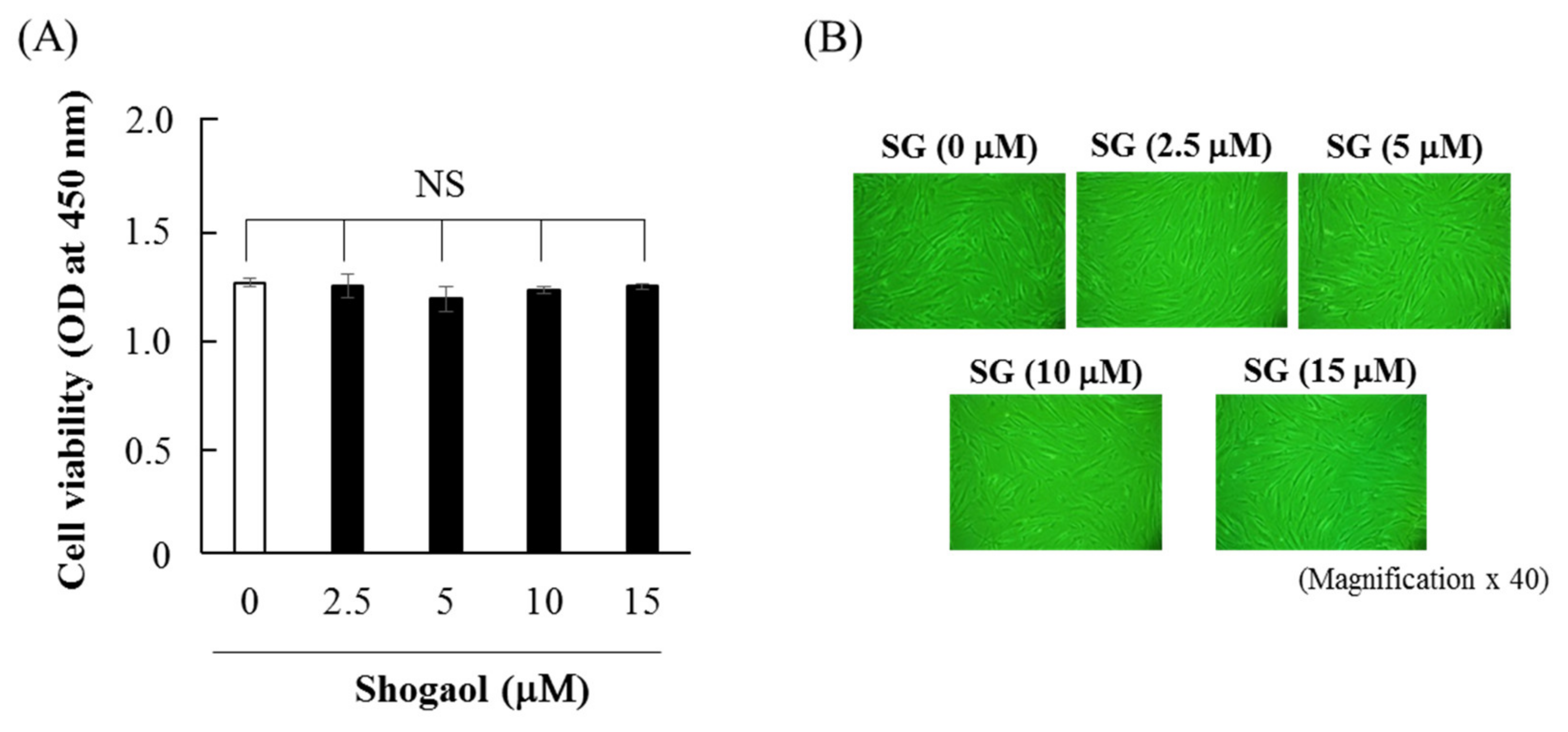
2.1. Effects of 6-shogaol on Cell Viability and Morphology of HGFs
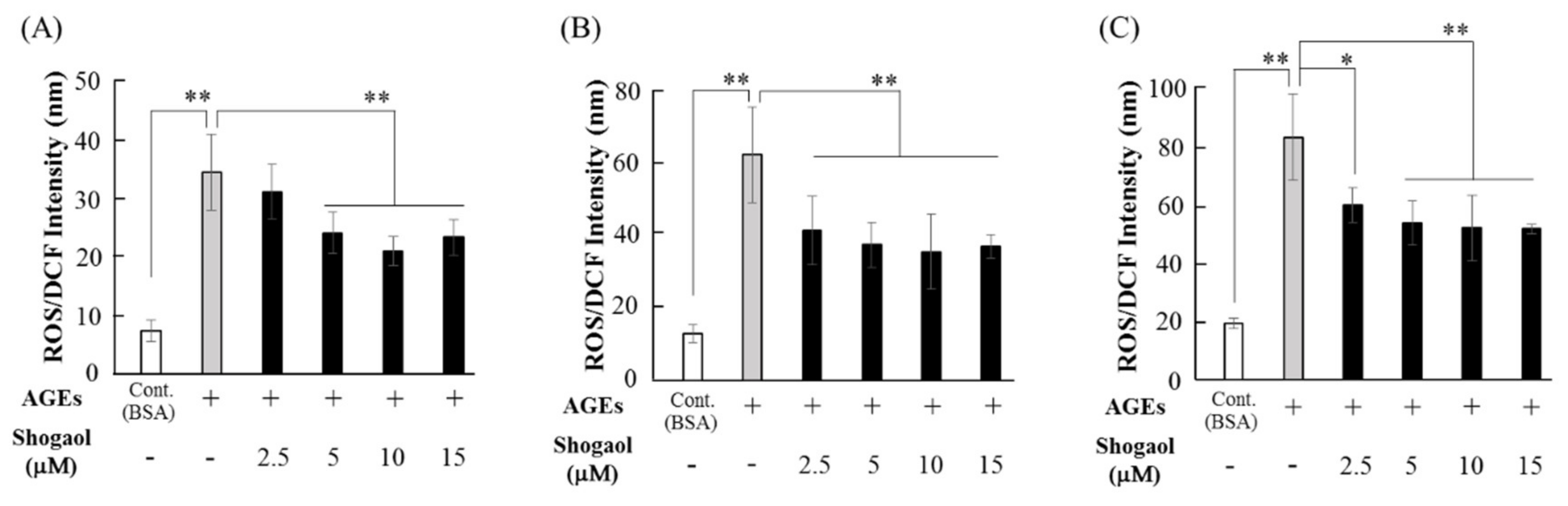
2.2. 6-Shogaol Inhibits AGEs-induced ROS Production in HGFs
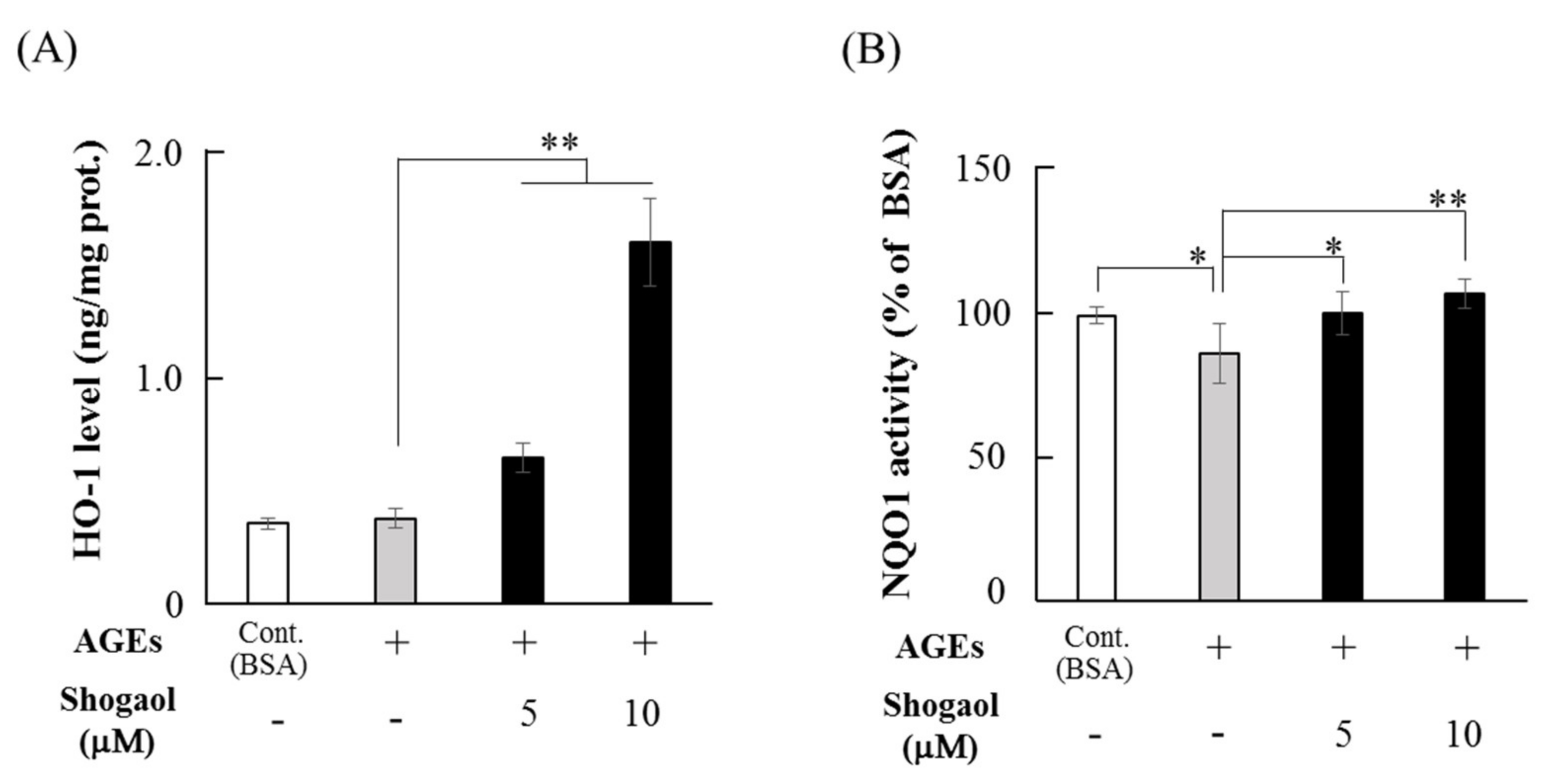
2.3. 6-Shogaol Increases the Levels of HO-1 and NQO1 in HGFs
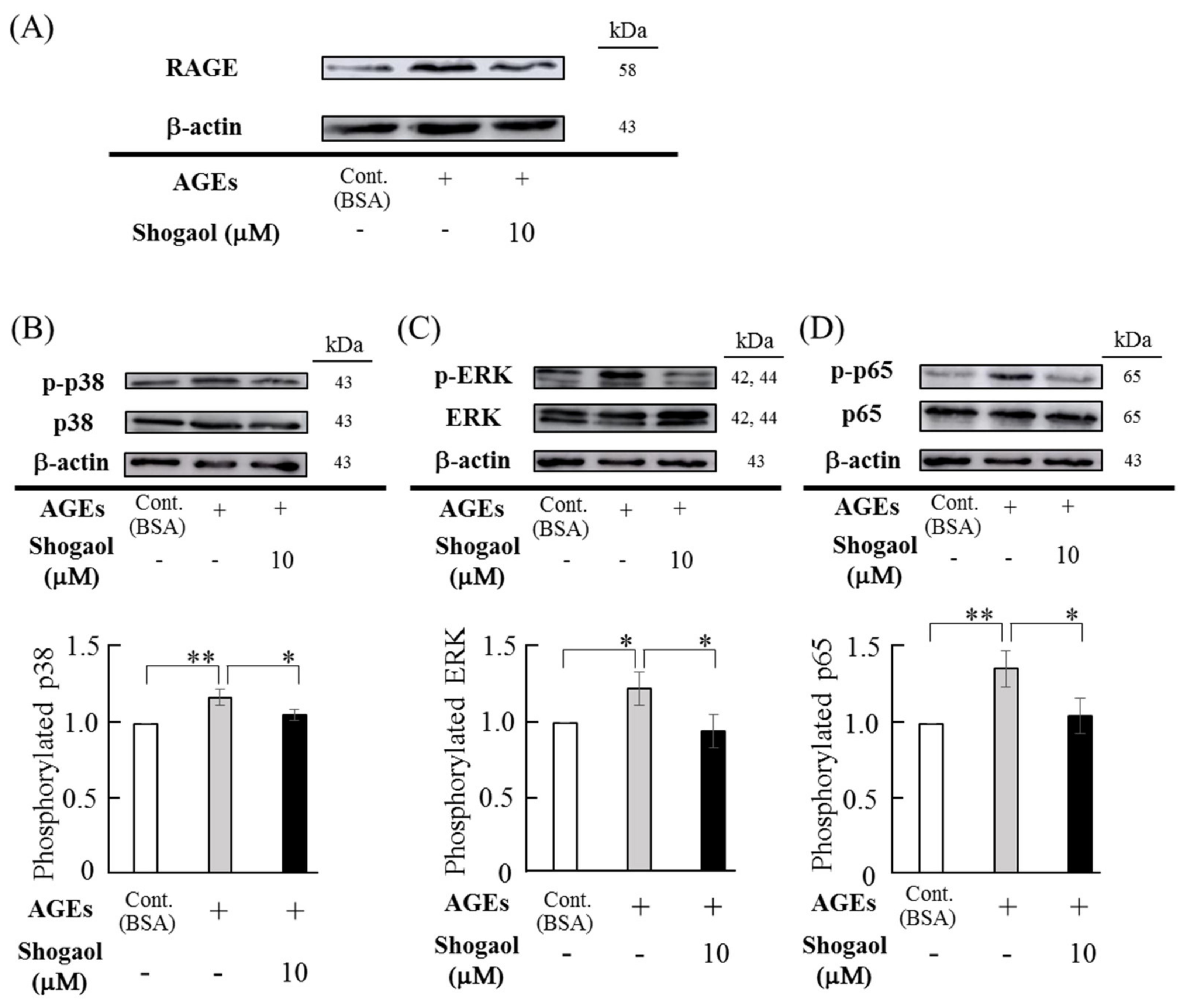
2.4. Effects of 6-Shogaol on AGEs-Induced RAGE Expression and Antivations of MAPKs and NF-κB Signaling Pathways in HGFs
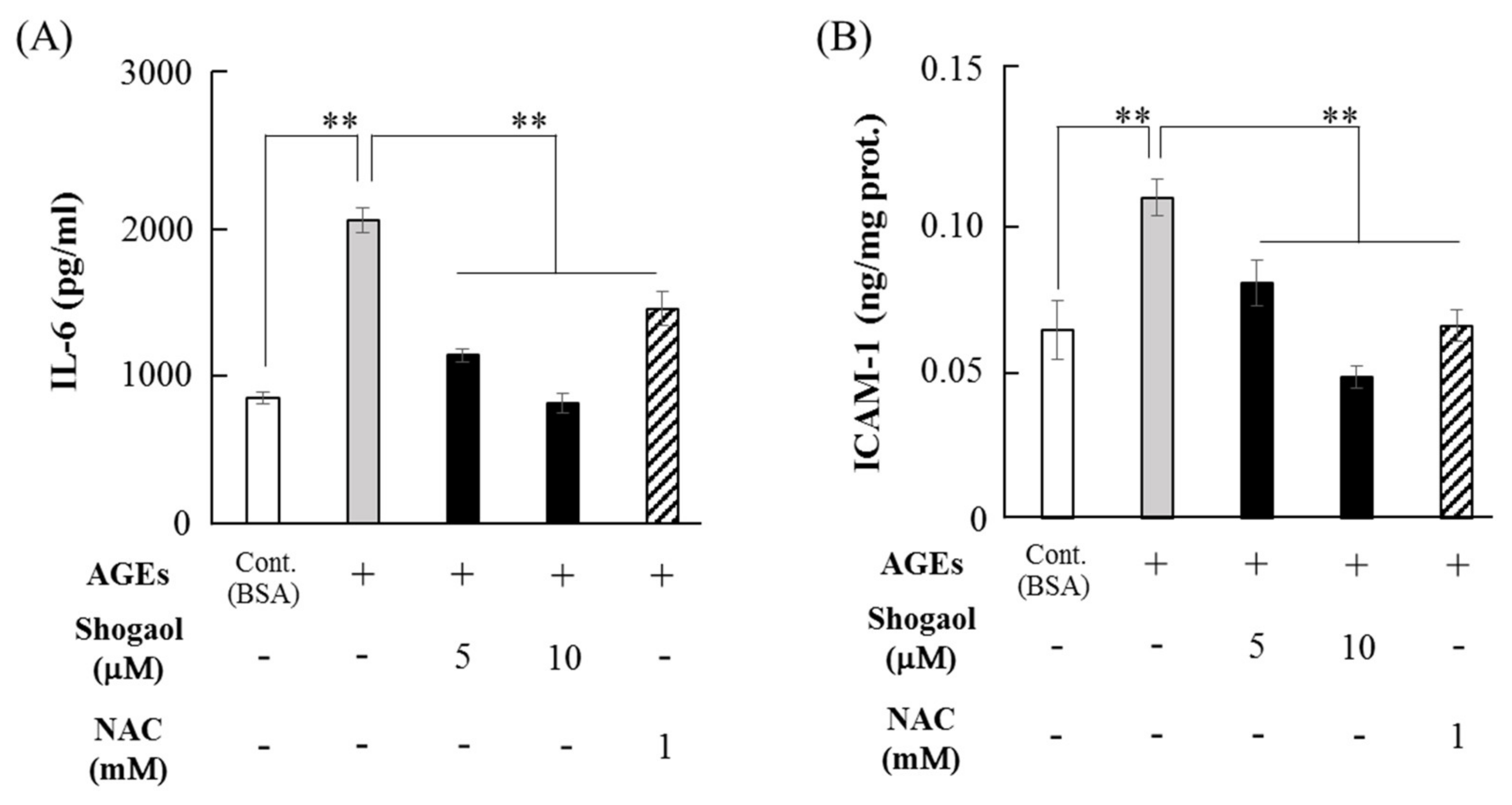
2.5. 6-Shogaol Inhibits AGEs-Induced IL-6 and ICAM-1 Expression in HGFs
3. Discussion
4. Materials and Methods
4.1. AGEs and Reagents
4.2. Cell Culture
4.3. Cell viability Assay and Observation of Cellular Morphology
4.4. ROS Measurement
4.5. HO-1 and NQO1 Measurement
4.6. Western Blotting
4.7. Enzyme-Linked Immunosorbent Assay
4.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Lakschevitz, F.; Aboodi, G.; Tenenbaum, H.; Glogauer, M. Diabetes and periodontal diseases: interplay and links. Curr. Diabetes Rev. 2011, 7, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Asadipooya, K.; Uy, E.M. Advanced glycation end products (AGEs), receptor for AGEs, diabetes, and bone: review of the literature. J. Endocr. Soc. 2019, 3, 1799–1818. [Google Scholar] [CrossRef] [PubMed]
- Goh, S.Y.; Cooper, M.E. The role of advanced glycation end products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products. Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Weidman, E.; Lalla, E.; Yan, S.D.; Hori, O.; Cao, R.; Brett, J.G.; Lamster, I.B. Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: A potential mechanism underlying accelerated periodontal disease associated with diabetes. J. Periodontal Res. 1996, 31, 508–515. [Google Scholar] [CrossRef]
- Zizzi, A.; Tirabassi, G.; Aspriello, S.D.; Piemontese, M.; Rubini, C.; Lucarini, G. Gingival advanced glycation end-products in diabetes mellitus-associated chronic periodontitis: An immunohistochemical study. J. Periodont Res. 2013, 48, 293–301. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Y.; Ye, C.; Wu, W.; Liao, G.; Lu, Y.; Huang, P. Changes in advanced glycation end products, beta-defensin-3, and interleukin-17 during diabetic periodontitis development in rhesus monkeys. Exp. Biol. Med. 2018, 243, 684–694. [Google Scholar] [CrossRef]
- Katz, J.; Bhattacharyya, I.; Farkhondeh-Kish, F.; Perez, F.M.; Caudle, R.M.; Heft, M.W. Expression of the receptor of advanced glycation end products in gingival tissues of type 2 diabetes patients with chronic periodontal disease: a study utilizing immunohistochemistry and RT-PCR. J. Clin. Periodontol. 2005, 32, 40–44. [Google Scholar] [CrossRef]
- Katz, J.; Caudle, R.M.; Bhattacharyya, I.; Stewart, C.M.; Cohen, D.M. Receptor for advanced glycation end product (RAGE) upregulation in human gingival fibroblasts incubated with nornicotine. J. Periodontol. 2005, 76, 1171–1174. [Google Scholar] [CrossRef]
- Ren, L.; Fu, Y.; Deng, Y.; Qi, L.; Jin, L. Advanced glycation end products inhibit the expression of collagens type I and III by human gingival fibroblasts. J. Periodontol. 2009, 80, 1166–1173. [Google Scholar] [CrossRef]
- Yu, S.; Li, H.; Ma, Y.; Fu, Y. Matrix metalloproteinase-1 of gingival fibroblasts influenced by advanced glycation end products (AGEs) and their association with receptor for AGEs and nuclear factor-kB in gingival connective tissue. J. Periodontol. 2012, 83, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, K.; Kajiura, Y.; Bando, M.; Sakamoto, E.; Inagaki, Y.; Lew, J.H.; Naruishi, K.; Ikuta, T.; Yoshida, K.; Kobayashi, T.; et al. Advanced glycation end-products increase IL-6 and ICAM-1 expression via RAGE, MAPK and NF-κB pathways in human gingival fibroblasts. J. Periodontal Res. 2018, 53, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Waddington, R.J.; Moseley, R.; Embery, G. Reactive oxygen species: a potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000, 6, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Wada, S.; Narimiya, T.; Yamaguchi, Y.; Katsumata, Y.; Itohiya, K.; Fukaya, S.; Miyamoto, Y.; Nakamura, Y. Pathways that regulate ROS scavenging enzymes, and their role in defense against tissue destruction in periodontitis. Front Physiol. 2017, 8, 351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Andrukhov, O.; Rausch-Fan, X. Oxidative Stress and Antioxidant System in Periodontitis. Front. Physiol. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, S.; Suzuki, G.; Kusunoki, Y.; Yamane, K.; Egusa, G.; Kohno, N. Increasing of oxidative stress from mitochondria in type 2 diabetic patients. Diabetes Metab. Res. Rev. 2004, 20, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Sabbahy, M.El.; Vaidya, V.S. Ischemic kidney injury and mechanisms of tissue repair. Wiley Interdiscip. Rev. Syst. Biol. Med. 2011, 3, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative stress and cardiovascular disease: new insights. Kardiol. Pol. 2018, 76, 713–722. [Google Scholar] [CrossRef]
- Chapple, I.Ll.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000 2007, 43, 160–232. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Yang, H.W.; Wang, X.G.; Zhang, H. Hepatocyte growth factor prevents advanced glycation end products-induced injury and oxidative stress through a PI3K/Akt-dependent pathway in human endothelial cells. Life Sci. 2009, 85, 670–677. [Google Scholar] [CrossRef]
- Dobi, A.; Bravo, S.B.; Veeren, B.; Paradela-Dobarro, B.; Alvarez, E.; Meilhac, O.; Viranaicken, W.; Baret, P.; Devin, A.; Rondeau, P. Advanced glycation end-products disrupt human endothelial cells redox homeostasis: new insights into reactive oxygen species production. Free Radic. Res. 2019, 53, 150–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Wu, W.J.; Zhou, Q.; Jie, J.P.; Chen, X.; Wang, F.; Gong, X.H. Advanced glycation end-products induce oxidative stress through the Sirt1/Nrf2 axis by interacting with the receptor of AGEs under diabetic conditions. J. Cell. Biochem. 2019, 120, 2129–2170. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.S.; Patil, V.P.; Gokhale, N.; Acharya, A.; Kangokar, P. Chronic periodontitis in type 2 diabetes mellitus: oxidative stress as a common factor in periodontal tissue injury. J. Clin. Diagn. Res. 2016, 10, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.B.; Wright, H.J.; Roberts, A.; Cooper, P.R.; Chapple, I.L. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clin. Exp. Immunol. 2007, 147, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Damgaard, C.; Kantarci, A.; Holmstrup, P.; Hasturk, H.; Nielsen, C.H.; Van Dyke, T.E. Porphyromonas gingivalis-induced production of reactive oxygen species, tumor necrosis factor-α, interleukin-6, CXCL8 and CCL2 by neutrophils from localized aggressive periodontitis and healthy donors: modulating actions of red blood cells and resolvin E1. J. Periodontal Res. 2017, 52, 246–254. [Google Scholar] [CrossRef]
- Fuchs, B.; Schiller, J. Glycosaminoglycan degradation by selected reactive oxygen species. Antioxid Redox Signal 2014, 21, 1044–1062. [Google Scholar] [CrossRef]
- Bax, B.E.; Alam, A.S.; Banerji, B.; Bax, C.M.; Bevis, P.J.; Stevens, C.R.; Moonga, B.S.; Blake, D.R.; Zaidi, M. Stimulation of osteoclastic bone resorption by hydrogen peroxide. Biochem. Biophys. Res. Commun. 1992, 183, 1153–1158. [Google Scholar] [CrossRef]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharm. 2012, 3, 119. [Google Scholar] [CrossRef]
- Atia, A.; Alrawaiq, N.; Abdullah, A. A Review of NAD(P)H: quinone oxidoreductase 1(NQO1); A multifunctional antioxidant enzyme. J. App. Pharm. Sci. 2014, 4, 118–122. [Google Scholar]
- Bhattarai, G.; Poudel, S.B.; Kook, S.H.; Lee, J.C. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta. Biomater. 2016, 29, 398–408. [Google Scholar] [CrossRef]
- He, M.; Siow, R.C.; Sugden, D.; Gao, L.; Cheng, X.; Mann, G.E. Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetes. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Shukla, Y.; Singh, M. Cancer preventive properties of ginger: a brief review. Food Chem. Toxicol. 2007, 45, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Yang, H.; Tan, S.H.; Chui, W.K.; Chew, E.H. 6-Shogaol, an active constituent of ginger, inhibits breast cancer cell invasion by reducing matrix metalloproteinase-9 expression via blockade of nuclear factor-κB activation. Br. J. Pharm. 2010, 161, 1763–1777. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Gu, M.Y.; Xu, J.L.; Zhang, L.J.; Ryu, S.Y.; Yang, H.O. Anti-neuroinflammatory effects of 12-dehydrogingerdione in LPS-activated microglia through inhibiting Akt/IKK/NF-κB pathway and activating Nrf-2/HO-1 pathway. Biomol. Ther. 2019, 27, 92. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Kim, J.K.; Jang, H.D. 6-shogaol attenuates H2O2-induced oxidative stress via upregulation of Nrf2-mediated γ-glutamylcysteine synthetase and heme oxygenase expression in HepG2 cells. Food Sci. Biotechnol. 2016, 25, 319–327. [Google Scholar] [CrossRef]
- Bak, M.J.; Ok, S.; Jun, M.; Jeong, W.S. 6-Shogaol-rich extract from ginger up-regulates the antioxidant defense systems in cells and mice. Molecules 2012, 17, 8037–8055. [Google Scholar] [CrossRef]
- Yi, J.K.; Ryoo, Z.Y.; Ha, J.J.; Oh, D.Y.; Kim, M.O.; Kim, S.H. Beneficial effects of 6-shogaol on hyperglycemia, islet morphology and apoptosis in some tissues of streptozotocin-induced diabetic mice. Diabetol. Metab. Syndr. 2019, 11, 15. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, L.; Chen, X.; Li, Y.; Qin, Y.; Meng, X.; Zhang, Q. 6-Shogaol ameliorates diabetic nephropathy through anti-inflammatory, hyperlipidemic, anti-oxidative activity in db/db mice. Biomed. Pharm. 2018, 97, 633–641. [Google Scholar] [CrossRef]
- Li, Y.; Tran, V.H.; Duke, C.C.; Roufogalis, B.D. Preventive and protective properties of Zingiber officinale (Ginger) in diabetes mellitus, diabetic complications, and associated lipid and other metabolic disorders: A brief review. Evid Based Complement. Alternat. Med. 2012, 516870. [Google Scholar]
- Saraswat, M.; Suryanarayana, P.; Reddy, P.Y.; Patil, M.A.; Balakrishna, N.; Reddy, G.B. Antiglycating potential of Zingiber officinalis and delay of diabetic cataract in rats. Mol. Vis. 2010, 16, 1525–1537. [Google Scholar] [PubMed]
- Zhu, Y.; Zhao, Y.; Wang, P.; Ahmedna, M.; Sang, S. Bioactive ginger constituents alleviate protein glycation by trapping methylglyoxal. Chem. Res. Toxicol. 2015, 9, 9–1849. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, P.; Zhu, Y.; Lv, L.; Sang, S. Additive capacity of [6]-shogaol and epicatechin to trap methylglyoxal. J. Agric. Food Chem. 2017, 65, 8356–8362. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Hong, Y.J.; Yao, Y.H.; Huang, X.M.; Liu, X.B.; Zhang, C.Y.; Zhang, L.; Xu, X.L. 6-Shogaol protects against oxidized LDL-induced endothelial injuries by inhibiting oxidized LDL-evoked LOX-1 signaling. Evid Based Complement. Alternat. Med. 2013, 503521. [Google Scholar]
- Park, G.; Oh, D.S.; Lee, M.G.; Lee, C.E.; Kim, Y.U. 6-Shogaol, an active compound of ginger, alleviates allergic dermatitis-like skin lesions via cytokine inhibition by activating the Nrf2 pathway. Toxicol. Appl. Pharm. 2016, 310, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ramlagan, P.; Rondeau, P.; Planesse, C.; Neergheen-Bhujun, V.S.; Bourdon, E.; Bahorun, T. Comparative suppressing effects of black and green teas on the formation of advanced glycation end products (AGEs) and AGE-induced oxidative stress. Food Funct. 2017, 8, 4194–4209. [Google Scholar] [CrossRef]
- Zhang, M.H.; Feng, L.; Zhu, M.M.; Gu, J.F.; Jiang, J.; Cheng, X.D.; Ding, S.M.; Wu, C.; Jia, X.B. The anti-inflammation effect of Moutan Cortex on advanced glycation end products-induced rat mesangial cells dysfunction and High-glucose-fat diet and streptozotocin-induced diabetic nephropathy rats. J. Ethnopharmacol. 2014, 151, 591–600. [Google Scholar] [CrossRef]
- Yan, H.D.; Li, X.Z.; Xie, J.; Li, M. Effects of advanced glycation end products on renal fibrosis and oxidative stress in cultured NRK-49F cells. Chin. Med. J. (Engl) 2007, 120, 787–793. [Google Scholar] [CrossRef]
- Sampath, C.; Zhu, Y.; Sang, S.; Ahmedna, M. Bioactive compounds isolated from apple, tea, and ginger protect against dicarbonyl induced stress in cultured human retinal epithelial cells. Phytomedicine 2016, 23, 200–213. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, C.; Bae, H.; Lee, J.H.; Baek, S.H.; Nam, D.; Chung, W.S.; Shim, B.S.; Lee, S.G.; Kim, S.H.; et al. 6-Shogaol exerts anti-proliferative and pro-apoptotic effects through the modulation of STAT3 and MAPKs signaling pathways. Mol. Carcinog. 2015, 54, 1132–1146. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fu, J.; Chen, H.; Hu, Y.; Soroka, D.N.; Prigge, J.R.; Schmidt, E.E.; Yan, F.; Major, M.B.; Chen, X.; et al. Ginger compound [6]-shogaol and its cysteine-conjugated metabolite (M2) activate Nrf2 in colon epithelial cells in vitro and in vivo. Chem. Res. Toxicol. 1575. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Yu, X.; Yang, H.; Wu, X. Advanced glycation ends products induce human corneal epithelial cells apoptosis through generation of reactive oxygen species and activation of JNK and p38 MAPK pathways. PLoS ONE 2013, 8, e66781. [Google Scholar] [CrossRef] [PubMed]
- Rhode, J.; Fogoros, S.; Zick, S.; Wahl, H.; Griffith, K.A.; Huang, J.; Liu, J.R. Ginger inhibits cell growth and modulates angiogenic factors in ovarian cancer cells. BMC Complement. Altern Med. 2007, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; McGrath, K.C.; Nammi, S.; Heather, A.K.; Roufogalis, B.D. Attenuation of liver pro-inflammatory responses by Zingiber officinale via inhibition of NF-kappa B activation in high-fat diet-fed rats. Basic Clin. Pharm. Toxicol 2012, 110, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.S.; Simon, O.; Shelly, J.; Gardener, M. 6-Shogaol reduced chronic inflammatory response in the knees of rats treated with complete Freund’s adjuvant. BMC Pharm. 2006, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, C.; Liu, D.; Han, M.K.; Wang, L.; Merlin, D. Oral delivery of nanoparticles loaded with ginger active compound, 6-shogaol, attenuates ulcerative colitis and promotes wound healing in a murine model of ulcerative colitis. J. Crohns Colitis 2018, 12, 217–229. [Google Scholar] [CrossRef]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Timmermann, B.N. Comparative effects of two gingerol-containing Zingiber officinale extracts on experimental rheumatoid arthritis. J. Nat. Prod. 2009, 72, 403–407. [Google Scholar] [CrossRef]
- Mahyari, S.; Mahyari, B.; Emami, S.A.; Malaekeh-Nikouei, B.; Jahanbakhsh, S.P.; Sahebkar, A.; Mohammadpour, A.H. Evaluation of the efficacy of a polyherbal mouthwash containing Zingiber officinale, Rosmarinus officinalis and Calendula officinalis extracts in patients with gingivitis: A randomized double-blind placebo-controlled trial. Complement. Clin. Pr. 2016, 22, 93–98. [Google Scholar] [CrossRef]
- Park, M.; Bae, J.; Lee, D.S. Antibacterial activity of [10]-gingerol and [12]-gingerol isolated from ginger rhizome against periodontal bacteria. Phytother. Res. 2008, 22, 1446–1449. [Google Scholar] [CrossRef]
- Takeuchi, M.; Makita, Z.; Bucala, R.; Suzuki, T.; Koike, T.; Kameda, Y. Immunological evidence that non-carboxymethyllysine advanced glycation end-products are produced from short chain sugars and dicarbonyl compounds in vivo. Mol. Med. 2000, 6, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Huang, J.; Xie, X.; Wang, S.; Cheng, C.; Shen, X.; Liu, P.; Huang, H. Sirt1 resists advanced glycation end products-induced expressions of fibronectin and TGF-β1 by activating the Nrf2/ARE pathway in glomerular mesangial cells. Free Radic. Biol. Med. 2013, 65, 528–540. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nonaka, K.; Bando, M.; Sakamoto, E.; Inagaki, Y.; Naruishi, K.; Yumoto, H.; Kido, J.-i. 6-Shogaol Inhibits Advanced Glycation End-Products-Induced IL-6 and ICAM-1 Expression by Regulating Oxidative Responses in Human Gingival Fibroblasts. Molecules 2019, 24, 3705. https://doi.org/10.3390/molecules24203705
Nonaka K, Bando M, Sakamoto E, Inagaki Y, Naruishi K, Yumoto H, Kido J-i. 6-Shogaol Inhibits Advanced Glycation End-Products-Induced IL-6 and ICAM-1 Expression by Regulating Oxidative Responses in Human Gingival Fibroblasts. Molecules. 2019; 24(20):3705. https://doi.org/10.3390/molecules24203705
Chicago/Turabian StyleNonaka, Kohei, Mika Bando, Eijiro Sakamoto, Yuji Inagaki, Koji Naruishi, Hiromichi Yumoto, and Jun-ichi Kido. 2019. "6-Shogaol Inhibits Advanced Glycation End-Products-Induced IL-6 and ICAM-1 Expression by Regulating Oxidative Responses in Human Gingival Fibroblasts" Molecules 24, no. 20: 3705. https://doi.org/10.3390/molecules24203705
APA StyleNonaka, K., Bando, M., Sakamoto, E., Inagaki, Y., Naruishi, K., Yumoto, H., & Kido, J.-i. (2019). 6-Shogaol Inhibits Advanced Glycation End-Products-Induced IL-6 and ICAM-1 Expression by Regulating Oxidative Responses in Human Gingival Fibroblasts. Molecules, 24(20), 3705. https://doi.org/10.3390/molecules24203705





