Raman Spectroscopy for the Competition of Hydrogen Bonds in Ternary (H2O–THF–DMSO) Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussion
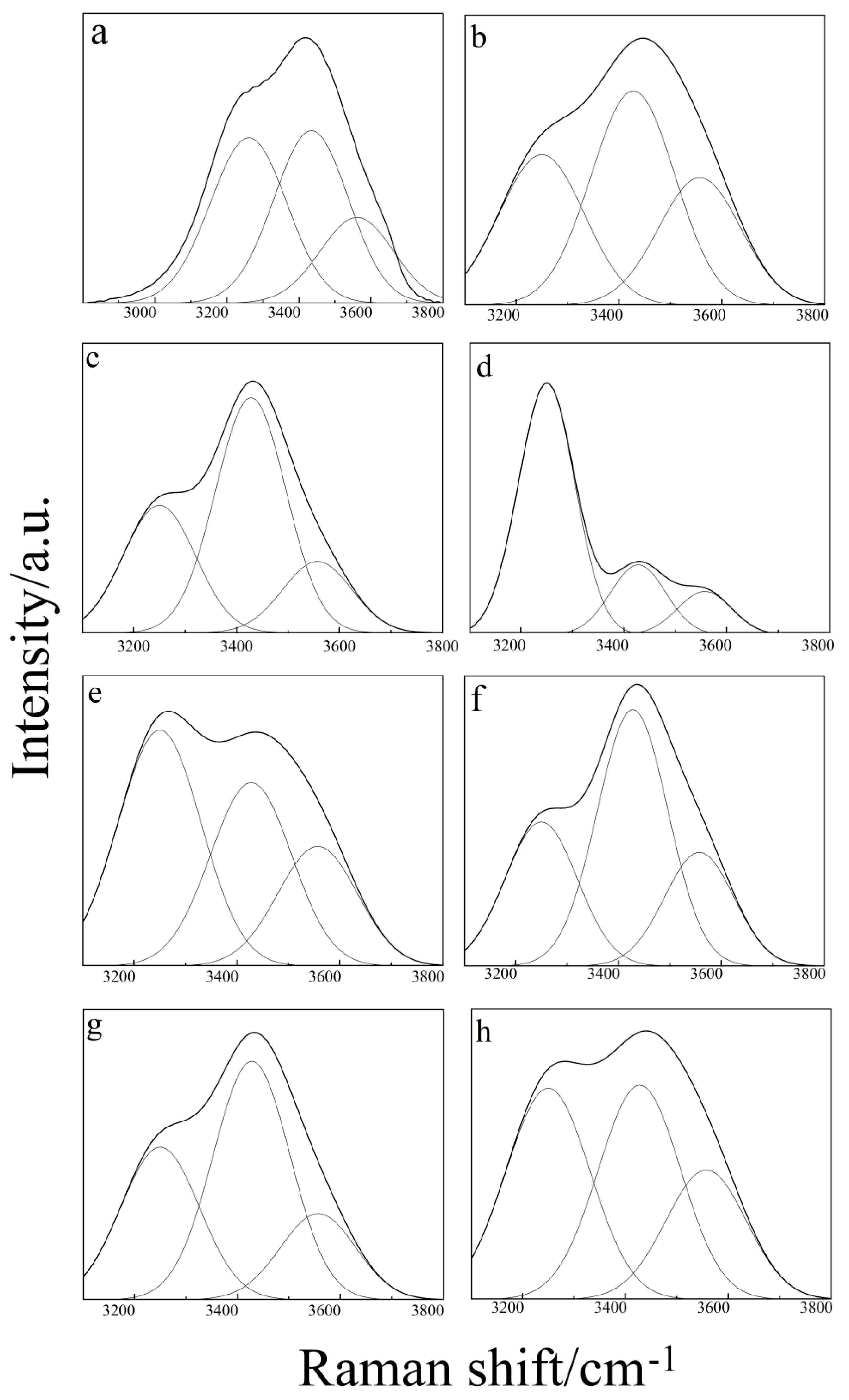
2.1. Raman Spectroscopy of Binary Aqueous Solution
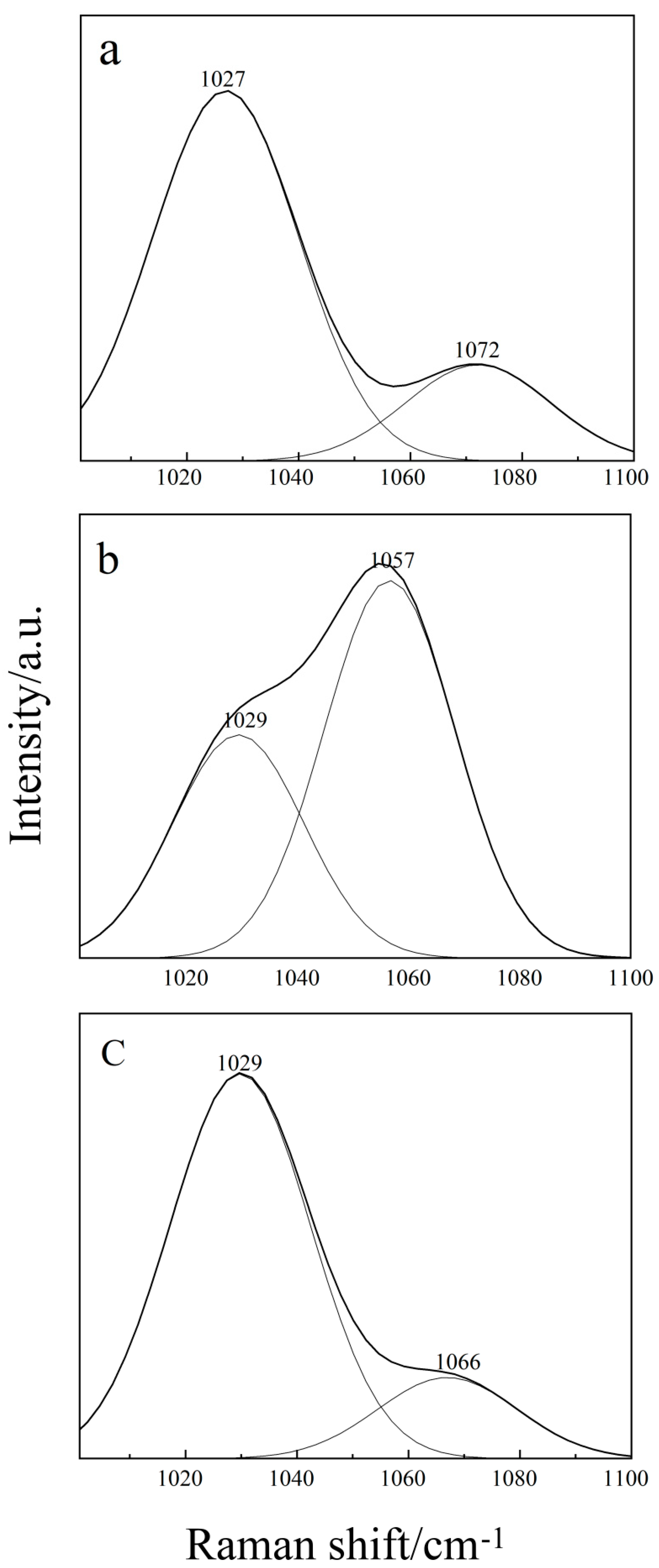
2.2. Raman Spectroscopy of the Binary THF–DMSO Solution
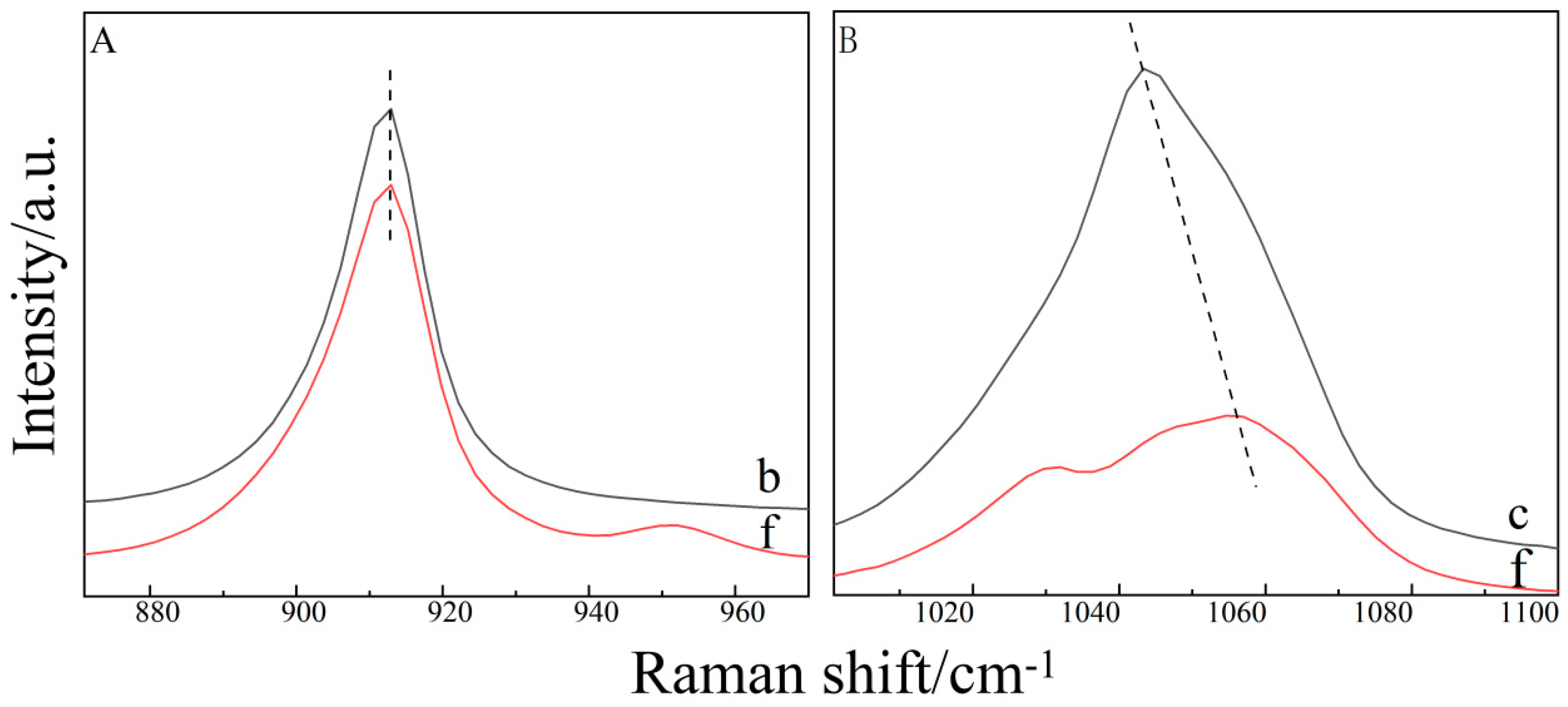
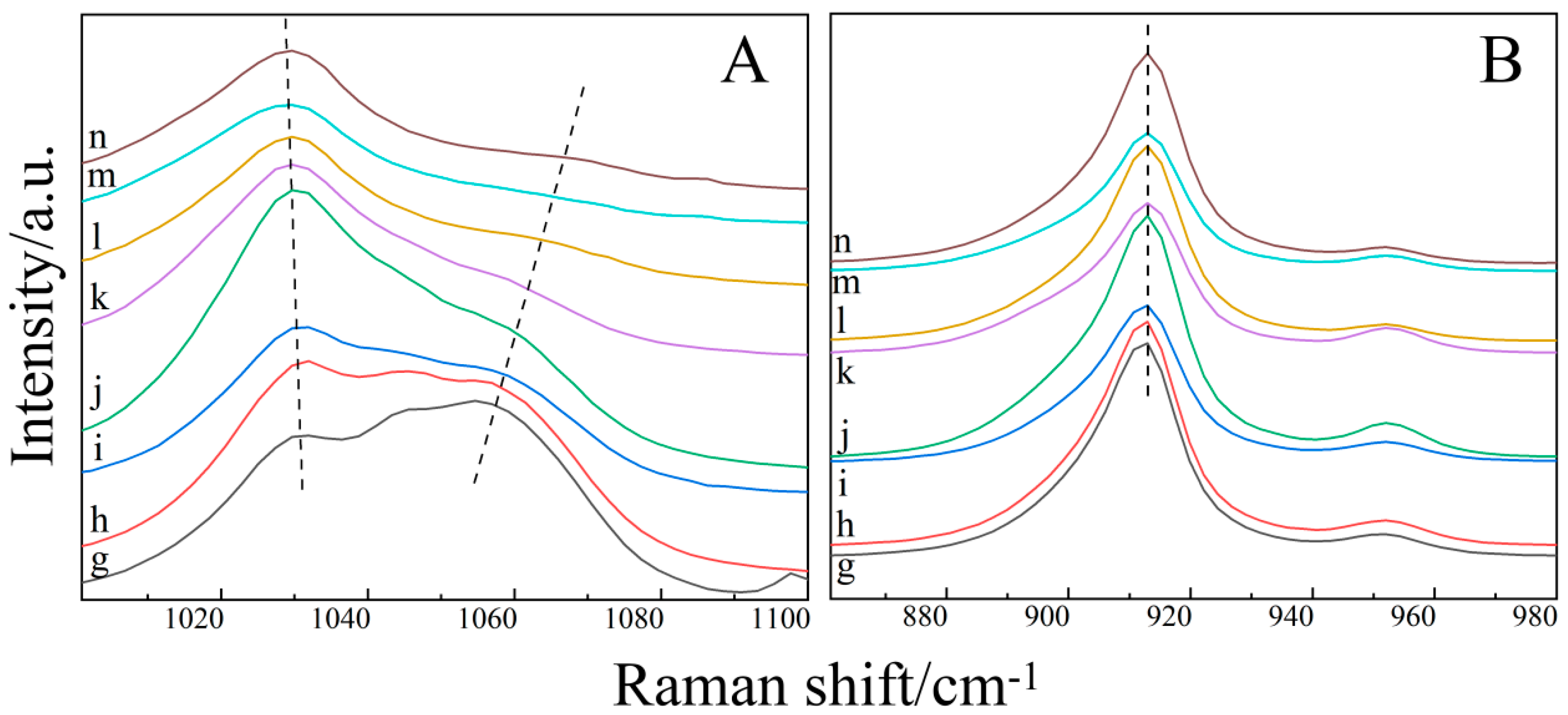
2.3. Raman Spectroscopy of the Ternary Aqueous Solution
3. Experimental
3.1. Reagents and Instruments
3.2. Sample Preparation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sastry, S. Water structure: Order and oddities. Nature 2001, 409, 300–301. [Google Scholar] [CrossRef]
- Franks, F. Water: A Matrix of Life, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2000. [Google Scholar]
- Ball, P. Water-An enduring mystery. Nature 2008, 452, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Ball, P. H2O: A Biography of Water; Weidenfeld & Nicolson: London, UK, 2000. [Google Scholar]
- Zhong, D.P.; Pal, S.K.; Zewail, A.H. Biological water: A critique. Chem. Phys. Lett. 2011, 503, 1–11. [Google Scholar] [CrossRef]
- Russo, J.; Tanaka, H. Understanding water,s anomalies with locally favoured structures. Nat. Commun. 2014, 5, 3556. [Google Scholar] [CrossRef] [PubMed]
- Laage, D.; Elsaesser, T.; Hynes, J.T. Water Dynamics in the Hydration Shells of Biomolecules. Chem. Rev. 2017, 117, 10694–10718. [Google Scholar] [CrossRef]
- Geissler, P.L.; Dellago, C.; Chandler, D.; Hutter, J.; Parrinello, M. Autoionization in Liquid Water. Science 2001, 291, 2121–2124. [Google Scholar] [CrossRef]
- Tokmakpff, A. Shining Light on the Rapidly Evolving Structure of Water. Science 2007, 317, 54–55. [Google Scholar] [CrossRef]
- Tu, Y.S.; Fang, H.P. The micro structure of liquid water. Physics 2010, 39, 79–84. [Google Scholar]
- Conferring, J.R.; Benedictine, P.G. Relationship between structural order and the anomalies of liquid water. Nature 2001, 409, 318–321. [Google Scholar]
- Li, F.B.; Li, Z.L.; Wang, S.H.; Li, S.; Men, Z.W.; Ouyang, S.L. Structure of water molecules from Raman measurements of cooling different concentrations of NaOH solutions. Spectrochim. Acta A 2017, 183, 425–430. [Google Scholar] [CrossRef]
- Kazuhiko Ozutsumi, M.K. The Number of Water-Water Hydrogen Bonds in Water-Tetrahydrofuran and Water-Acetone Binary Mixtures Determined by Means of X-Ray Scattering. J. Solution Chem. 2008, 37, 841–856. [Google Scholar]
- Ouyang, S.L.; Zhou, M.; Cao, B.; Lu, G.H.; Gao, S.Q.; Li, Z.W. Raman Spectral Analysis of the Anti-freezing Mechanism of Dimethyl Sulfoxide. Chem. J. Chin. U 2008, 29, 2055–2058. [Google Scholar]
- Ouyang, S.L.; Wu, N.N.; Sun, C.L.; Liu, J.Y.; Li, Z.W. Investigation of inter-molecular hydrogen bonding in the binary mixture (acetone + water) by concentration dependent Raman study and ab initio calculations. Chin. Phys. B 2010, 19, 093103. [Google Scholar] [CrossRef]
- Charisiadis, P.; Kontogianni, V.G.; Tsiafoulis, C.G.; Tzakos, A.G.; Siskos, M.; Gerothanassis, I.P. 1H-NMR as a Structural and Analytical Tool of Intra- and Intermolecular Hydrogen Bonds of Phenol-Containing Natural Products and Model Compounds. Molecules 2014, 19, 13643–13682. [Google Scholar] [CrossRef]
- Yee, A.A.; Savchenko, A.Y.; Ignachenko, A.; Lukin, J.A.; Xu, X.H.; Skarina, T.; Evdokimova, E.G.; Liu, C.; Semesi, A.; Guido, V.; et al. NMR and X-ray crystallography, complementary tools in structural proteomics of small proteins. J. Am. Chem. Soc. 2005, 127, 16512–16517. [Google Scholar] [CrossRef]
- Gilbert, A.S. Hydrogen Bonding and Other Physicochemical Interactions Studied By IR and Raman Spectroscopy. Encycl. Spectrosc. Spectrom. 1999, 837–843. [Google Scholar]
- Wu, N.N.; Zhang, X.F.; Ouyang, S.L.; Han, X.G. Effect of Hydrogen Bond on Line Width of Raman Spectra in Three Aqueous Solutions, Spectrosc. Spect. Anal. 2012, 32, 3031–3034. [Google Scholar]
- Hu, Q.C.; Ouyang, S.L.; Li, J.; Zhao, C. Raman spectroscopic investigation on pure D2O/H2O from 303 to 573 K: Interpretation and implications for water structure. J. Raman Spectrosc. 2017, 19, 21540–21547. [Google Scholar] [CrossRef]
- Solomonov, B.N.; Varfolomeev, M.A.; Abaidullina, D.I. Cooperative hydrogen bonding in solution: Influence of molecule structure. Vib. Spectrosc. 2007, 43, 380–386. [Google Scholar] [CrossRef]
- Alves, W.A.; Antunes, Q.A.C. Lewis acid-base adducts: A quantitative Raman analysis of formamide and dimethylsulfoxide mixtures. Spectrochim. Acta A 2007, 67, 847–851. [Google Scholar] [CrossRef]
- Ivana, D.; Rémy, C.; Patrice, B.; Mario, M.; Jean-Marie, C.; Marc, D.F. Water-ice phase transition probed by Raman Spectroscopy. J. Raman Spectrosc. 2011, 42, 1408–1412. [Google Scholar]
- Li, F.; Skinner, J.L. Infrared and Raman line shapes for ice Ih. II. H2O and D2O. J. Chem. Phys. 2010, 132, 204505. [Google Scholar] [CrossRef] [PubMed]
- Auer, B.; Kumar, R.; Schmidt, J.R.; Skinner, J.L. Hydrogen bonding and Raman, IR, and 2D-IR spectroscopy of dilute HOD in liquid D2O. Proc. Natl. Acad. Sci. USA 2007, 104, 14215. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, U.; Nordlund, D.; Wernet, P.; Odelius, M.; Pettersson, L.G.M.; Nilsson, A. Isotope effects in liquid water probed by X-ray Raman spectroscopy. Phys. Rev. B 2007, 76, 4202. [Google Scholar] [CrossRef]
- Weng, S.F. Fourier Transform Infrared Spectrum Analysis, 2nd ed.; Chemical Industry Press: Beijing, China, 2010; pp. 2–41. [Google Scholar]
- Madigosky, W.M.; Warfield, R.W. Ultrasonic measurements and liquid structure of DMSO-water mixture. J. Chem. Phys. 1983, 78, 1912–1916. [Google Scholar] [CrossRef]
- Benevenuto, R.L.; Alves, W.A. Acid-base interactions between formamide and tetrahydrofuran molecules: Raman investigation of speciation in a binary system of biological interest. J. Raman Spectrosc. 2008, 39, 490–494. [Google Scholar] [CrossRef]
- Alves, W.A. Preferential interactions in the formamide/tetrahydrofuran/dioxane system: A study by Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 366–369. [Google Scholar] [CrossRef]
- Nayak, J.N.; Aralaguppi, M.I.; Naidu, B.V.K.; Aminabhavi, T.M. Thermodynamic Properties of Water + Tetrahydrofuran and Water + 1,4-Dioxane Mixtures at (303.15, 313.15, and 323.15) K. J. Chem. Eng. 2004, 49, 468–474. [Google Scholar] [CrossRef]
- Strajbl, M.; Baumruk, V.; Florian, J. Scaled Quantum Mechanical Force Fields and Vibrational Spectra of Nucleic Acid Constituents. 9. Tetrahydrofuran. J. Phys. Chem. B 1998, 102, 1314–1319. [Google Scholar] [CrossRef]
- Singh, R.K.; Bhriguvansh, P.; Asthana, B.P.; Verma, A.L. Raman study of vibrational dephasing in hydrogen-bonded binary and ternary complexes of C6H5Cl and methanol. Chem. Phys. Lett. 1998, 296, 611–618. [Google Scholar] [CrossRef]
- Kirchner, B.; Reiher, M. The Secret of Dimethyl Sulfoxide-Water Mixtures. A Quantum Chemical Study of 1DMSO-nWater Clusters. J. Am. Chem. Soc. 2002, 124, 6206–6215. [Google Scholar] [CrossRef] [PubMed]

| Sample | a | d | e | g | h | j | k | m |
|---|---|---|---|---|---|---|---|---|
| (IC2 + IC3)/IC1 | 1.58 | 2.20 | 2.40 | 0.45 | 1.30 | 2.55 | 2.14 | 1.60 |
| Sample | n(H2O):n(THF):n(DMSO) | Sample | n(H2O):n(THF):n(DMSO) |
|---|---|---|---|
| a | 1:0:0 | h | 0.3:1:1 |
| b | 0:1:0 | i | 0.5:1:1 |
| c | 0:0:1 | j | 1:1:1 |
| d | 1:1:0 | k | 1.5:1:1 |
| e | 1:0:1 | l | 2:1:1 |
| f | 0:1:1 | m | 2.5:1:1 |
| g | 0.1:1:1 | n | 3:1:1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhang, M.; Huang, B.; Wu, N.; Ouyang, S. Raman Spectroscopy for the Competition of Hydrogen Bonds in Ternary (H2O–THF–DMSO) Aqueous Solutions. Molecules 2019, 24, 3666. https://doi.org/10.3390/molecules24203666
Liu S, Zhang M, Huang B, Wu N, Ouyang S. Raman Spectroscopy for the Competition of Hydrogen Bonds in Ternary (H2O–THF–DMSO) Aqueous Solutions. Molecules. 2019; 24(20):3666. https://doi.org/10.3390/molecules24203666
Chicago/Turabian StyleLiu, Shiliang, Mingzhe Zhang, Baokun Huang, Nannan Wu, and Shunli Ouyang. 2019. "Raman Spectroscopy for the Competition of Hydrogen Bonds in Ternary (H2O–THF–DMSO) Aqueous Solutions" Molecules 24, no. 20: 3666. https://doi.org/10.3390/molecules24203666
APA StyleLiu, S., Zhang, M., Huang, B., Wu, N., & Ouyang, S. (2019). Raman Spectroscopy for the Competition of Hydrogen Bonds in Ternary (H2O–THF–DMSO) Aqueous Solutions. Molecules, 24(20), 3666. https://doi.org/10.3390/molecules24203666





