Unambiguous Characterization of p-Cresyl Sulfate, a Protein-Bound Uremic Toxin, as Biomarker of Heart and Kidney Disease
Abstract
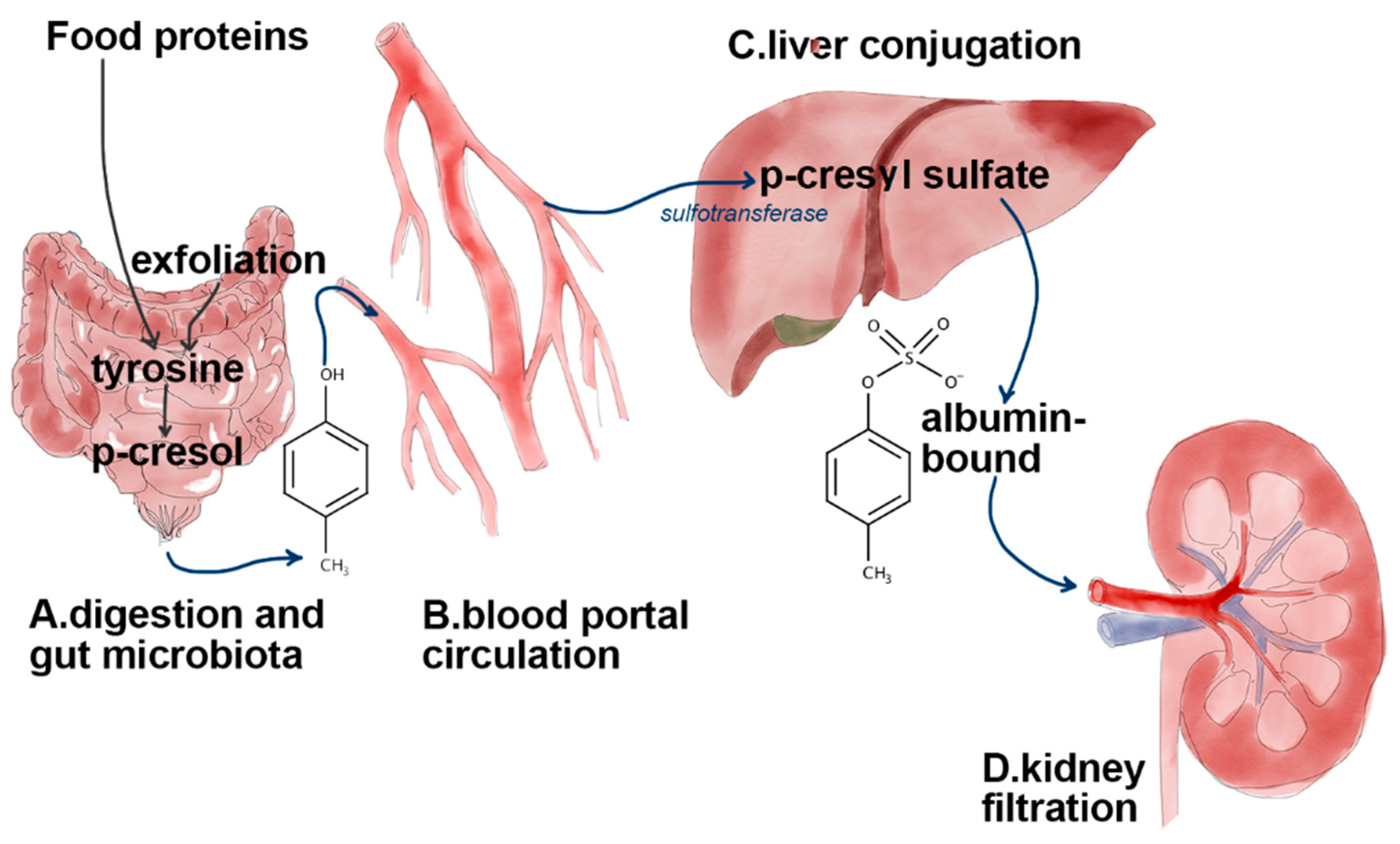
1. Introduction
2. Results and Discussion
2.1. Compound Preparation
2.2. Compound Characterization
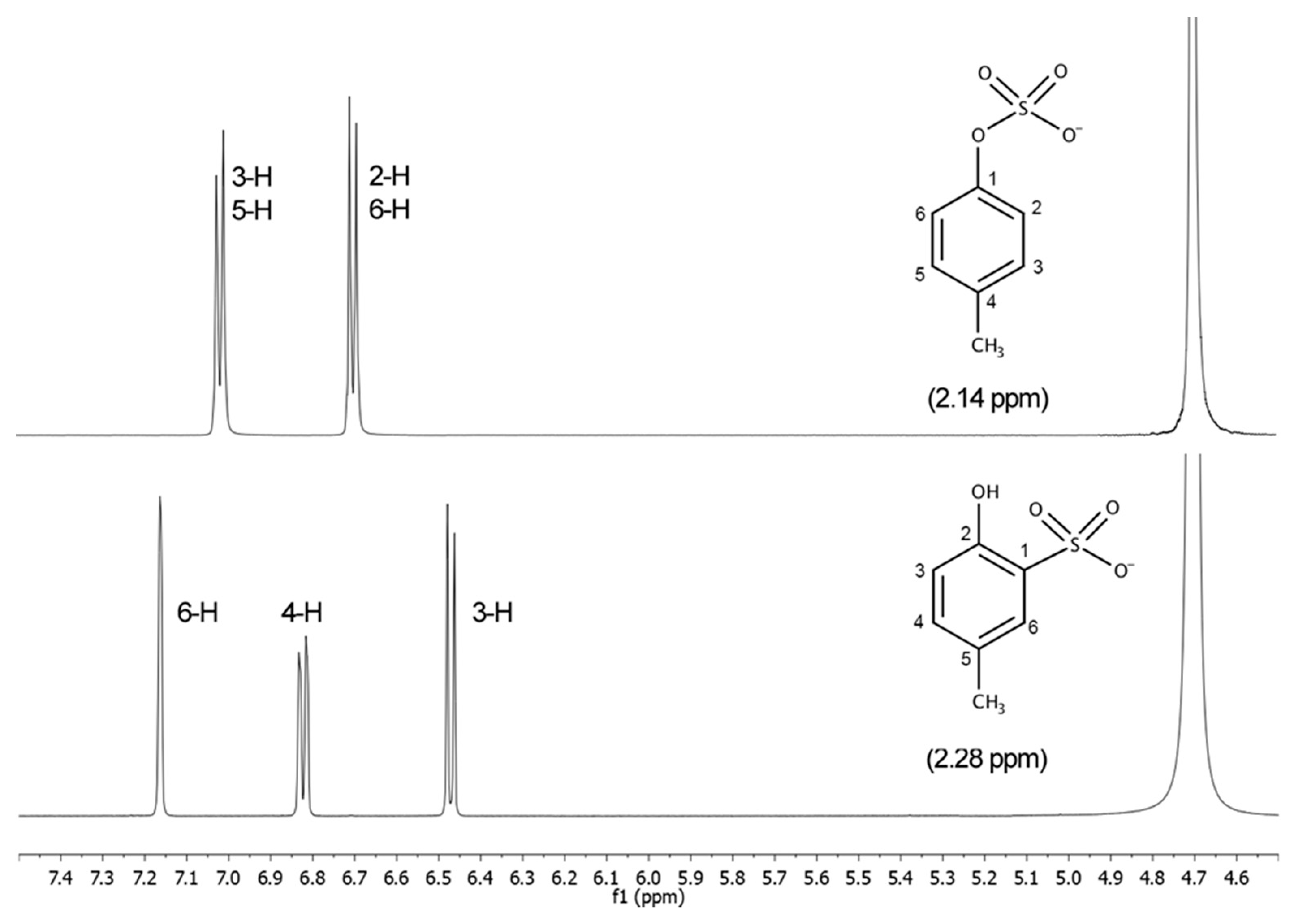
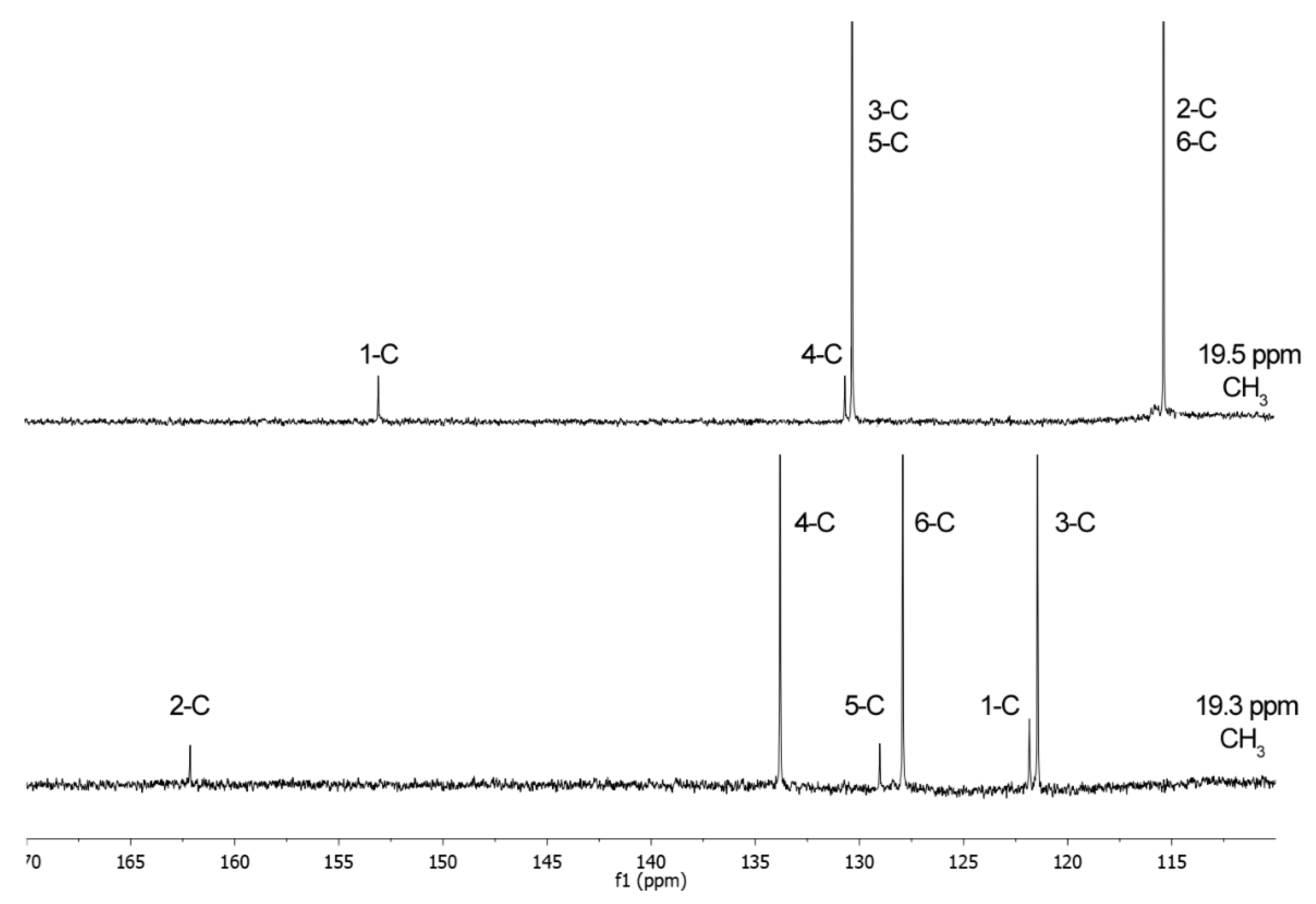
2.2.1. NMR Analysis
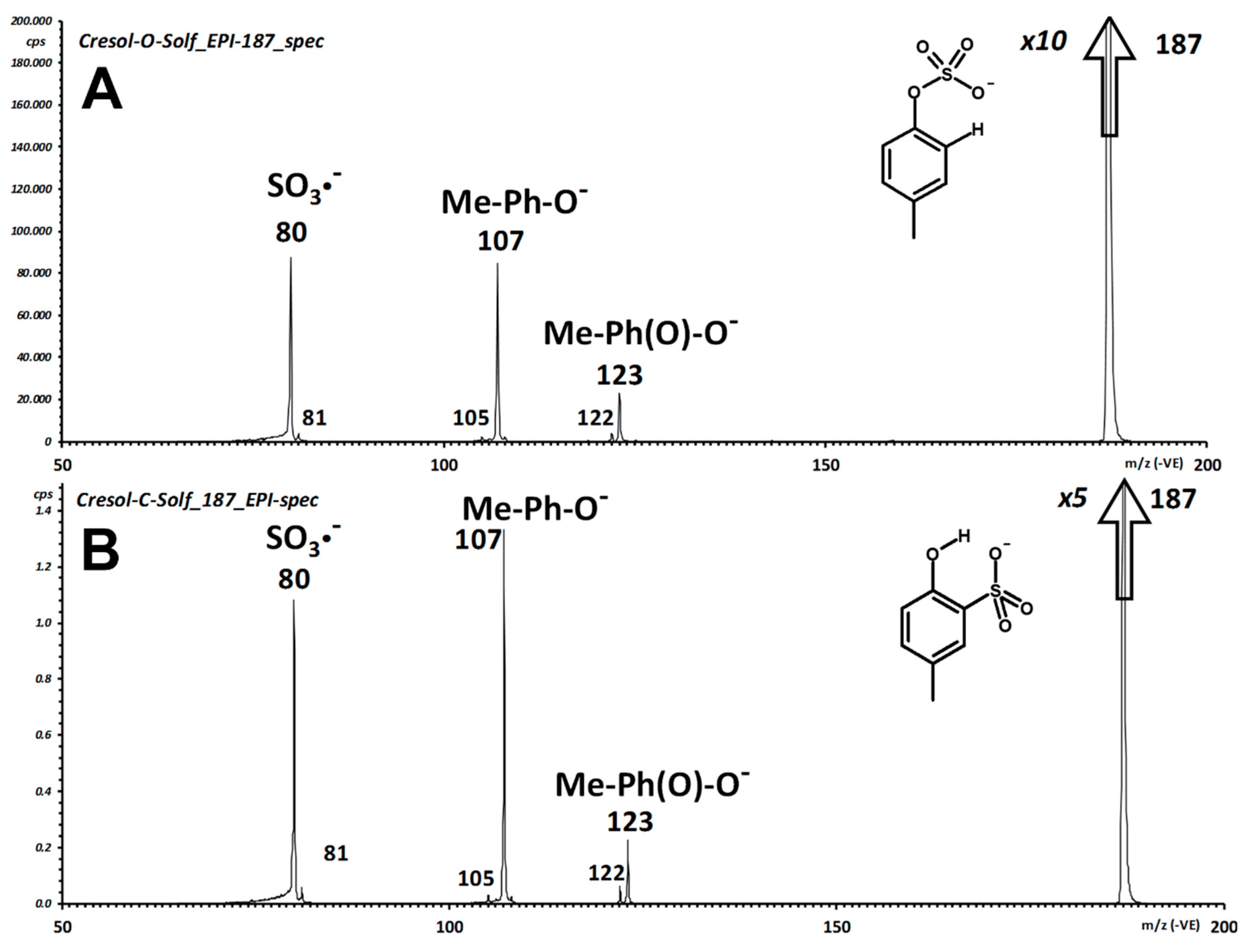
2.2.2. Mass Spectrometry
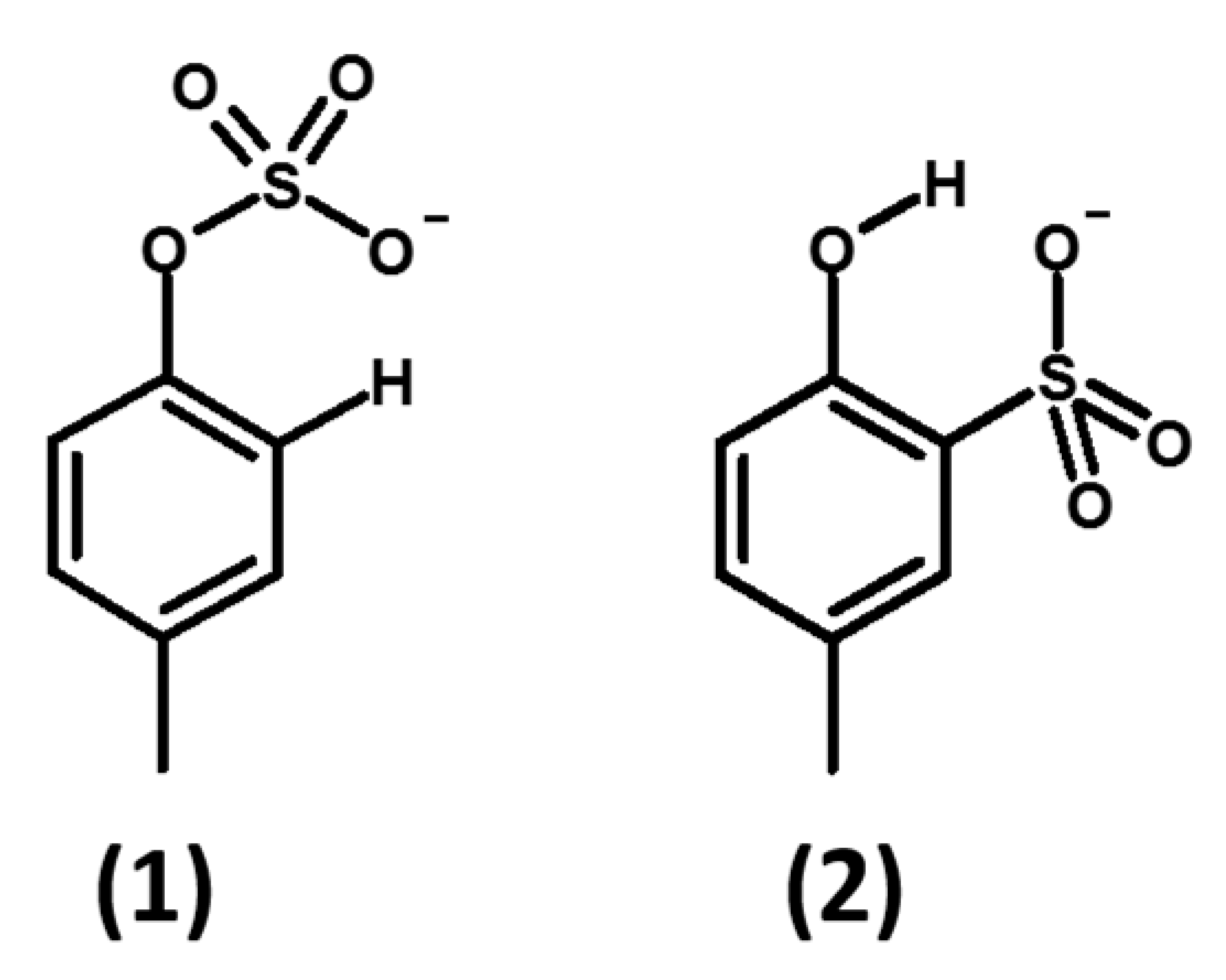
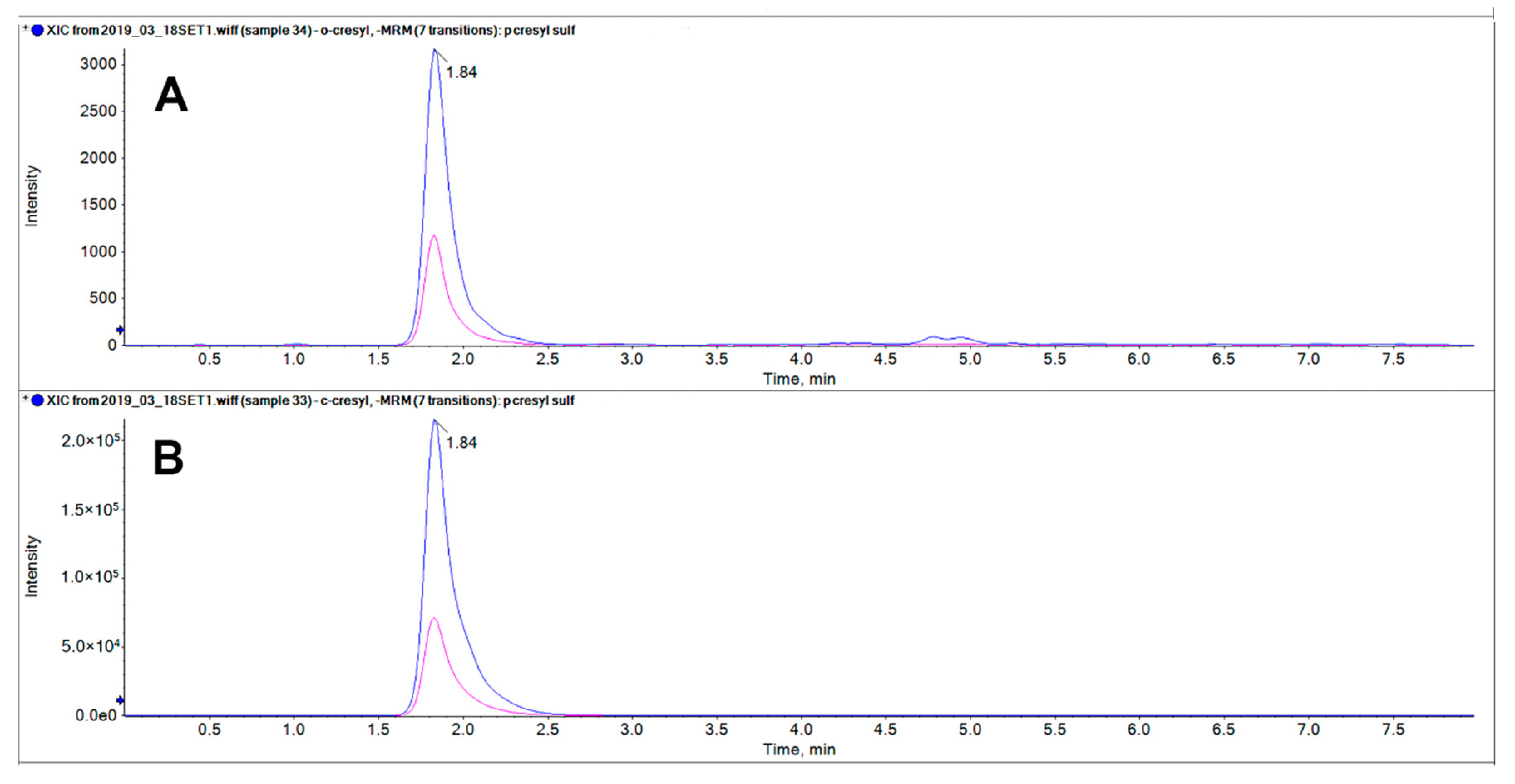
2.3. Studies for Separation of pCS (1) and 2-Hydroxy-5-Methylbenzenesulfonic Acid (2) by LC-MS/MS
3. Material and Methods
3.1. Chemistry
3.2. Synthesis of p-Cresyl Sulfate (1)
3.3. 2-Hydroxy-5-methylbenzenesulfonic Acid (2) Formation
3.3.1. General Procedure
3.3.2. 2-Hydroxy-5-methylbenzenesulfonic Acid (2) Formation Using Different Bases
3.4. NMR Analysis
3.4.1. p-Cresyl Sulfate (pCS, 1)
3.4.2. 2-Hydroxy-5-methylbenzenesulfonic Acid (2)
3.5. Mass Spectrometry
3.6. LC-MS/MS Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Meert, N.; Glorieux, G.; Schepers, E.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transplant. 2010, 25, 1183–1191. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Serum Indoxyl Sulfate Is Associated with Vascular Disease and Mortality in Chronic Kidney Disease Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef]
- Lin, C.-J.; Chen, H.-H.; Pan, C.-F.; Chuang, C.-K.; Wang, T.-J.; Sun, F.-J.; Wu, C.-J. p-cresylsulfate and indoxyl sulfate level at different stages of chronic kidney disease. J. Clin. Lab. Anal. 2011, 25, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-T.; Shu, K.-H.; Cheng, C.-H.; Wu, M.-J.; Yu, T.-M.; Chuang, Y.-W.; Chen, C.-H. Serum Total p-Cresol and Indoxyl Sulfate Correlated With Stage of Chronic Kidney Disease in Renal Transplant Recipients. Transplant. Proc. 2012, 44, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Gryp, T.; Vanholder, R.; Vaneechoutte, M.; Glorieux, G. p-Cresyl Sulfate. Toxins 2017, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, S.; Sacco, R.; Altieri, L.; Neri, C.; Urbani, A.; Bravaccio, C.; Riccio, M.P.; Iovene, M.R.; Bombace, F.; De Magistris, L.; et al. Slow intestinal transit contributes to elevate urinary p-cresol level in Italian autistic children. Autism Res. 2016, 9, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Cheng, M.-L.; Liu, M.-H.; Shiao, M.-S.; Hsu, K.-H.; Huang, Y.-Y.; Lin, C.-C.; Lin, J.-F. Increased p-cresyl sulfate level is independently associated with poor outcomes in patients with heart failure. Heart Vessels 2016, 31, 1100–1108. [Google Scholar] [CrossRef]
- Stone, R.W.; Machamer, H.E. Production of p-cresol and phenol from tyrosine by marine mud cultures. J. Bacteriol. 1947, 54, 39. [Google Scholar]
- Passmore, I.J.; Letertre, M.P.M.; Preston, M.D.; Bianconi, I.; Harrison, M.A.; Nasher, F.; Kaur, H.; Hong, H.A.; Baines, S.D.; Cutting, S.M.; et al. Para-cresol production by Clostridium difficile affects microbial diversity and membrane integrity of Gram-negative bacteria. PLOS Pathog. 2018, 14, e1007191. [Google Scholar] [CrossRef]
- Rajakovich, L.J.; Balskus, E.P. Metabolic functions of the human gut microbiota: The role of metalloenzymes. Nat. Prod. Rep. 2019, 36, 593–625. [Google Scholar] [CrossRef]
- Broderick, J.B.; Duffus, B.R.; Duschene, K.S.; Shepard, E.M. Radical S-Adenosylmethionine Enzymes. Chem. Rev. 2014, 114, 4229–4317. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and Pathologic Concentrations of Uremic Toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.W.; Hostetter, T.H. Uremic solutes from colon microbes. Kidney Int. 2012, 81, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Yoshida, M. Protein-Bound Uremic Toxins: New Culprits of Cardiovascular Events in Chronic Kidney Disease Patients. Toxins 2014, 6, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Viaene, L.; Annaert, P.; de Loor, H.; Poesen, R.; Evenepoel, P.; Meijers, B. Albumin is the main plasma binding protein for indoxyl sulfate and p-cresyl sulfate. Biopharm. Drug Dispos. 2013, 34, 165–175. [Google Scholar] [CrossRef]
- Pretorius, C.J.; McWhinney, B.C.; Sipinkoski, B.; Johnson, L.A.; Rossi, M.; Campbell, K.L.; Ungerer, J.P.J. Reference ranges and biological variation of free and total serum indoxyl- and p-cresyl sulphate measured with a rapid UPLC fluorescence detection method. Clin. Chim. Acta 2013, 419, 122–126. [Google Scholar] [CrossRef]
- Schepers, E.; Meert, N.; Glorieux, G.; Goeman, J.; Van der Eycken, J.; Vanholder, R. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol. Dial. Transplant. 2006, 22, 592–596. [Google Scholar] [CrossRef]
- de Loor, H.; Meijers, B.K.I.; Meyer, T.W.; Bammens, B.; Verbeke, K.; Dehaen, W.; Evenepoel, P. Sodium octanoate to reverse indoxyl sulfate and p-cresyl sulfate albumin binding in uremic and normal serum during sample preparation followed by fluorescence liquid chromatography. J. Chromatogr. A 2009, 1216, 4684–4688. [Google Scholar] [CrossRef]
- Cuoghi, A.; Caiazzo, M.; Bellei, E.; Monari, E.; Bergamini, S.; Palladino, G.; Ozben, T.; Tomasi, A. Quantification of p-cresol sulphate in human plasma by selected reaction monitoring. Anal. Bioanal. Chem. 2012, 404, 2097–2104. [Google Scholar] [CrossRef]
- Watanabe, H.; Miyamoto, Y.; Enoki, Y.; Ishima, Y.; Kadowaki, D.; Kotani, S.; Nakajima, M.; Tanaka, M.; Matsushita, K.; Mori, Y.; et al. p -Cresyl sulfate, a uremic toxin, causes vascular endothelial and smooth muscle cell damages by inducing oxidative stress. Pharmacol. Res. Perspect. 2015, 3, e00092. [Google Scholar] [CrossRef]
- Watanabe, H.; Miyamoto, Y.; Honda, D.; Tanaka, H.; Wu, Q.; Endo, M.; Noguchi, T.; Kadowaki, D.; Ishima, Y.; Kotani, S.; et al. p-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int. 2013, 83, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Noguchi, T.; Miyamoto, Y.; Kadowaki, D.; Kotani, S.; Nakajima, M.; Miyamura, S.; Ishima, Y.; Otagiri, M.; Maruyama, T. Interaction between Two Sulfate-Conjugated Uremic Toxins, p-Cresyl Sulfate and Indoxyl Sulfate, during Binding with Human Serum Albumin. Drug Metab. Dispos. 2012, 40, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Feigenbaum, J.; Neuberg, C.A. Simplified Method for the Preparation of Aromatic Sulfuric Acid Esters. J. Am. Chem. Soc. 1941, 63, 3529–3530. [Google Scholar] [CrossRef]
- Deno, N.C.; Newman, M.S. Mechanism of Sulfation of Alcohols 1,2. J. Am. Chem. Soc. 1950, 72, 3852–3856. [Google Scholar] [CrossRef]
- Dado, G.; Bernhardt, R. Sulfonation and Sulfation. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–30. [Google Scholar]
- Suter, C.M.; Oberg, E. A Quantitative Study of the Reaction between Some Primary Aliphatic Alcohols and Sulfuric Acid. J. Am. Chem. Soc. 1934, 56, 677–679. [Google Scholar] [CrossRef]
- Cerfontain, H.; Koeberg-Telder, A.; Lambrechts, H.J.A.; De Wit, P. Aromatic sulfonation. 90. Sulfonation of three symmetrical 2,6-dialkylphenols, 2,6-dimethylanisole. Sulfation and sulfonation product distributions and mechanisms. J. Org. Chem. 1984, 49, 4917–4923. [Google Scholar] [CrossRef]
- Cerfontain, H.; Koeberg-telder, A. Sulfonation and sulfation in the reaction of 4-methylphenol with concentrated sulfuric acid. Phosphorus. Sulfur. Silicon Relat. Elem. 1989, 42, 223–225. [Google Scholar] [CrossRef]
- Goossens, H.D.; Lambrechts, H.J.A.; Cerfontain, H.; de Wit, P. Sulfonation and sulfation in reactions of C-methylated phenols and anisoles with sulfur trioxide. 4-Substituted phenyl hydrogen sulfates: Effective reagents for transsulfonation. Recl. Des Trav. Chim. Des Pays-Bas 2010, 107, 426–430. [Google Scholar] [CrossRef]
- Ragan, M.A. Phenol sulfate esters: Ultraviolet, infrared, 1 H and 13 C nuclear magnetic resonance spectroscopic investigation. Can. J. Chem. 1978, 56, 2681–2685. [Google Scholar] [CrossRef]
- Karimi-Jaberi, Z.; Poolodian, B.; Moradi, M.; Ghasemi, E. 1,3,5-Tris (hydrogensulfato) Benzene: A New and Efficient Catalyst for Synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol) Derivatives. Chin. J. Catal. 2012, 33, 1945–1949. [Google Scholar] [CrossRef]
- Duffel, M.W.; Marshall, A.D.; McPhie, P.; Sharma, V.; Jakoby, W.B. Enzymatic aspects of the phenol (aryl) sulfotransferases. Drug Metab. Rev. 2001, 33, 369–395. [Google Scholar] [CrossRef] [PubMed]
- Ciuffreda, P.; Brizzolari, A.; Casati, S.; Eberini, I.; Palazzolo, L.; Parravicini, C.; Santaniellob, E. 2,4-Furfurylidene-D-sorbitol and its tetra-methyl ether: Synthesis, conformational studies, and radical scavenging activity. Arkivoc 2016, 2016, 50. [Google Scholar]
- Ciuffreda, P.; Casati, S.; Santaniello, E. The action of adenosine deaminase (E.C. 3.5.4.4.) on adenosine and deoxyadenosine acetates: The crucial role of the 5’-hydroxy group for the enzyme activity. Tetrahedron 2000, 56, 3239–3243. [Google Scholar] [CrossRef]
- Attygalle, A.B.; García-Rubio, S.; Ta, J.; Meinwald, J. Collisionally-induced dissociation mass spectra of organic sulfate anions. J. Chem. Soc. Perkin Trans. 2001, 2, 498–506. [Google Scholar] [CrossRef]
- Casati, S.; Manzocchi, A.; Ottria, R.; Ciuffreda, P. 1H, 13C and 15N NMR assignments for N6-isopentenyladenosine/inosine analogues. Magn. Reson. Chem. 2010, 48, 745–748. [Google Scholar] [CrossRef]
- Casati, S.; Manzocchi, A.; Ottria, R.; Ciuffreda, P. 1H, 13C and 15N NMR spectral assignments of adenosine derivatives with different amino substituents at C6-position. Magn. Reson. Chem. 2011, 49, 279–283. [Google Scholar] [CrossRef]
- Chinthakindi, P.K.; Rath, S.K.; Singh, J.; Singh, S.; Koul, S.; Sangwan, P.L. Isolation of isoxanthanol and synthesis of novel derivatives as potential cytotoxic agents. Med. Chem. Res. 2017, 26, 2499–2513. [Google Scholar] [CrossRef]
- Rubino, F.M.; Pitton, M.; Caneva, E.; Pappini, M.; Colombi, A. Thiol-disulfide redox equilibria of glutathione metaboloma compounds investigated by tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 3935–3948. [Google Scholar] [CrossRef]
- Rubino, F.M.; Pitton, M.; Brambilla, G.; Colombi, A. A study of the glutathione metaboloma peptides by energy-resolved mass spectrometry as a tool to investigate into the interference of toxic heavy metals with their metabolic processes. J. Mass Spectrom. 2006, 41, 1578–1593. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1 and 2 are available from the authors, upon reasonable request. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paroni, R.; Casati, S.; Dei Cas, M.; Bignotto, M.; Rubino, F.M.; Ciuffreda, P. Unambiguous Characterization of p-Cresyl Sulfate, a Protein-Bound Uremic Toxin, as Biomarker of Heart and Kidney Disease. Molecules 2019, 24, 3704. https://doi.org/10.3390/molecules24203704
Paroni R, Casati S, Dei Cas M, Bignotto M, Rubino FM, Ciuffreda P. Unambiguous Characterization of p-Cresyl Sulfate, a Protein-Bound Uremic Toxin, as Biomarker of Heart and Kidney Disease. Molecules. 2019; 24(20):3704. https://doi.org/10.3390/molecules24203704
Chicago/Turabian StyleParoni, Rita, Silvana Casati, Michele Dei Cas, Monica Bignotto, Federico Maria Rubino, and Pierangela Ciuffreda. 2019. "Unambiguous Characterization of p-Cresyl Sulfate, a Protein-Bound Uremic Toxin, as Biomarker of Heart and Kidney Disease" Molecules 24, no. 20: 3704. https://doi.org/10.3390/molecules24203704
APA StyleParoni, R., Casati, S., Dei Cas, M., Bignotto, M., Rubino, F. M., & Ciuffreda, P. (2019). Unambiguous Characterization of p-Cresyl Sulfate, a Protein-Bound Uremic Toxin, as Biomarker of Heart and Kidney Disease. Molecules, 24(20), 3704. https://doi.org/10.3390/molecules24203704







