Cu(II)-Catalyzed Homocouplings of (Hetero)Arylboronic Acids with the Assistance of 2-O-Methyl-d-Glucopyranose
Abstract
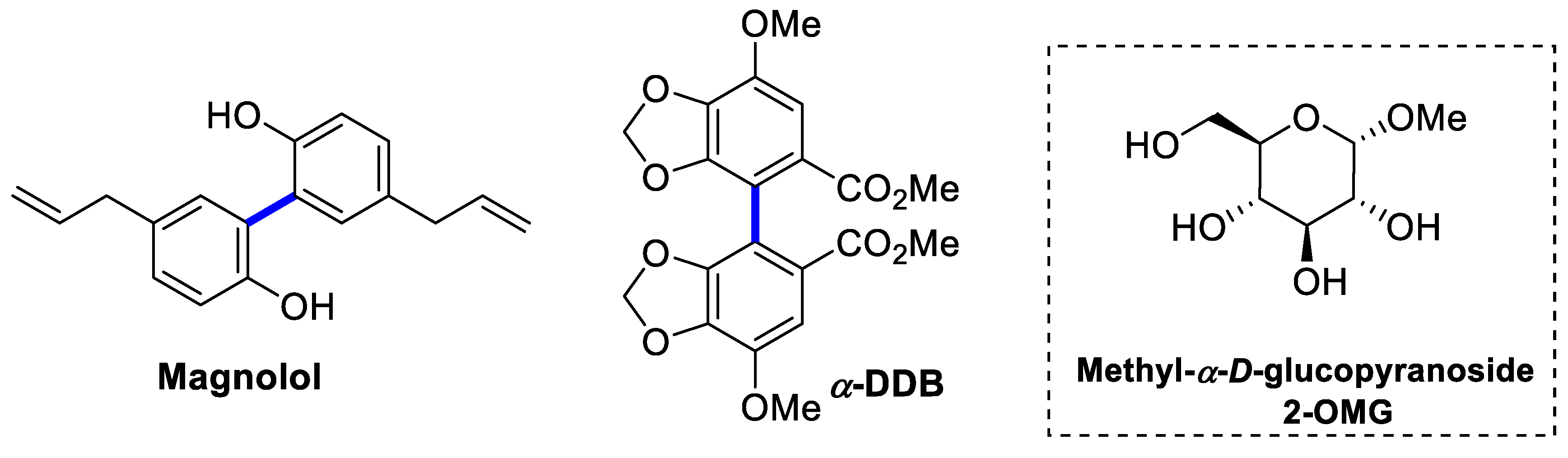
1. Introduction
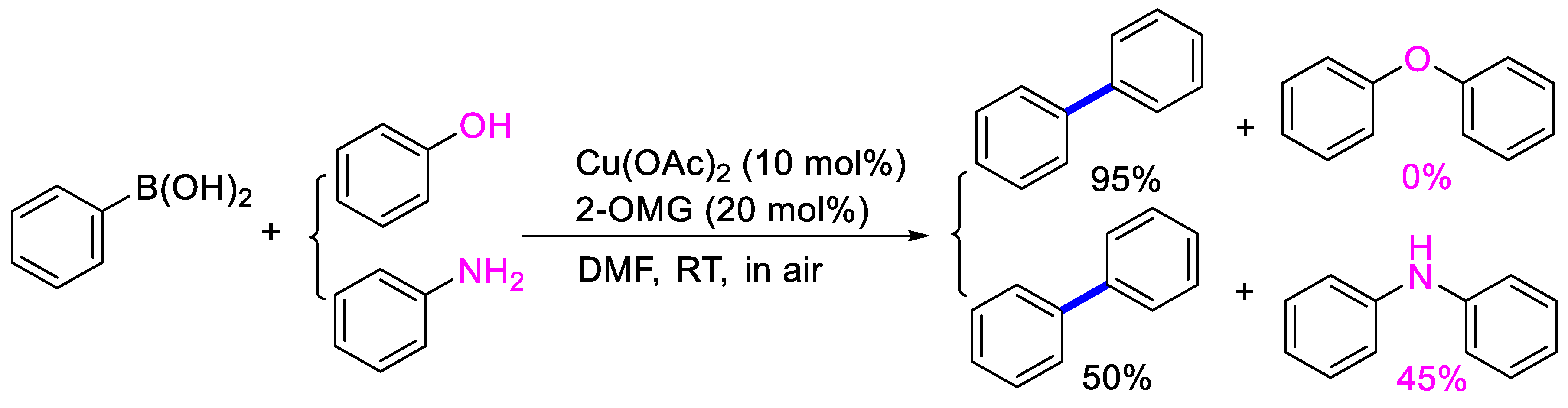
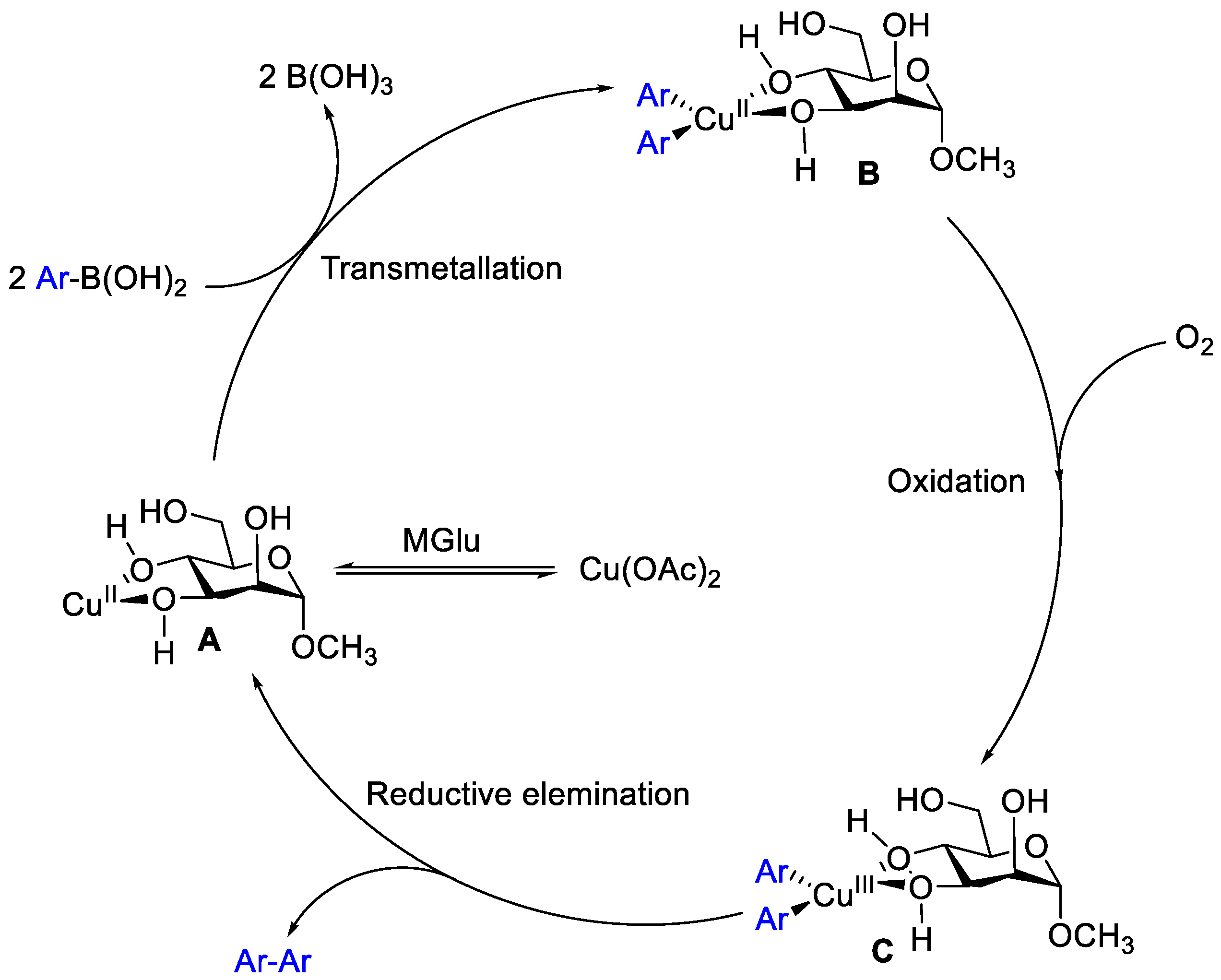
2. Results and Discussion
3. Materials and Methods
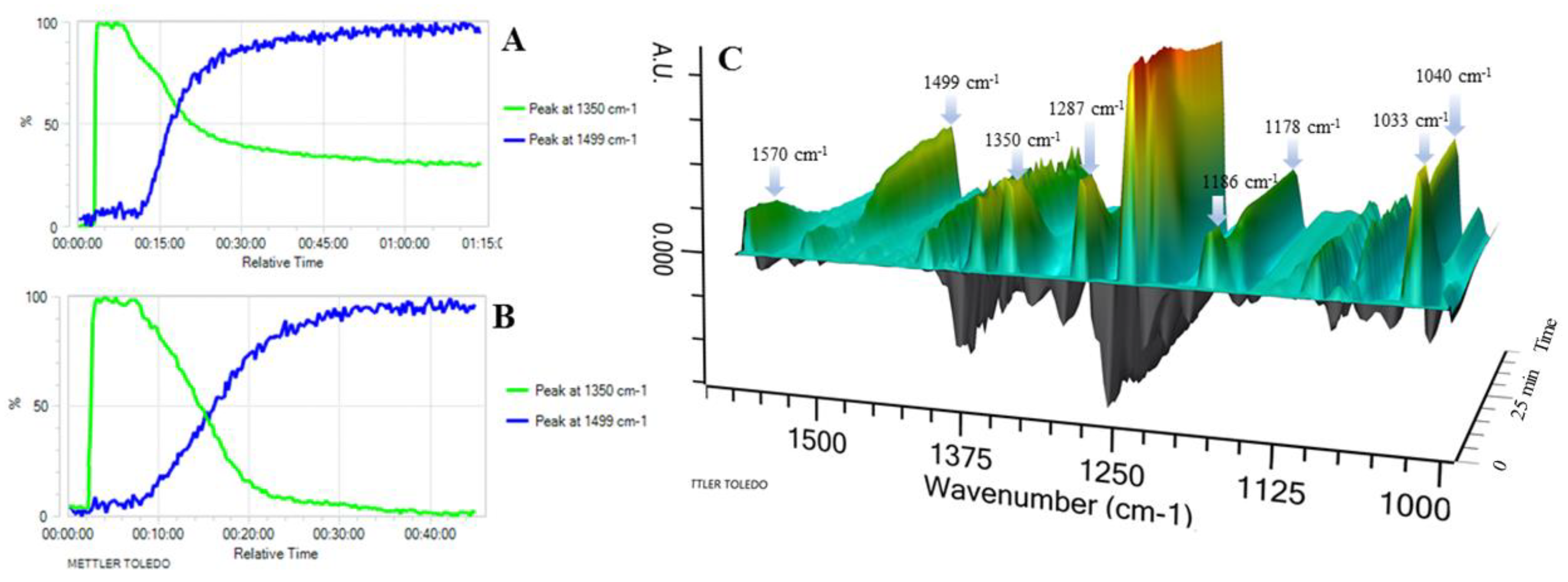
3.1. React IR Experiment
3.2. General Procedure for Catalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Agharahimi, M.R.; Lebel, N.A. Synthesis of (−)-monoterpenylmagnolol and magnolol. J. Org. Chem. 1995, 60, 1856–1863. [Google Scholar] [CrossRef]
- Wu, G.; Guo, H.; Gao, K.; Liu, Y.; Bastow, K.F.; Morris-Natschke, S.L.; Lee, K.H.; Xie, L. Synthesis of unsymmetrical biphenyls as potent cytotoxic agents. Bioorg. Med. Chem. Lett. 2008, 18, 5272–5276. [Google Scholar] [CrossRef]
- Ullmann, F. Ueber eine neue Darstellungsweise von Phenyläthersalicylsäure. Ber. Dtsch. Chem. Ges. 1904, 37, 853–857. [Google Scholar] [CrossRef]
- Miyake, Y.; Wu, M.; Rahman, M.J.; Kuwatani, Y.; Iyoda, M. Efficient construction of biaryls and macrocyclic cyclophanes via electron-transfer oxidation of lipshutz cuprates. J. Org. Chem. 2006, 71, 6110–6117. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cheng, D.; Pei, W. Iron-catalyzed homocoupling of bromide compounds. J. Org. Chem. 2006, 71, 6637–6639. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Mao, J.; Zhang, Y. Pd (OAc) 2-catalyzed room temperature homocoupling reaction of arylboronic acids under air without ligand. Catal. Commun. 2008, 9, 97–100. [Google Scholar] [CrossRef]
- Cahiez, G.; Moyeux, A.; Buendia, J.; Duplais, C. Manganese- or iron-catalyzed homocoupling of grignard reagents using atmospheric oxygen as an oxidant. J. Am. Chem. Soc. 2007, 129, 13788–13789. [Google Scholar] [CrossRef]
- Robinson, M.K.; Kochurina, V.S.; Hanna, J.M. Palladium-catalyzed homocoupling of arenediazonium salts: An operationally simple synthesis of symmetrical biaryls. Tetrahedron Lett. 2007, 48, 7687–7690. [Google Scholar] [CrossRef]
- Ostrowska, S.; Rogalski, S.; Lorkowski, J.; Walkowiak, J.; Pietraszuk, C. Efficient homocoupling of aryl- and alkenylboronic acids in the presence of low loadings of [{Pd (μ-OH) Cl (IPr)} 2]. Synlett 2018, 29, 1735–1740. [Google Scholar]
- Valiente, A.; Carrasco, S.; Sanz-Marco, A.; Tai, C.W. Aerobic homocoupling of arylboronic acids catalyzed by regenerable Pd (II) @MIL-88B-NH 2 (Cr). ChemCatChem 2019, 11, 3933–3940. [Google Scholar] [CrossRef]
- Demir, A.S.; Reis, O.; Emrullahoglu, M. Role of copper species in the oxidative dimerization of arylboronic acids: Synthesis of symmetrical biaryls. J. Org. Chem. 2003, 68, 10130–10134. [Google Scholar] [CrossRef] [PubMed]
- Kirai, N.; Yamamoto, Y. Homocoupling of arylboronic acids catalyzed by 1, 10-phenanthroline-ligated copper complexes in air. Eur. J. Org. Chem. 2009, 12, 1864–1867. [Google Scholar] [CrossRef]
- Tyagi, D.; Binnani, C.; Rai, R.K.; Dwivedi, A.D.; Gupta, K.; Li, P.; Zhao, Y.; Singh, S.K. Ruthenium-catalyzed oxidative homocoupling of arylboronic acids in water: Ligand tuned reactivity and mechanistic study. Inorg. Chem. 2016, 55, 6332–6343. [Google Scholar] [CrossRef] [PubMed]
- Kaboudin, B.; Abedi, Y.; Yokomatsu, T. Cu-II–β-cyclodextrin complex as a nanocatalyst for the homo- and crosscoupling of arylboronic acids under ligand-and base-free conditions in air: Chemoselective cross-coupling of arylboronic acids in water. Eur. J. Org. Chem. 2011, 2011, 6656–6662. [Google Scholar] [CrossRef]
- Kaboudin, B.; Mostafalu, R.; Yokomatsu, T. Fe3O4 nanoparticle-supported Cu(II)-β-cyclodextrin complex as a magnetically recoverable and reusable catalyst for the synthesis of symmetrical biaryls and 1,2,3-triazoles from aryl boronic acids. Green Chem. 2013, 15, 2266–2274. [Google Scholar] [CrossRef]
- Modak, A.; Mondal, J.; Sasidharan, M.; Bhaumik, A. Triazine functionalized ordered mesoporous polymer: A novel solid support for Pd-mediated C-C cross-coupling reactions in water. Green Chem. 2011, 13, 1317–1331. [Google Scholar] [CrossRef]
- Cheng, J.W.; Luo, F.T. Coupling of aryl Grignard reagents by electron transfer to 2,3-dichloropropene. Tetrahedron Lett. 1988, 29, 1293–1294. [Google Scholar] [CrossRef]
- Zeng, M.; Du, Y.; Qi, C.; Zuo, S.; Li, X.; Shao, L.; Zhang, X. An efficient and recyclable heterogeneous palladiumcatalyst utilizing naturally abundant pearl shell waste. Green Chem. 2011, 13, 350–356. [Google Scholar] [CrossRef]
- Endo, Y.; Shudo, K.; Okamoto, T. Acid-catalyzed solvolysis of N-sulfonyl- and N-acyl-O-arylhydroxylamines. Phenoxenium ions. J. Am. Chem. Soc. 1982, 104, 6393–6397. [Google Scholar] [CrossRef]
- Allen, C.F.H.; Burnss, D.M. The chemistry of o-terphenyl. III. Sulfonic acids. J. Org. Chem. 1949, 14, 163–169. [Google Scholar] [CrossRef]
- Gilman, H.; Ingham, R.K. Some tetraorganosilanes. J. Am. Chem. Soc. 1955, 77, 1680–1681. [Google Scholar] [CrossRef]
- Lee, K.; Lee, P.H. Efficient homo-coupling reactions of heterocyclic aromatic bromides catalyzed by Pd (OAc) 2 using indium. Tetrahedron Lett. 2008, 49, 4302–4305. [Google Scholar] [CrossRef]
- Leowanawat, P.; Zhang, N.; Resmerita, A.M.; Rosen, B.M.; Percec, V. Ni(COD)2/PCy3 catalyzed cross-coupling of aryl and heteroaryl neopentylglycolboronates with aryl and heteroaryl mesylates and sulfamates in THF at room temperature. J. Org. Chem. 2011, 76, 9946–9955. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Entry | Catalyst (mol %) | Base | Solvent | Temp (°C) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | CuI (10) | K2CO3 | DMF | 40 | -- c |
| 2 | CuO (10) | K2CO3 | DMF | 40 | -- c |
| 3 | Powdered Cu (10) | K2CO3 | DMF | 40 | -- c |
| 4 | CuSO4 (10) | K2CO3 | DMF | 40 | 10 |
| 5 | Cu(OAc)2 (10) | K2CO3 | DMF | 40 | 85 |
| 6 | Cu(OAc)2 (10) | Cs2CO3 | DMF | 40 | 80 |
| 7 | Cu(OAc)2 (10) | Et3N | DMF | 40 | 78 |
| 8 | Cu(OAc)2 (10) | -- | DMF | 40 | 99 |
| 9 | Cu(OAc)2 (10) | -- | Dioxane | 40 | -- c |
| 10 | Cu(OAc)2 (10) | -- | H2O | 40 | -- c |
| 11 | Cu(OAc)2 (10) | -- | MeOH | 40 | 14 |
| 12 | Cu(OAc)2 (10) | -- | DMF | RT d | 98 |
| 13 e | Cu(OAc)2 (10) | -- | DMF | 40 | 58 |
| 14 f | Cu(OAc)2 (5) | -- | DMF | 40 | 96 |
| 15 | Cu(OAc)2 (15) | -- | DMF | 40 | 98 |
| 16 g | Cu(OAc)2 (5) | -- | DMF | RT d | 98 |

| Entry | Substance | Product | Time (min) | Yield (%) b |
|---|---|---|---|---|
| 1 | 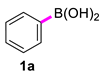 |  | 60 | 98 |
| 2 | 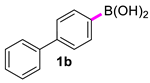 | 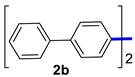 | 60 | 97 |
| 3 | 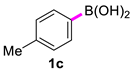 | 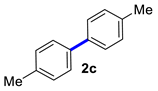 | 60 | 94 |
| 4 | 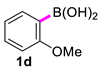 | 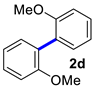 | 90 | 84 |
| 5 | 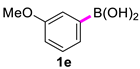 | 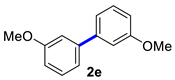 | 90 | 90 |
| 6 | 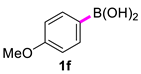 | 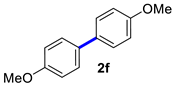 | 60 | 91 |
| 7 | 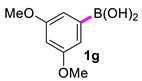 | 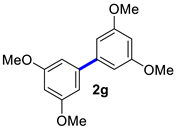 | 90 | 89 |
| 8 | 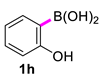 | 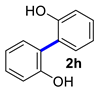 | 90 | 91 |
| 9 |  | 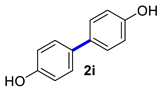 | 90 | 93 |
| 10 | 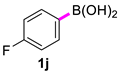 | 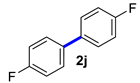 | 60 | 98 |
| 11 | 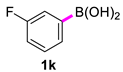 |  | 60 | 98 |
| 12 | 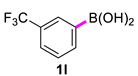 | 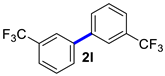 | 60 | 99 |
| 13 | 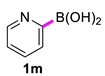 | 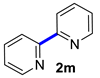 | 90 | 94 |
| 14 | 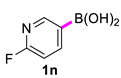 |  | 90 | 95 |
| 15 | 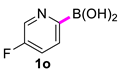 | 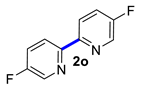 | 90 | 93 |
| 16 |  | 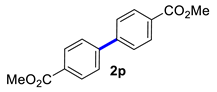 | 90 | 90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, C.; Zheng, L.; Zhao, Y. Cu(II)-Catalyzed Homocouplings of (Hetero)Arylboronic Acids with the Assistance of 2-O-Methyl-d-Glucopyranose. Molecules 2019, 24, 3678. https://doi.org/10.3390/molecules24203678
Yuan C, Zheng L, Zhao Y. Cu(II)-Catalyzed Homocouplings of (Hetero)Arylboronic Acids with the Assistance of 2-O-Methyl-d-Glucopyranose. Molecules. 2019; 24(20):3678. https://doi.org/10.3390/molecules24203678
Chicago/Turabian StyleYuan, Chunling, Li Zheng, and Yingdai Zhao. 2019. "Cu(II)-Catalyzed Homocouplings of (Hetero)Arylboronic Acids with the Assistance of 2-O-Methyl-d-Glucopyranose" Molecules 24, no. 20: 3678. https://doi.org/10.3390/molecules24203678
APA StyleYuan, C., Zheng, L., & Zhao, Y. (2019). Cu(II)-Catalyzed Homocouplings of (Hetero)Arylboronic Acids with the Assistance of 2-O-Methyl-d-Glucopyranose. Molecules, 24(20), 3678. https://doi.org/10.3390/molecules24203678





