3. Results and Discussion
Physical appearance, % yield, NMR, IR and MS data for each of the synthesized indicator dyes are summarized below:
(1E,5E)-1,6-diphenyl-1,5-hexadiene-3,4-dione (3a) Orange crystal; 10.20% yield; 1H NMR (400 MHz, CDCl3) δ = 7.87 (d, J = 16.2 Hz, 2H), 7.66 (dd, J = 7.7, 1.9 Hz, 4H), 7.48 (d, J = 16.2 Hz, 2H), 7.46–7.40 (m, 6H) ppm; 13C NMR (101 MHz, CDCl3) δ = 189.22, 148.00, 134.56, 131.55, 129.20, 129.14, 119.78 ppm; IR (neat) νmax = 1668, 1591, 1572, 1447, 986, 752, 719, 687 cm−1; mass (electrospray ionization (ESI) LC-MS): 263.1045 (M + 1).
(1E,5E)-1,6-bis(4-methylphenyl)-1,5-hexadiene-3,4-dione (3b) Orange crystal; 8.51% yield; 1H NMR (400 MHz, CDCl3) δ = 7.84 (d, J = 16.1 Hz, 2H), 7.55 (d, J = 8.0 Hz, 4H), 7.42 (d, J = 16.1 Hz, 2H), 7.23 (d, J = 8.0 Hz, 4H), 2.40 (s, 6H) ppm; 13C NMR (101 MHz, CDCl3) δ = 189.57, 148.01, 142.25, 131.89, 129.93, 129.16, 118.95, 21.76 ppm; IR (neat) νmax = 1668, 1593, 1510, 1327, 1182, 995, 800, 683 cm−1; mass (ESI LC-MS): 291.1361 (M + 1).
(1E,5E)-1,6-bis(4-methoxyphenyl)-1,5-hexadiene-3,4-dione (3c) Orange crystal; 9.43% yield; 1H NMR (400 MHz, CDCl3) δ = 7.82 (d, J = 15.9 Hz, 2H), 7.61 (d, J = 9.0 Hz, 4H), 7.33 (d, J = 15.9 Hz, 2H), 6.94 (d, J = 9.0 Hz, 4H), 3.86 (s, 6H) ppm; 13C NMR (101 MHz, CDCl3) δ = 189.69, 162.48, 147.67, 131.03, 127.43, 117.74, 114.66, 55.59 ppm; IR (neat) νmax = 1678, 1593, 1506, 1417, 1248, 1175, 1028, 991, 829, 812, 787, 687 cm−1; mass (ESI LC-MS): 323.1273 (M + 1).
2,3-Bis[(E)-2-phenylvinyl]-quinoxaline (5a) Yellow powder; 82.71% yield; 1H NMR (400 MHz, DMSO-d6) δ = 8.09 (d, J = 15.2 Hz, 2H), 8.06–8.04 (m, 2H), 8.01 (d, J = 15.2 Hz, 2H), 7.94 (d, J = 7.4 Hz, 4H), 7.80-7.78 (m, 2H), 7.48 (t, J = 7.4 Hz, 4H), 7.41 (t, J = 7.4 Hz, 2H) ppm; 13C NMR (101 MHz, DMSO-d6) δ = 148.67, 141.00, 137.37, 136.11, 129.91, 129.16, 128.82, 128.59, 128.07, 122.46 ppm; IR (neat) νmax = 1624, 1518, 1495, 1194, 1130, 964, 745, 688 cm−1; mass (ESI LC-MS): 335.1512 (M + 1).
2,3-Bis[(E)-2-(4-methylphenyl)vinyl]-quinoxaline (5b) Yellow powder; 84.84% yield; 1H NMR (400 MHz, DMSO-d6) δ = 8.04-8.00 (m, 4H), 7.97 (d, J = 15.6 Hz, 2H), 7.83 (d, J = 8.2 Hz, 4H), 7.78-7.75 (m, 2H), 7.29 (d, J = 8.2 Hz, 4H), 2.37 (s, 6H) ppm; 13C NMR (101 MHz, DMSO-d6) δ = 148.77, 140.93, 138.87, 137.34, 133.42, 129.71, 129.42, 128.52, 128.06, 121.36, 21.02 ppm; IR (neat) νmax = 1622, 1510, 1190, 1128, 966, 799, 752 cm−1; mass (ESI LC-MS): 363.1850 (M + 1).
2,3-Bis[(E)-2-(4-methoxyphenyl)vinyl]-quinoxaline (5c) Yellow powder; 75.70% yield; 1H NMR (400 MHz, DMSO-d6) δ = 8.01–7.99 (m, 2H), 7.99–7.92 (m, 4H), 7.88 (d, J = 8.7 Hz, 4H), 7.75–7.73 (m, 2H), 7.03 (d, J = 8.8 Hz, 4H), 3.83 (s, 6H) ppm; 13C NMR (101 MHz, DMSO-d6) δ = 160.17, 148.90, 140.86, 137.01, 129.67, 129.48, 128.90, 128.43, 119.99, 114.27, 55.27 ppm; IR (neat) νmax = 1620, 1597, 1508, 1419, 1248, 1173, 1022, 970, 830, 810, 762 cm−1; mass (ESI LC-MS): 395.1746 (M + 1).
The yields for the cinnamil derivatives (
3a–
c) were between 8.51 to 10.20%, similar to those reported in the literature [
11]. The low yield can be explained on the basis of the intrinsic chemical properties of the substrates. Since diacetyl is a Brønsted acid, this reaction is initiated via the enolization of diacetyl’s carbonyl group under alkaline conditions provided by piperidinium acetate. In theory, the addition reaction between one mole of enolized diacetyl and two moles of benzoic aldehydes (
2a–
c) should result in one mole of
3a–
c. However, in this alkaline solution, the non-enolizable benzoic aldehydes would undergo a self-redox reaction known as Cannizzaro reaction, generating equal molar amount of benzoic alcohol and benzoic acid [
26]. Moreover, self-condensation of diacetyl could occur as another side reaction. Preliminary experiments with piperidinium acetate gave the highest yield over other catalysts tested, including sodium acetate, sodium hydroxide, and sodium citrate. Hence, piperidinium acetate was selected as the base in this reaction. Considering the low costs of the reaction substrates and the readily achievable experimental protocol, the overall 10% yield is probably a fair compromise. In contrast, the synthesis of quinoxaline derivatives (
5a–
c) via 1,2-diamine and 1,2-diketone condensation had higher yields ranging from 75–84%, similar to that reported in a previous study involving the same reaction [
10].
3.1. Structural Confirmation
The molecular structures of the synthesized cinnamil and quinoxaline derivatives were confirmed by MS, FT-IR and NMR spectroscopies. The molecular weights of 3a–c and 5a–c were confirmed from MS results. In FT-IR spectroscopy, the carbonyl groups of cinnamil derivatives (3a–c) were shown as strong IR stretching bands at 1678–1668 cm−1. As compared to 3a and 3b, the carbonyl band of 3c shifted to higher frequency due to the presence of the ether group. The C-O stretching band of 3c appeared at 1028 cm−1. For 3a–c, absorbance bands between 1600 and 1400 cm−1 could be attributed to C=C stretching of trans double bonds and aromatic rings. The strong IR absorbance around 990 cm−1 was due to the trans vinyl C=C-H out-of-plane bending. The substitution in the benzene ring could be identified by the aromatic C-H out-of-plane bending vibration at 800 cm−1 for 3b and 3c (para-di-substitution) and two strong bands at 752 and 719 cm−1 for 3a (mono-substitution). In 1H NMR, the trans carbon–carbon double bond can be identified as doublets with a large 3J coupling constant of approximately 16 Hz. All aromatic proton peaks appeared around 7–8 ppm. The methyl group proton peaks for 3b and 3c appeared as a singlet, at a lower chemical shift due to more diamagnetic shielding as compared to 3a. In 13C NMR, the carbonyl carbon peak was located at high chemical shift (189 ppm) due to strong electronegativity effect, while the methyl group carbons for 3b and 3c were at the lower end (21 ppm and 55 ppm, respectively).
For quinoxaline derivatives (
5a–
c), the imine (C=N) stretching band was located at 1624–1620 cm
−1 in the IR spectra. Similar to
3a–
c, the C=C stretching band for
5a–
c appeared around 1600–1400 cm
−1 and the
trans C=C-H out-of-plane bending at 970–964 cm
−1. The ether group of
5c can be identified by the C-O stretching band at 1022 cm
−1. In
1H NMR spectra, the
trans C=C bond were confirmed by the two doublets with a coupling constant of 15–16 Hz. The aromatic protons on the quinoxaline backbone appeared as multiplets, while the doublets with lower coupling (7–9 Hz) could be attributed to other aromatic protons. The two singlet peaks of
5b and
5c (at 2.37 ppm and 3.83 ppm, respectively) confirmed the conservation of the methyl groups. In
13C NMR spectra, the carbonyl carbon peaks of
5b and
5c disappeared, while the methyl groups appeared, at similar chemical shifts as in
3b and
3c. For
5c, the peak of the quaternary aromatic carbon next to the ether oxygen was observed at 160 ppm. Overall, the IR and NMR results corroborated well with those from previous research [
10].
3.2. Photophysical Properties
The synthesized cinnamil (
3a–
c) and quinoxaline (
5a–
c) derivatives appeared as orange and pale-yellow solids, respectively.
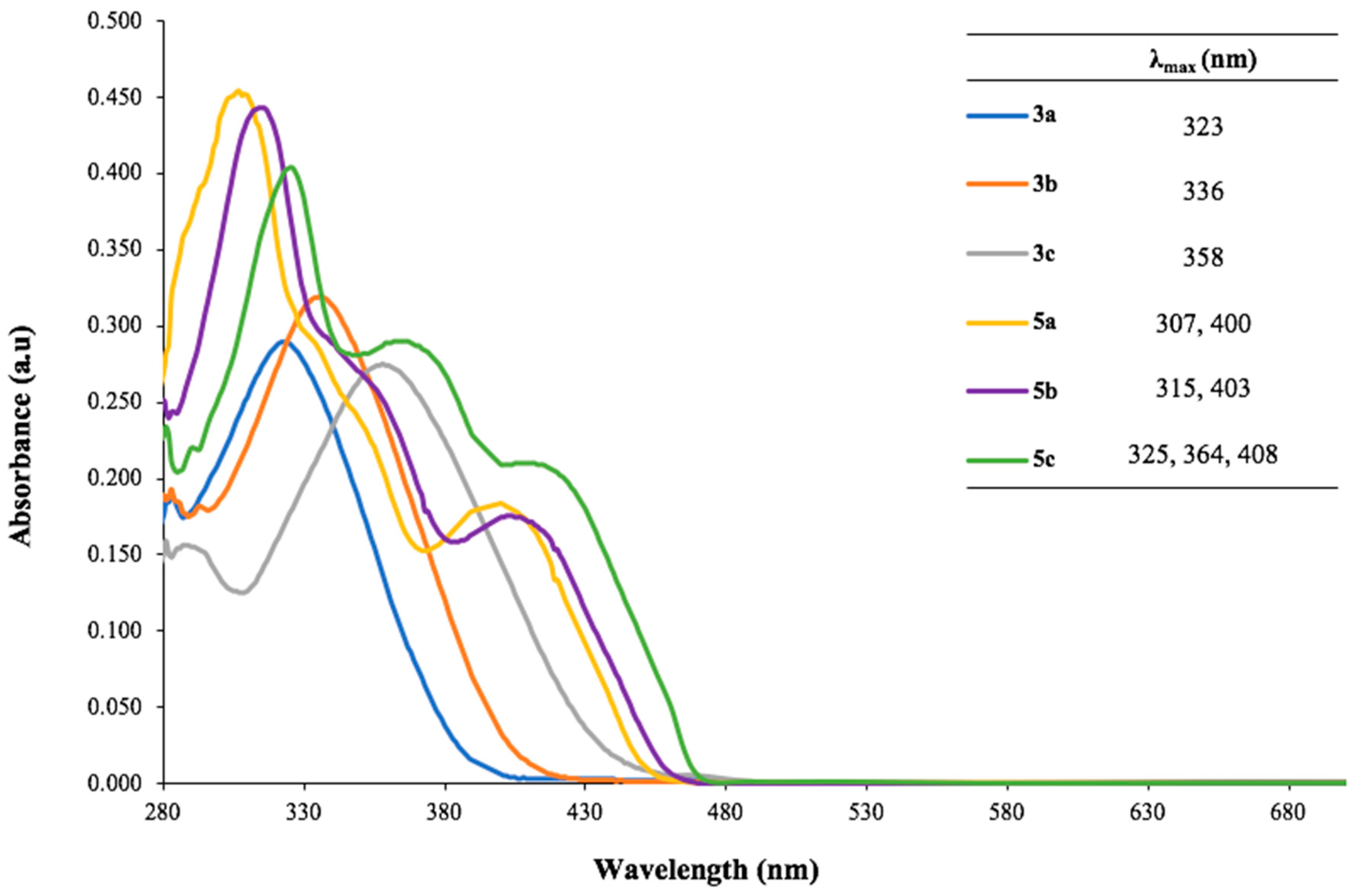
Figure 1 shows the absorption spectra in UV-Vis wavelength, along with the maximum absorbance wavelength (λ
max) values, of the dyes dissolved in DMSO. The highly conjugated moiety of all synthesized compounds induced a bathochromic shift, resulting in absorption of the visible light. The absorption peaks of
3a–
c are mainly attributed to π-π* electronic transition of the carbonyl group. The UV-Vis spectra of quinoxaline derivatives
5a–
c have two or more maxima, due to the electronic transitions of π-π* (305–325 nm) and n-π* (400–410 nm) of C=N bonds within the pyrazine ring. Moreover,
5c showed a maximum at 364 nm, suggesting the n-σ* transition of C-O bond, which was absent in
5a and
5b. Substitution of the aromatic rings with electron-donating groups resulted in red shifts for both
3a–
c and
5a–
c. These findings are consistent with those reported in the literature [
10].
The photophysical properties of cinnamil and quinoxaline derivatives were examined by reacting with acids and amines in aqueous solutions.
Figure 2A illustrated the color appearance of quinoxaline derivatives with excess amount of
p-TsOH as a dopant. The dopant was added at 5000:1 acid:dye molar ratio to completely protonate the nitrogen atoms within the pyrazine ring of
5a–
c. The colors of acid-doped quinoxaline solutions were strikingly different as shown in
Figure 2A, changing to bright yellow, orange and reddish orange for
5a,
5b, and
5c respectively. This color variation can be explained by the bathochromic shift of
5a to
5c, as shown in the absorbance spectra (
Figure 1). The pH values of 10 µM
5a,
5b and
5c with
p-TsOH in DMSO were determined to be 1.87, 1.82 and 1.62 respectively. The UV-Vis spectra of acid-doped
5a–
c were shown in the
supplementary file (Figure S25).
Cinnamil (
3a), being a 1,2-diketone with α-hydrogens, enolized under strong alkaline conditions, underwent yellow-to-dark brown transition when doped with TBAH (
Figure 2B). Specifically, the α-hydrogens can be deprotonated in the presence of a strong base, resulting in negatively charged α-carbons, lowering the energy gap of π-π* transition, and causing bathochromic shift. Increasing TBAH concentration raised the alkalinity of the solution, inducing the bathochromic shift and resulting in a darker solution. The photophysical property of cinnamil was further evaluated with ammonium hydroxide and other amines in aqueous solutions, including, tertiary amines, secondary amines and primary amines (
Figure 2C). Ammonium hydroxide and tertiary amines trimethylamine (TMA) and triethylamine (TEA) showed similar color effects as TBAH, due to their strong alkalinity and lacking nucleophilic properties of the protonated nitrogen. The increasing color intensity observed with NH
3, TMA and TEA was attributed to their different pKa values, which are 9.25, 9.80 and 10.81, respectively at room temperature [
27,
28]. Under these alkaline conditions, cinnamil’s diketone moiety would be converted to the corresponding enolate form. For secondary amines such as dimethylamine and piperidine, cinnamil solution remained yellow after 4 h (
Figure 2C). This phenomenon could be due to the excess secondary amine present, which served as a nucleophile that attacked the carbonyl centre of cinnamil, forming an unstable aminoalcohol structure that tended to convert back to cinnamil at equilibrium, giving the solution’s bright yellow color. In comparison, the cinnamil solution exhibited a reduced color intensity when doped with more reactive primary amines (e.g., triethylenetetramine (TETA), aniline, and hydrazine) by forming imine groups through ketone-amine condensation. On the other hand, the yellow color of cinnamil disappeared by reacting with hydrazine, which introduced an additional amino substitute to each imine group (i.e., hydrazone), leading to a further hypsochromic shift. In summary, cinnamil displayed different color responses to various classes of amines, suggesting that it is potentially useful for amine discrimination.
3.3. Volatile Amine Detection
3.3.1. Amine Detection by Quinoxaline Derivatives
The acid-doped quinoxaline derivative solutions, as shown in
Figure 2A, were used as colorimetric indicators for ammonia, trimethylamine, dimethylamine, triethylamine, piperidine and hydrazine. The relative basicity with different pKa values of ammonia, TMA and TEA have been discussed in
Section 3.2. By doping the quinoxaline derivatives
5a–
c with
p-TsOH (
Figure 2A), they were protonated and effectively functioning as pH indicators when exposed to these alkaline volatiles. The changes in color profiles for these acid-doped quinoxaline derivatives are shown in
Figure 3.
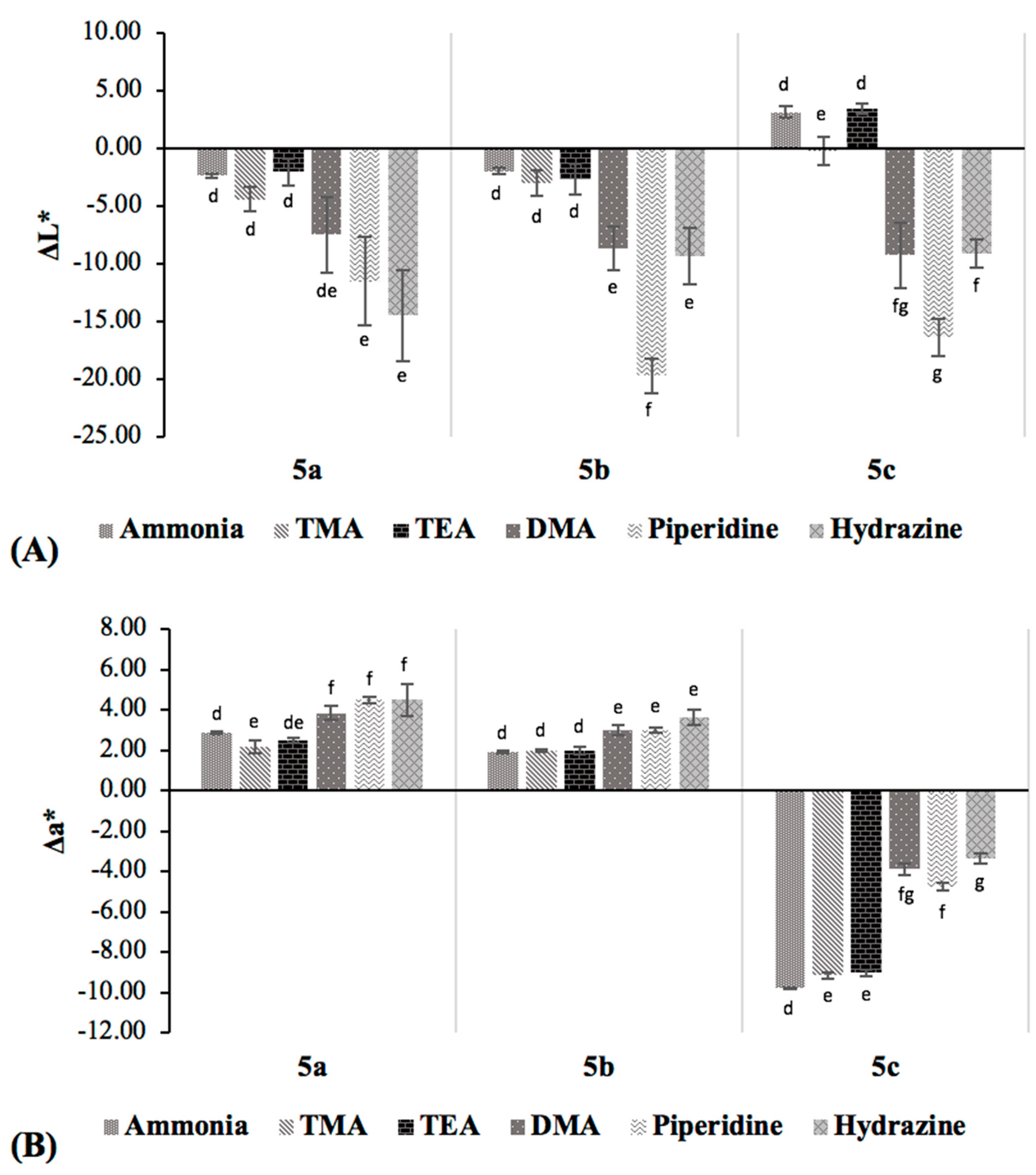
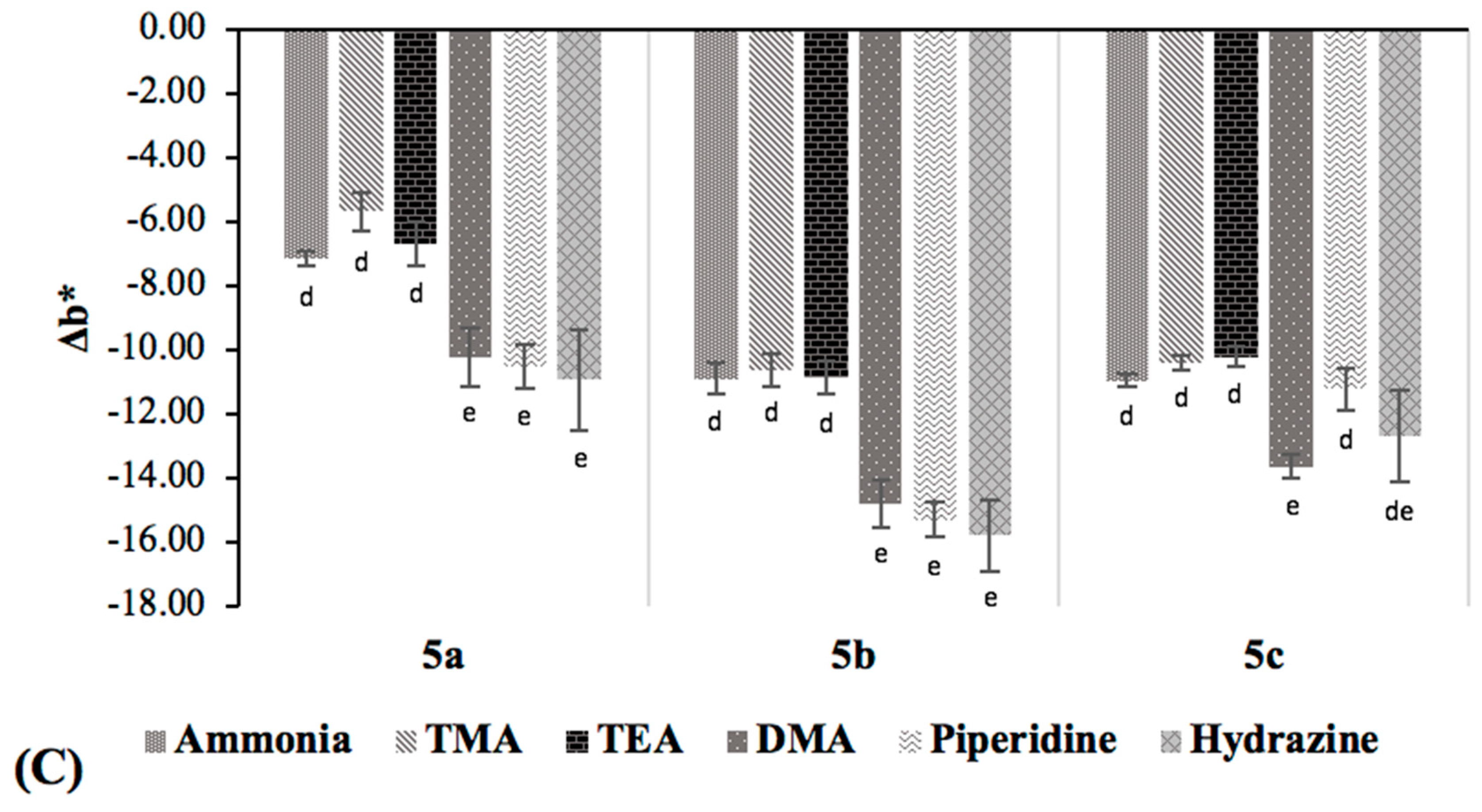
The different initial colors observed among the acid-doped quinoxaline solutions were due to the different aromatic substituents (
Figure 2A). In general, the color of the indicators became darker upon amine exposure, as indicated by the decreasing
L* value, except
5c. Quinoxaline derivative
5c showed a considerable decrease in
a* value, consistent with the fading of its orange reddish color when exposed to the alkalis. On the other hand, small increases in
a* value were detected for
5a and
5b. All samples exhibited decreases in
b* values (i.e., decreases in yellow color), the extent of which was less for tertiary amines (TMA and TEA) and ammonia, as compared to the primary and secondary amines. However, the color variations among
5a,
5b and
5c were not readily distinguished by the naked eye, implying that the quinoxaline compounds would not allow the discrimination of the six alkaline volatiles. Hence, these dyes are more useful for the detection of the volatile amine mixtures, such as those produced during the spoilage of fresh fish and fishery products.
3.3.2. Amine Detection by Cinnamil
The color response images of cinnamil-loaded cellulose papers to different amines are shown in
Figure 4A, and the changes of individual color parameters are summarized in
Figure 4B.
Figure 4B shows that ΔΕ value for hydrazine was significantly higher than those of the other four amines tested (i.e., piperidine, TEA, TMA and ammonia, except for dimethylamine (DMA)), although other amines could not be differentiated by relying on the ΔE values alone. However,
L* value increased when cinnamil was exposed to TEA, while all other amines showed decreasing trends. This observation implies that
L* value can potentially be considered for differentiating TEA from other amines. Similarly, the
a* value declined when cinnamil was exposed to piperidine, but increased for all the other amines, especially hydrazine and TMA. On the other hand, minimal change in
a* value was observed for ammonia, which can be used to differentiate it from other volatiles. Hydrazine resulted in a significant decrease in
b*, consistent with the visual decrease in yellow color (
Figure 4A), while insignificant changes in
b* values were observed for piperidine and TMA. Taken together, by investigating the
L*,
a* and
b* color parameters individually, all six amines tested can be differentiated by cinnamil. These results demonstrate that cinnamil can potentially be used as a colorimetric indicator for the discrimination of volatile amines. To stabilize the dye and prevent leaching into food product, encapsulating it within a protective matrix permeable to the amine analytes is important for real food packaging applications.
3.3.3. Limit of Detection (LOD) and Limit of Quantitation (LOQ) for Amine Detection
The LOD and LOQ values of
3a and acid-doped
5a–
c for detecting different volatile amines are summarized in
Table 1, showing that the detection and quantitation limits of cinnamil were higher than the acid-doped quinoxaline compounds. The lower sensitivity of
3a than quinoxaline compounds is consistent with findings in
Figure 3 and
Figure 4, i.e., when exposing to the same concentration of a given amine, the extent of color changes for
3a was lower than
5a–
c. Comparing the acid-doped quinoxaline dyes, the LOD and LOQ values were the lowest (i.e., most sensitive) for
5c when exposed to low concentrations of amines. Among the six amines, ammonia resulted in the lowest LOD and LOQ values for all indicators, while triethylamine the highest. Conceivably, the detection limit can be further improved by optimizing the concentration of the indicator dyes, the amount of acid dopant, and/or changing the substrates for dye deposition. Since trimethylamine, ammonia and dimethylamine were the predominant volatile compounds produced during the spoilage of fish, the low detection limits of these dyes for these compounds are promising for intelligent packaging applications to monitor fish freshness.
3.4. Preliminary Test on Fish Freshness Indication by Acid-Doped Quinoxaline
During the spoilage of fish, the predominate volatile amine formed is TMA, due to microbial activities from the bacteria such as
Shewanella putrifaciens,
Photobacterium phosphoreum, and
Vibrionacaea spp. [
29,
30]. These bacteria derive energy by converting trimethylamineoxide (TMAO), an osmoregulatory compound in fish tissue, to TMA that further breaks down to DMA [
31]. Apart from TMA and DMA, ammonia can also be generated during fish spoilage from the proliferation of aerobic bacteria [
29]. In view of the prevalence of these basic nitrogen volatiles during the spoilage of fish, a preliminary study was conducted to illustrate the application of an acid-doped quinoxaline derivative,
5b, as freshness indicator.
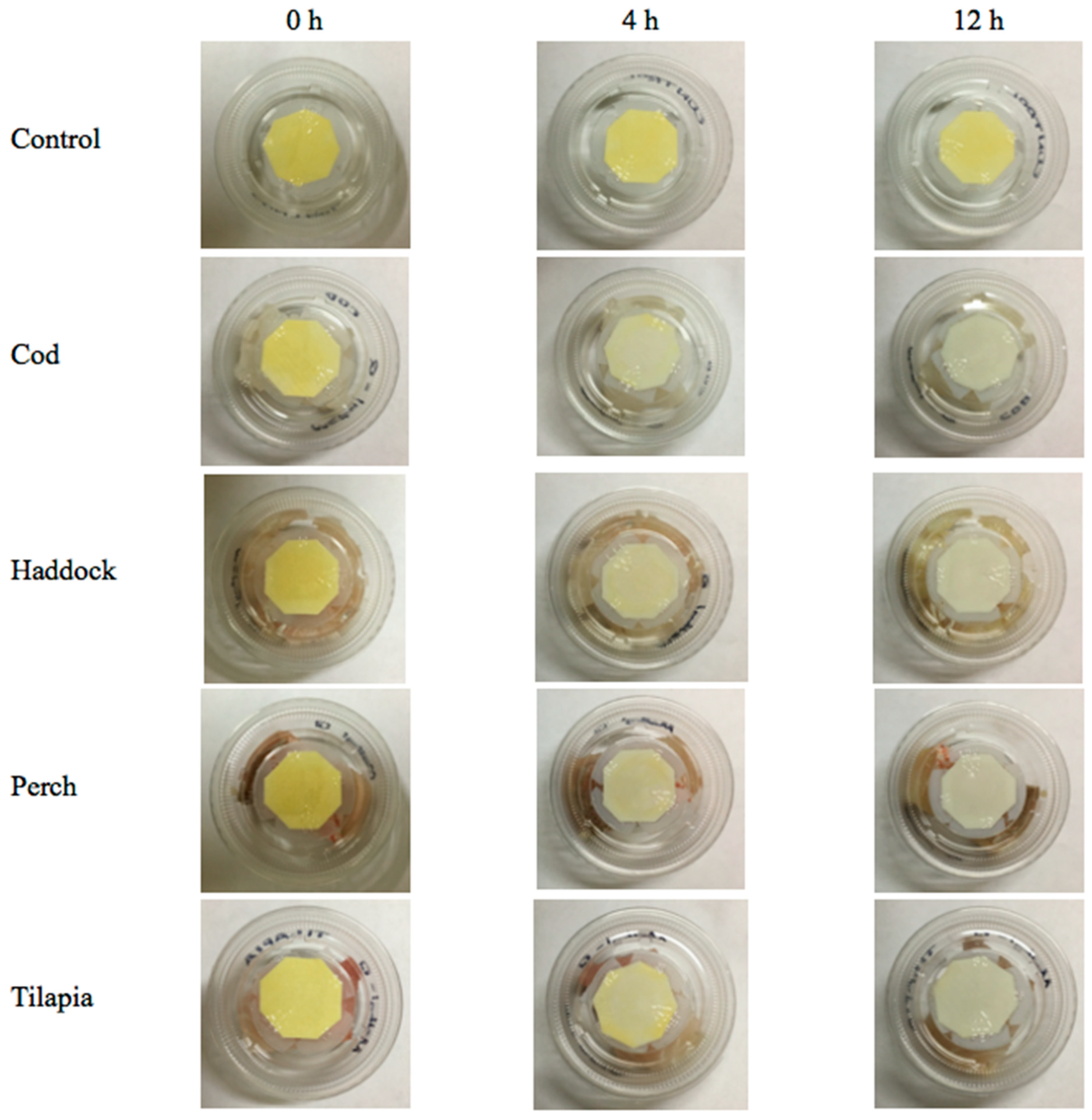
The color responses of the
5b indicators, prepared as described in
Section 2.5, are shown in
Figure 5. It can be seen that the
5b-containing cellulose paper was in bright yellowish color at the beginning. The yellow color faded notably after 4-h of exposure to various fish fillets stored at room temperature, and substantially faded after 12 h. This observation correlated well with the results presented in
Figure 3, where
b* value decreased dramatically when the acid-doped quinoxaline was exposed to volatile amines. The reduction in yellowness intensity was attributable to the interaction of basic amines present in the package headspace with the dopant,
p-TsOH. Accordingly, the extent of color change will be affected by the quantity of acid dopant in the dye; the higher the dopant concentration, the lower the indicator’s sensitivity towards the basic volatiles, and vice versa. This preliminary study shows that the acid-doped quinoxaline dyes are promising as colorimetric indicators for real-time detection of fish freshness. More future studies are needed to correlate the color profile of the dyes with the concentration of total volatile basic nitrogen compounds, as well as the quality parameters of the fish fillets (e.g., microbial loads, texture profiles, sensory properties), in order to accurately establish the color–freshness relationship.