Compounding MgCl2·6H2O with NH4Al(SO4)2·12H2O or KAl(SO4)2·12H2O to Obtain Binary Hydrated Salts as High-Performance Phase Change Materials
Abstract
:1. Introduction
2. Results and Discussion
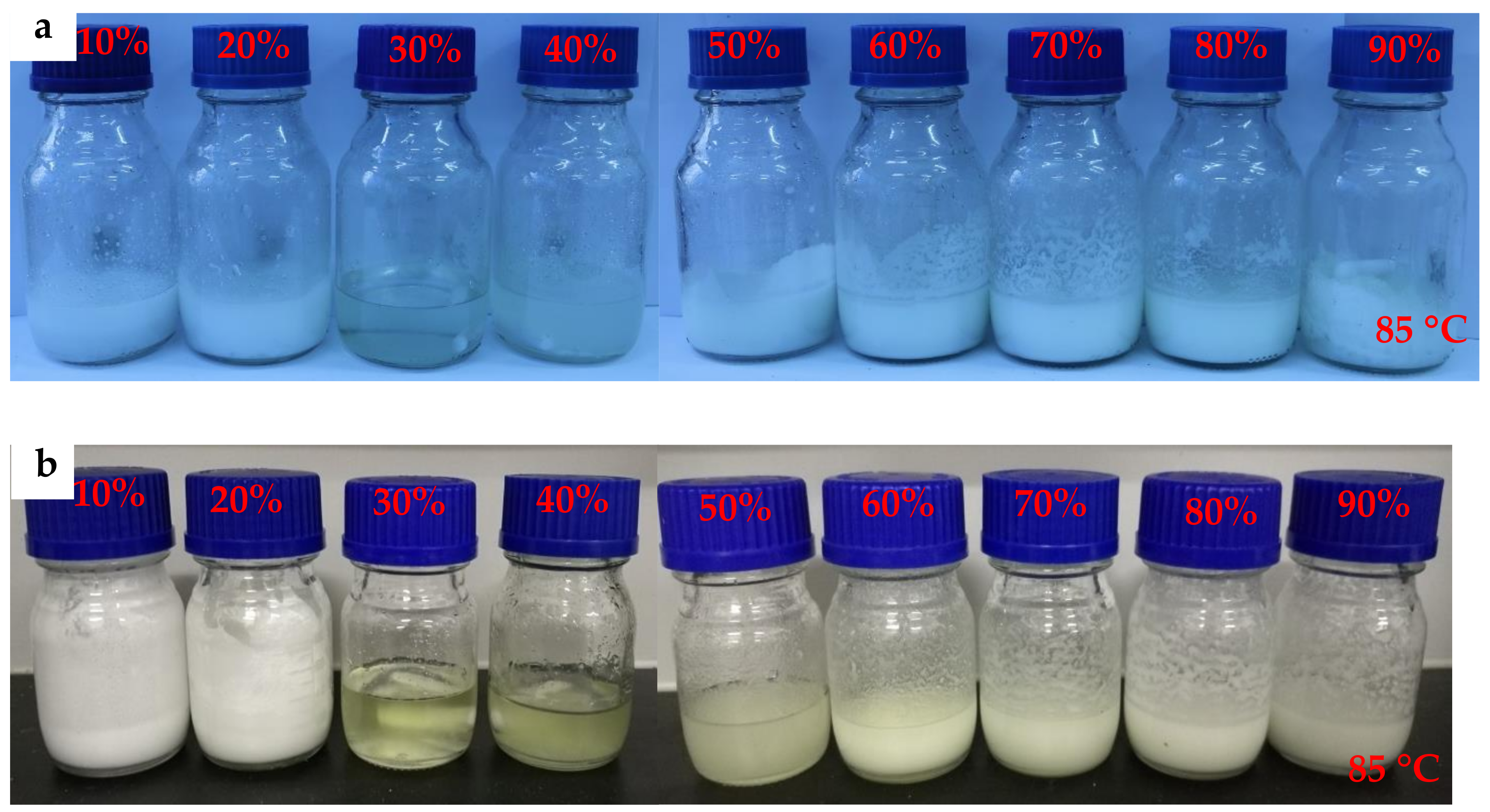
2.1. Determining the Mixture Composition
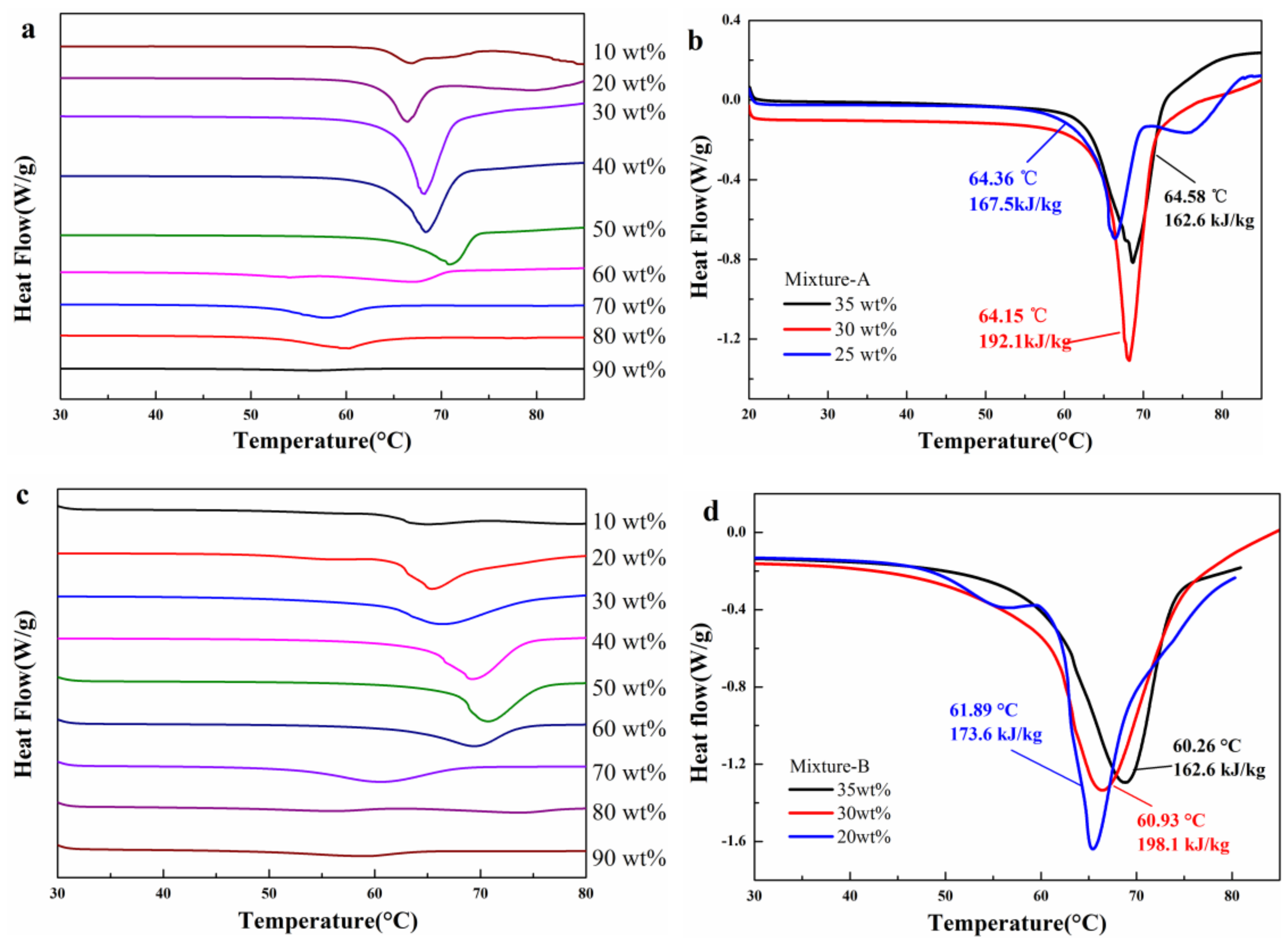
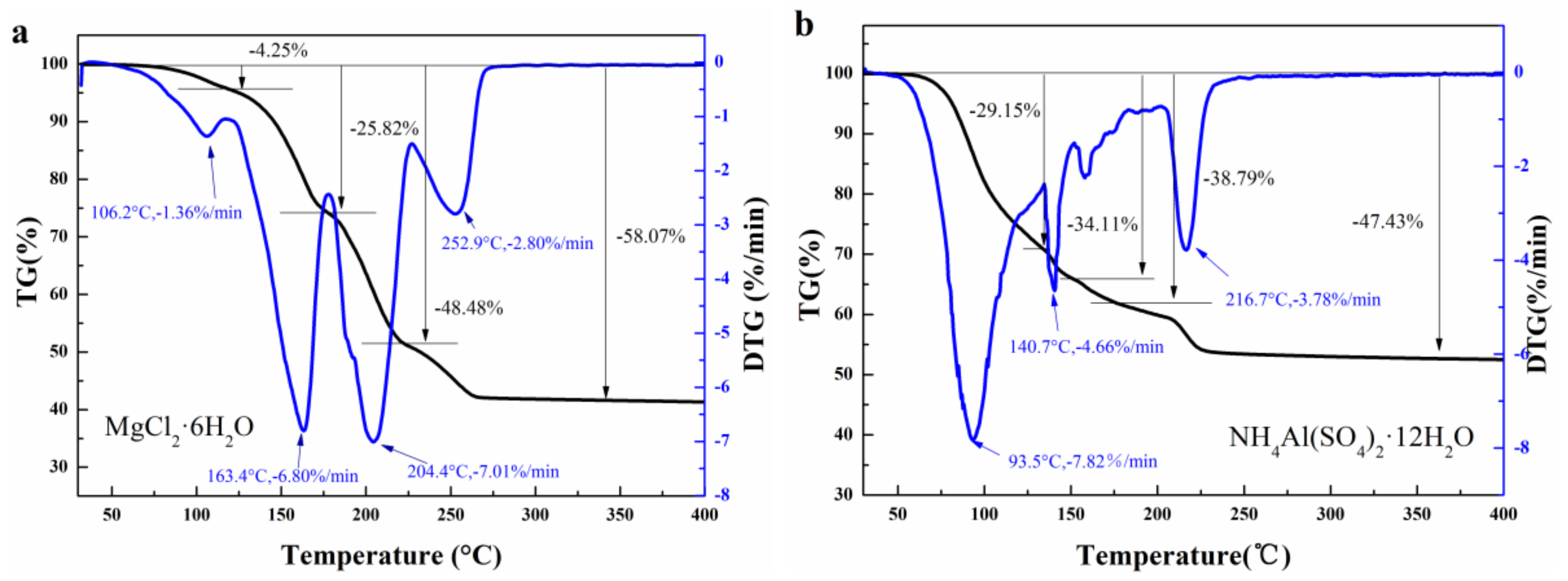
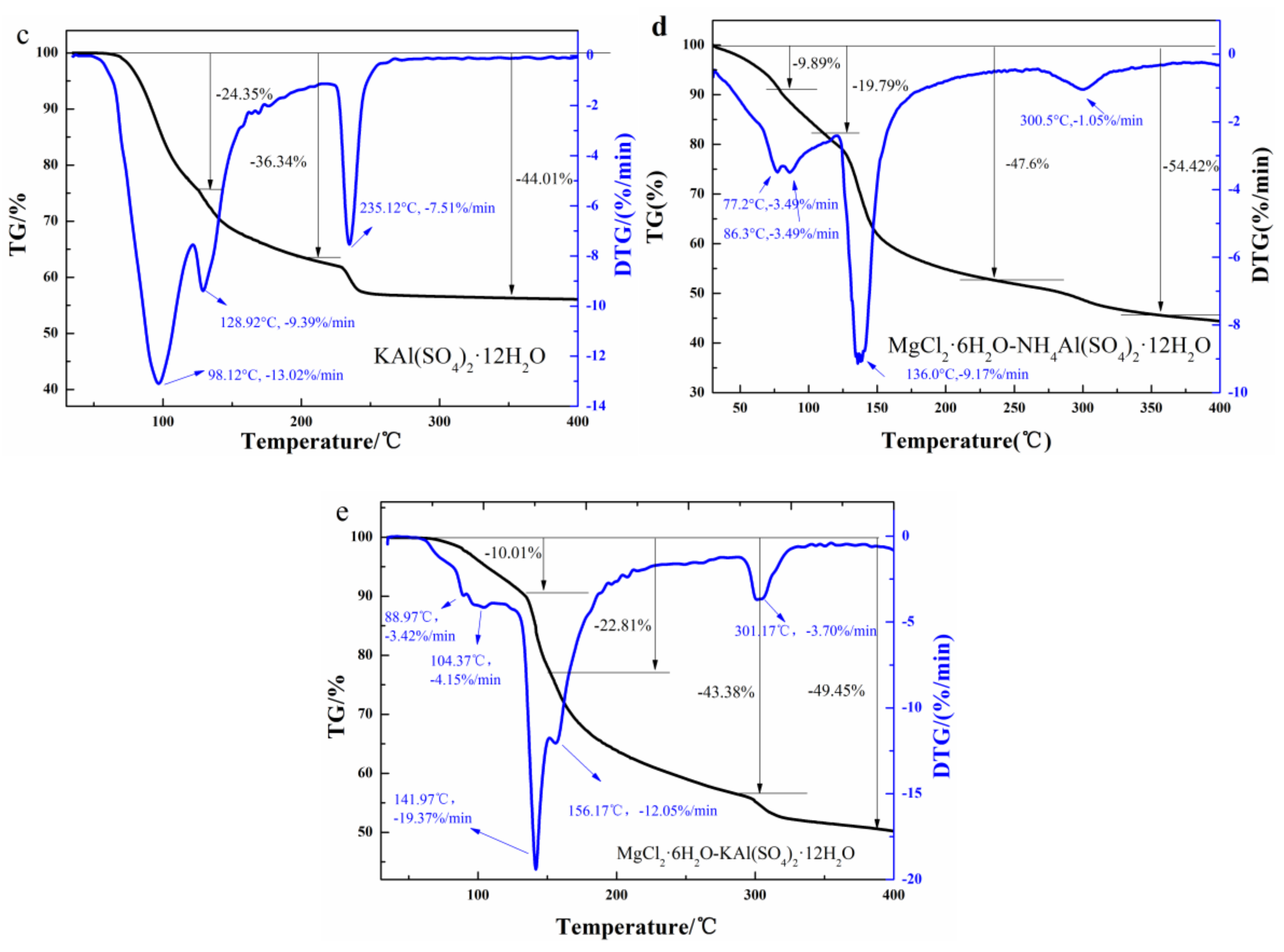
2.2. Structure and Thermal Properties of the Mixtures
2.3. Thermal Reliability of Mixture-A and Mixture-B
3. Materials and Methods
3.1. Materials and Reagents
3.2. Sample Preparation
3.2.1. MgCl2·6H2O-NH4Al(SO4)2·12H2O Mixtures
3.2.2. MgCl2·6H2O-KAl(SO4)2·12H2O Mixtures
3.3. Characterization
3.4. Measurement of Cooling Curves
4. Conclusions
- 1)
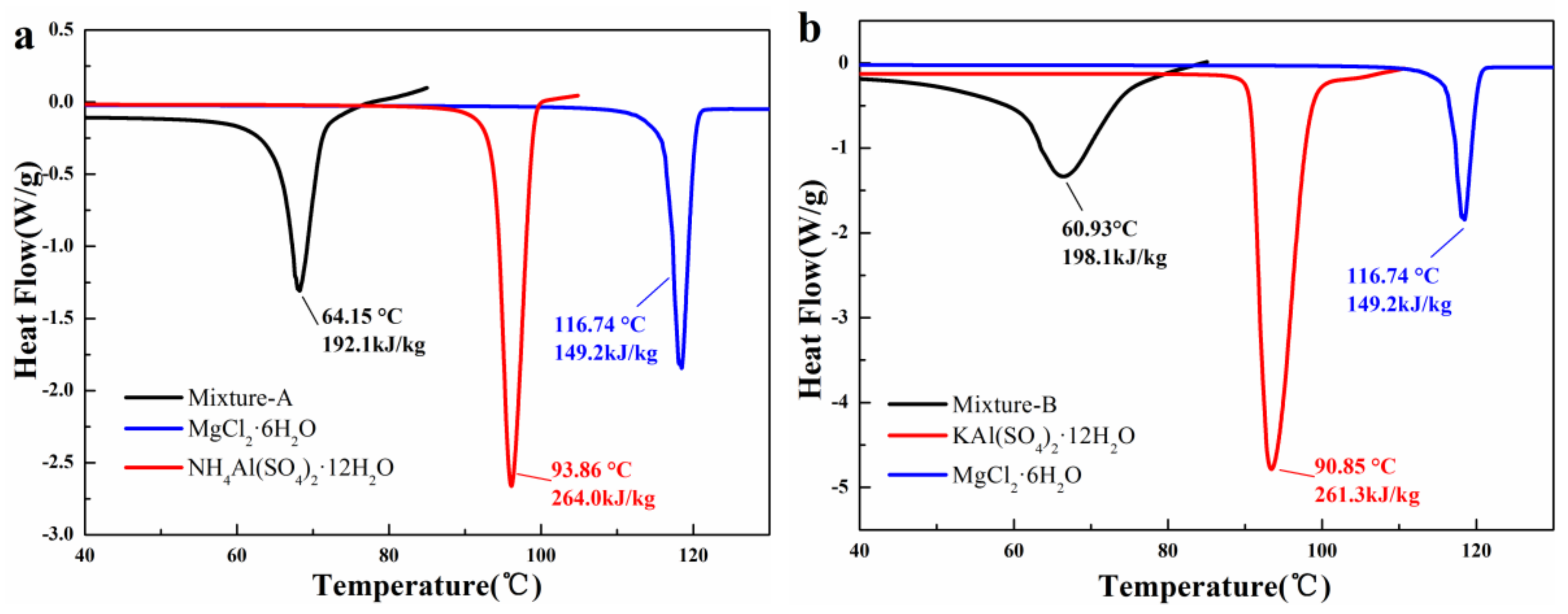
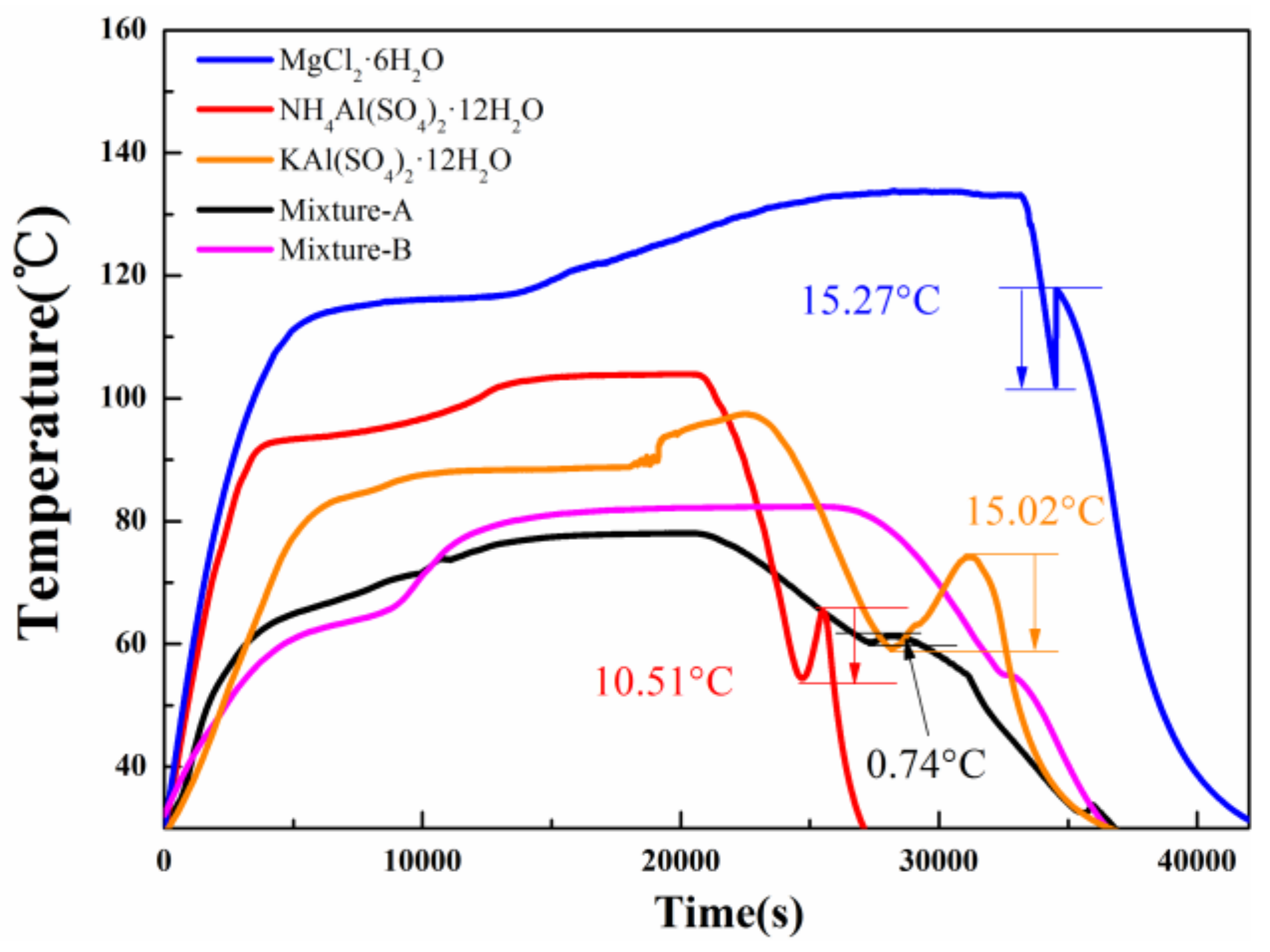
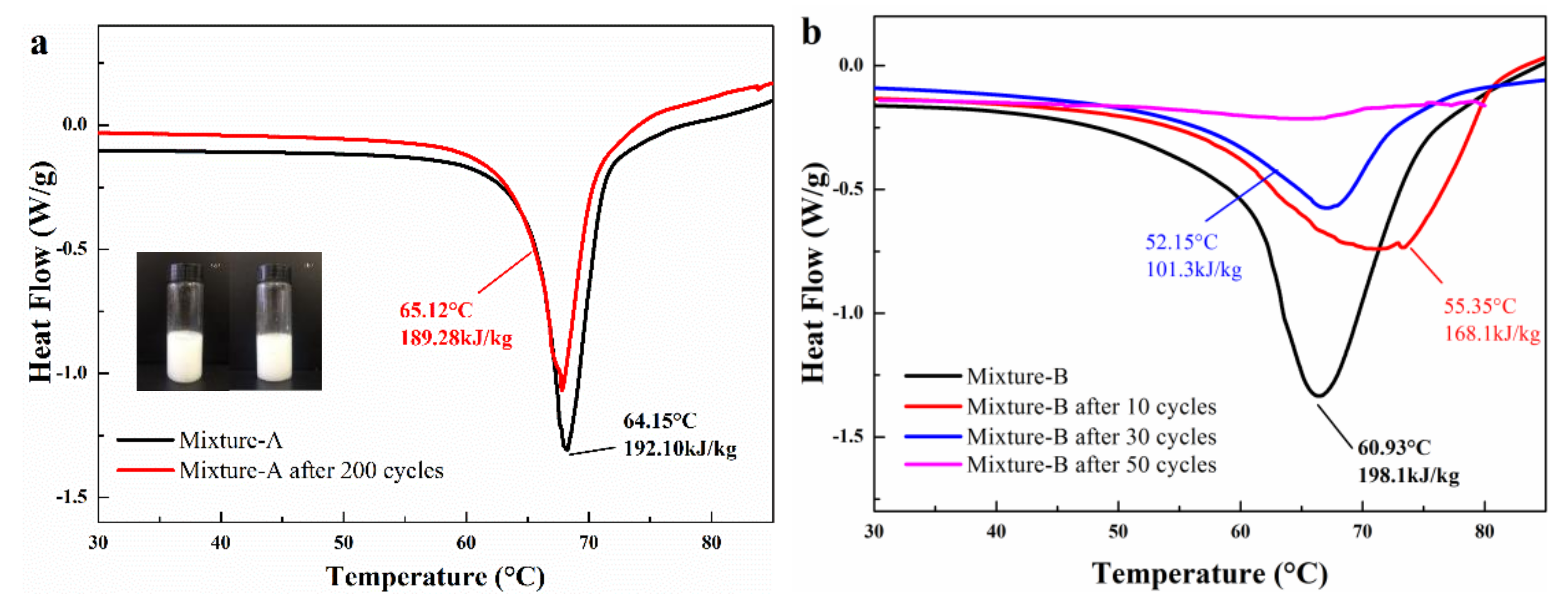
- The mass fraction of MgCl2·6H2O in mixture-A or mixture-B is determined to be 30 wt%. It is found that the mixture-A has a melting point of 64.15 °C, a melting enthalpy of 192.1 kJ/kg, and a very low supercooling degree (0.74 °C). On the other hand, the melting point and melting enthalpy of mixture-B without supercooling degree are 60.93 °C and 198.1 kJ/kg, respectively.
- 1)
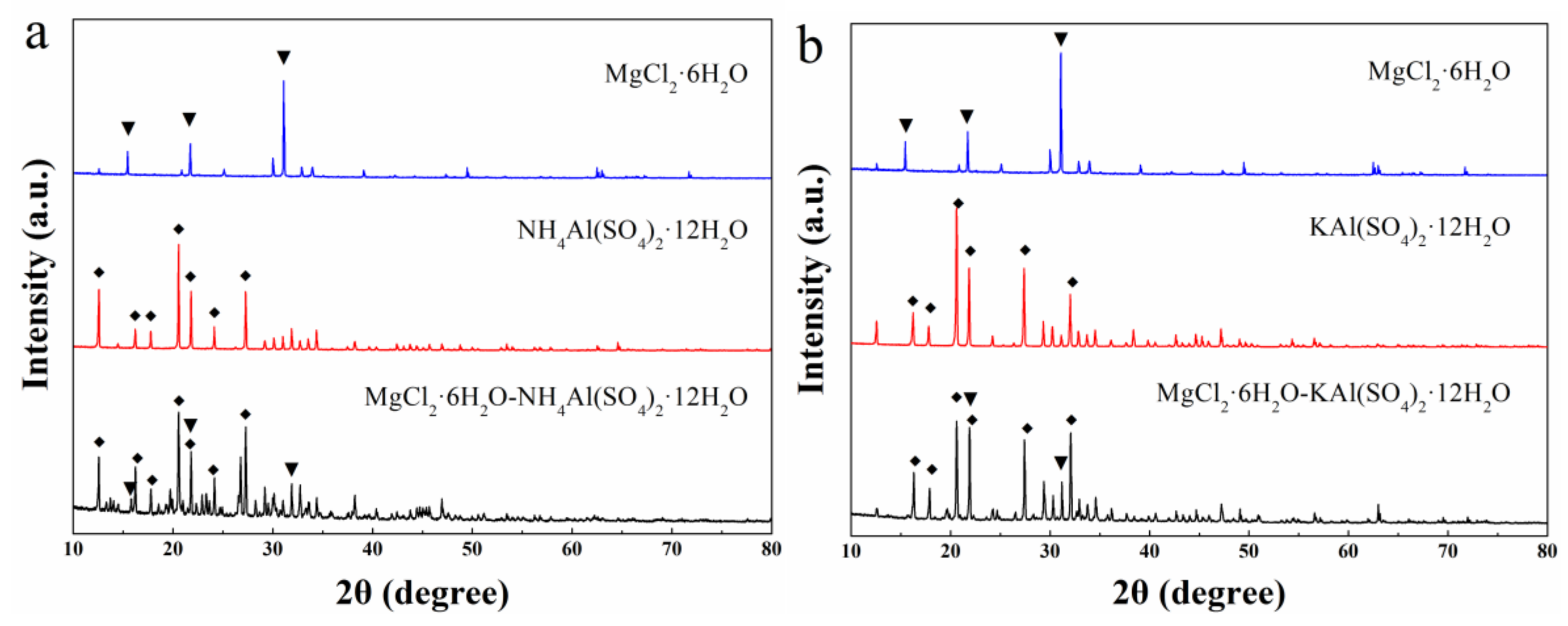
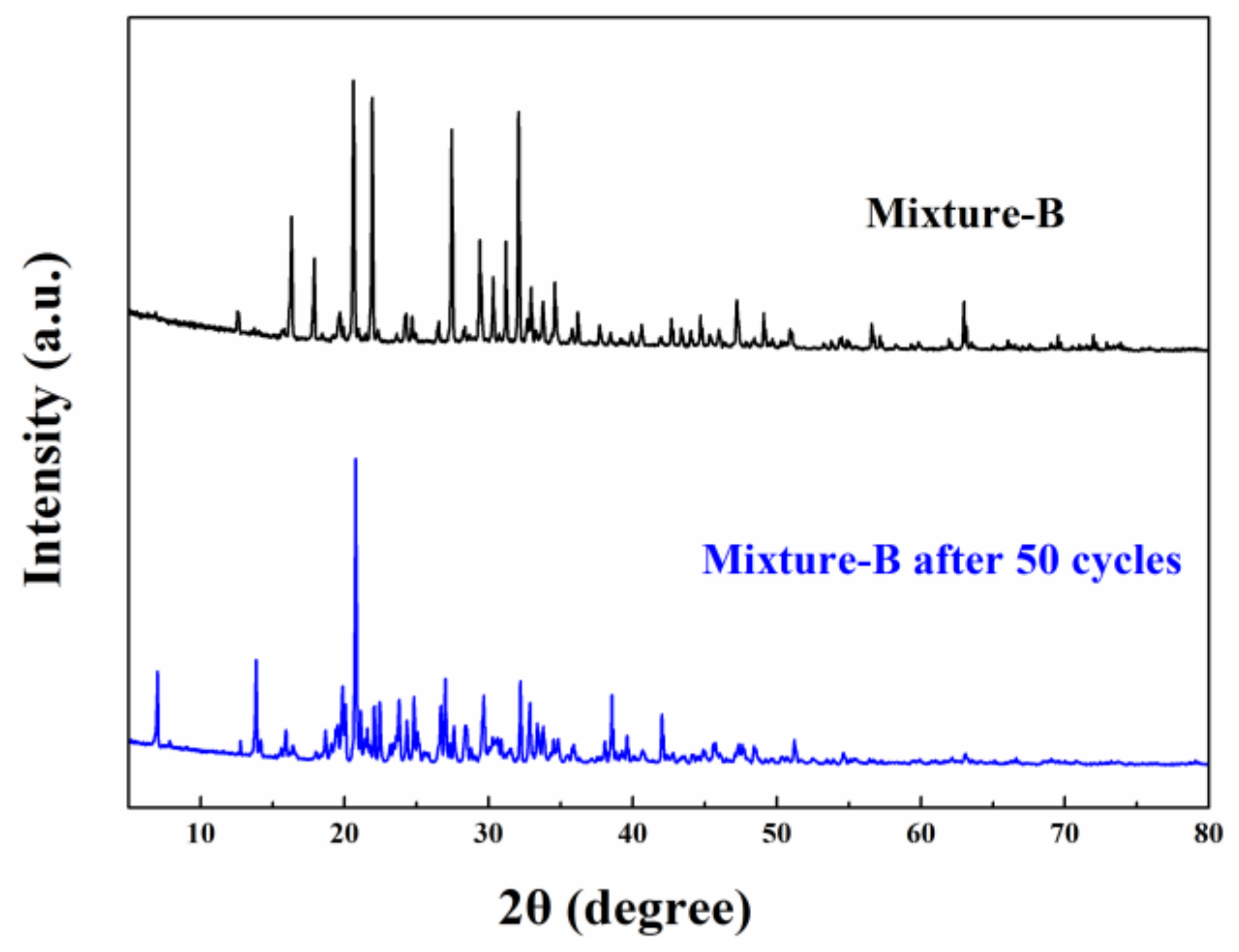
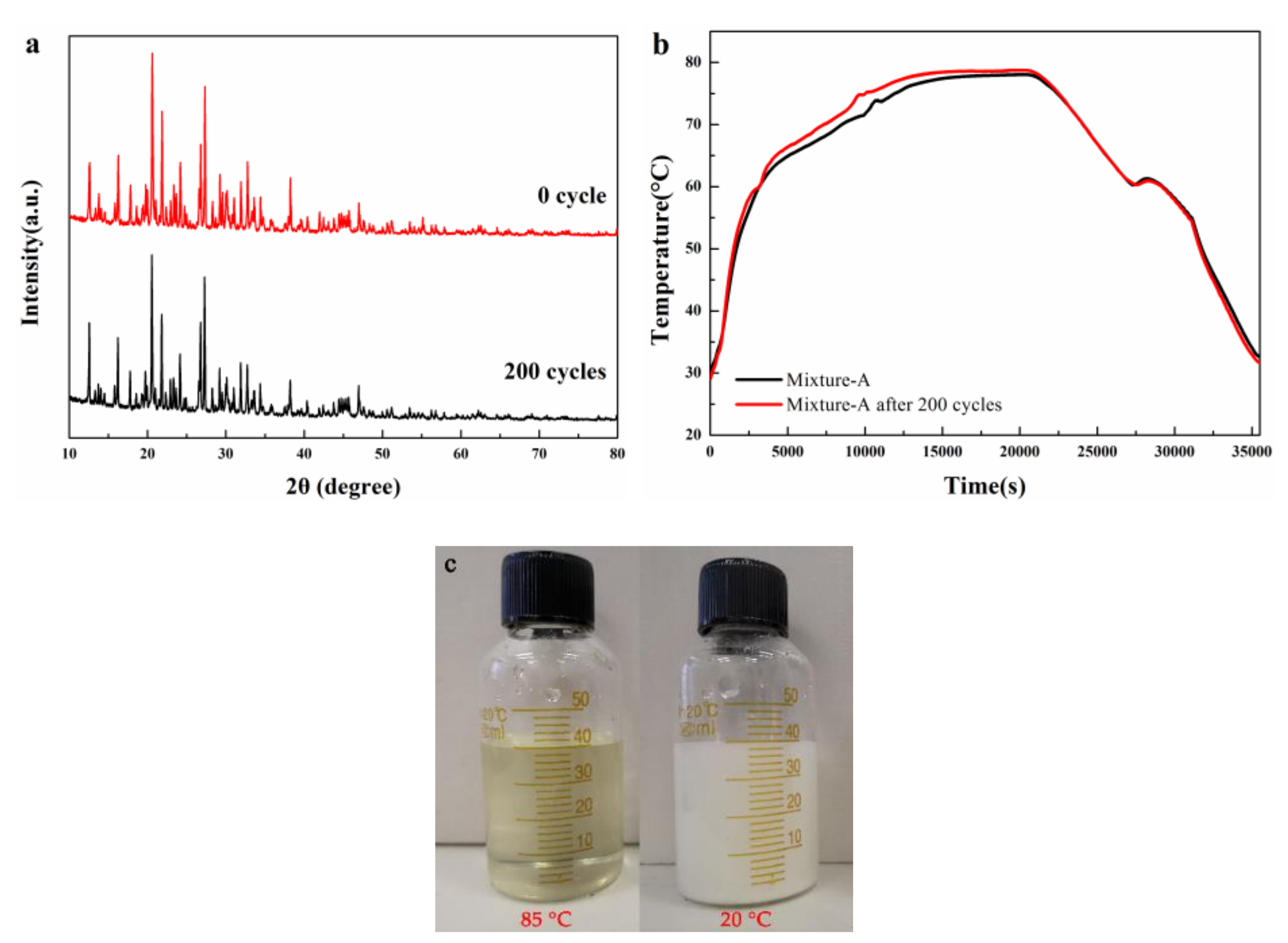
- The XRD pattern of mixture-A shows that MgCl2·6H2O and NH4Al(SO4)2·12H2O combined physically and have no chemical reaction, like mixture-B (MgCl2·6H2O and KAl(SO4)2·12H2O).
- 1)
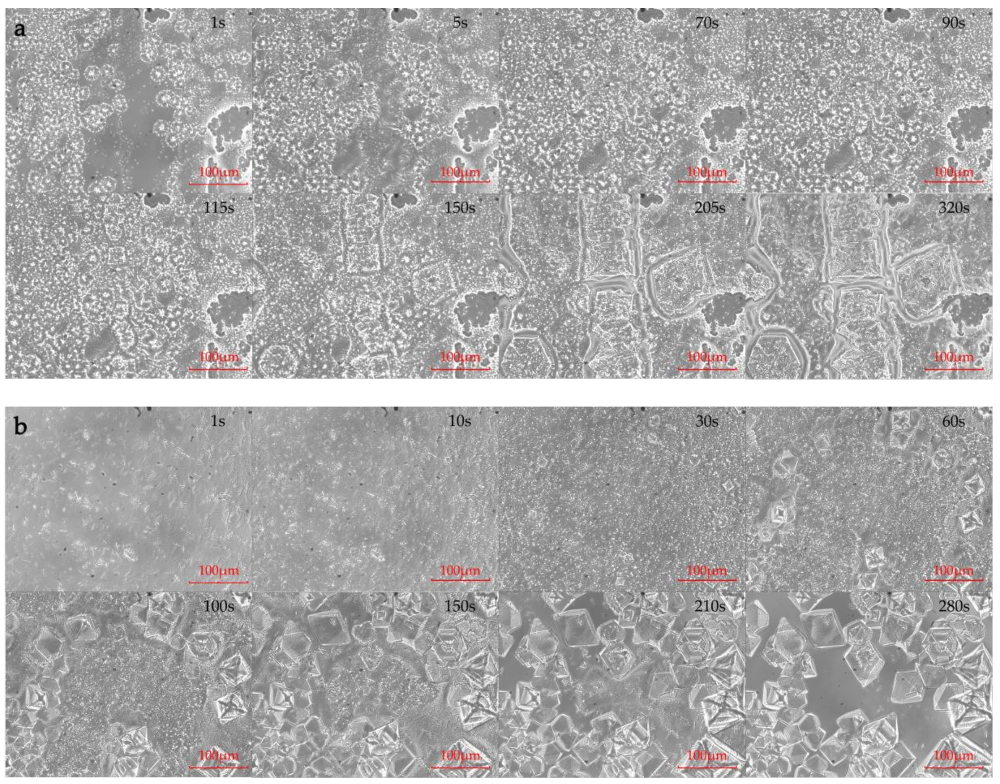
- The polarized microscope images show that the crystallization behaviors of mixture-A and mixture-B. The acicular crystals (MgCl2·6H2O) not only attach on the surface of the octahedral crystals (NH4Al(SO4)2·12H2O) but also stick into the gaps between octahedral crystals. Furthermore, the free acicular crystals (MgCl2·6H2O) in the mixture-B eventually only adhere to the surface of the octahedral crystals (KAl(SO4)2·12H2O).
- 1)
- The 200 heating-cooling cycle test reveals that the mixture-A possesses good structure stability and excellent thermal reliability while the mixture-B has poor thermal reliability. The suitable phase change temperature, high latent heat along with excellent thermal reliability make the MgCl2·6H2O-NH4Al(SO4)2·12H2O mixture show great potential in solar water heating systems.
- 1)
- In conclusion, with a suitable melting point, high latent heat, good structure stability and excellent thermal reliability, mixture-A is a highly promising thermal energy storage phase change material for SWH system. In future work, the heat transfer efficiency of mixture-A and its compatibility with devices will require further research and exploration to better apply materials to practical SWH system or other thermal storage system.
Author Contributions
Funding
Conflicts of Interest
References
- Alva, G.; Lin, Y.; Fang, G. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Farid, M.M.; Khudhair, A.M.; Razack, S.A.K.; Al-Hallaj, S. A review on phase change energy storage: Materials and applications. Energ. Convers. Manag. 2004, 45, 1597–1615. [Google Scholar] [CrossRef]
- Zalba, B.; Marı́n, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sust. Energ. Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, M.H.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.S.; Sahin, A.Z. A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sust. Energ. Rev. 2017, 70, 1072–1089. [Google Scholar] [CrossRef]
- Sun, W.C.; Huang, R.; Ling, Z.Y.; Fang, X.M.; Zhang, Z.G. Two types of composite phase change panels containing a ternary hydrated salt mixture for use in building envelope and ventilation system. Energ. Convers. Manag. 2018, 177, 306–314. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Y.; Zheng, S.; Park, Y.; Frost, R.L. Preparation and thermal energy storage properties of paraffin/calcined diatomite composites as form-stable phase change materials. Therm. Acta 2013, 558, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Özonur, Y.; Mazman, M.; Paksoy, H.Ö.; Evliya, H. Microencapsulation of coco fatty acid mixture for thermal energy storage with phase change material. Int. J. Energ. Res. 2006, 30, 741–749. [Google Scholar] [CrossRef]
- Alper Aydın, A. High-chain fatty acid esters of 1-octadecanol as novel organic phase change materials and mathematical correlations for estimating the thermal properties of higher fatty acid esters’ homologous series. Sol. Energ. Mater. Sol. Cells 2013, 113, 44–51. [Google Scholar] [CrossRef]
- Alkan, C.; Sari, A. Fatty acid/poly(methyl methacrylate) (PMMA) blends as form-stable phase change materials for latent heat thermal energy storage. Sol. Energ. 2008, 82, 118–124. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Wang, J.; Fang, J. From anisotropic graphene aerogels to electron- and photo-driven phase change composites. J. Mater. Chem. A 2016, 4, 17042–11704. [Google Scholar] [CrossRef]
- Ye, R.; Zhang, C.; Sun, W.; Fang, X.; Zhang, Z. Novel wall panels containing CaCl2·6H2O-Mg(NO3)2·6H2O/expanded graphite composites with different phase change temperatures for building energy savings. Energ. Buildings 2018, 176, 407–417. [Google Scholar] [CrossRef]
- da Cunha, J.P.; Eames, P. Thermal energy storage for low and medium temperature applications using phase change materials—A review. Appl. Energ. 2016, 177, 227–238. [Google Scholar] [CrossRef]
- Gasia, J.; Miró, L.; Cabeza, L.F. Review on system and materials requirements for high temperature thermal energy storage. Part 1: General requirements. Renew. Sust. Energ. Rev. 2017, 75, 1320–1338. [Google Scholar] [CrossRef]
- Sharma, A.; Chen, C.R. Solar water heating system with phase change materials. Int. Rev. Chem. Eng. Rapid Comm. 2009. [Google Scholar]
- Sarı, A.; Biçer, A. Preparation and thermal energy storage properties of building material-based composites as novel form-stable PCMs. Energ. Buildings 2012, 51, 73–83. [Google Scholar] [CrossRef]
- Zhou, G.; Yang, Y.; Wang, X.; Zhou, S. Numerical analysis of effect of shape-stabilized phase change material plates in a building combined with night ventilation. Appl. Energ. 2009, 86, 52–59. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energ. 2012, 92, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Lane, G.A. Phase change materials for energy storage nucleation to prevent supercooling. Sol. Energ. Mater. Sol. Cells 1992, 27, 135–160. [Google Scholar] [CrossRef]
- Hu, P.; Lu, D.-J.; Fan, X.-Y.; Zhou, X.; Chen, Z.-S. Phase change performance of sodium acetate trihydrate with AlN nanoparticles and CMC. Sol. Energ. Mater. Sol. Cells 2011, 95, 2645–2649. [Google Scholar] [CrossRef]
- Garay Ramirez, B.M.L.; Glorieux, C.; San Martin Martinez, E.; Flores Cuautle, J.J.A. Tuning of thermal properties of sodium acetate trihydrate by blending with polymer and silver nanoparticles. Appl. Therm. Eng. 2014, 62, 838–844. [Google Scholar] [CrossRef]
- Ze-Shao, L.D.-J.H.P.Z.B.-B.L.Y.C. Study on the performance of nanoparticles as nucleating agents for sodium acetate trihydrate. J. Eng. Thermophys. 2012, 33, 1279–1282. [Google Scholar]
- Li, G.; Zhang, B.; Li, X.; Zhou, Y.; Sun, Q.; Yun, Q. The preparation, characterization and modification of a new phase change material: CaCl2·6H2O–MgCl2·6H2O eutectic hydrate salt. Sol. Energ. Mater. Sol. Cells 2014, 126, 51–55. [Google Scholar] [CrossRef]
- Mao, J.; Dong, X.; Hou, P.; Lian, H. Preparation research of novel composite phase change materials based on sodium acetate trihydrate. Appl. Therm. Eng. 2017, 118, 817–825. [Google Scholar] [CrossRef]
- Mao, J.; Hou, P.; Liu, R.; Chen, F.; Dong, X. Preparation and thermal properties of SAT-CMC-DSP/EG composite as phase change material. Appl. Therm. Eng. 2017, 119, 585–592. [Google Scholar] [CrossRef]
- Li, T.X.; Wu, D.L.; He, F.; Wang, R.Z. Experimental investigation on copper foam/hydrated salt composite phase change material for thermal energy storage. Int. J. Heat Mass Transf. 2017, 115, 148–157. [Google Scholar] [CrossRef]
- Yuan, K.; Zhou, Y.; Sun, W.; Fang, X.; Zhang, Z. A polymer-coated calcium chloride hexahydrate/expanded graphite composite phase change material with enhanced thermal reliability and good applicability. Compos. Sci. Technol. 2018, 156, 78–86. [Google Scholar] [CrossRef]
- Ye, R.; Lin, W.; Fang, X.; Zhang, Z. A numerical study of building integrated with CaCl2·6H2O/expanded graphite composite phase change material. Appl. Therm. Eng. 2017, 126, 480–488. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A. Capric–myristic acid/expanded perlite composite as form-stable phase change material for latent heat thermal energy storage. Renew. Energ. 2008, 33, 2599–2605. [Google Scholar] [CrossRef]
- Fu, L.; Wang, Q.; Ye, R.; Fang, X.; Zhang, Z. A calcium chloride hexahydrate/expanded perlite composite with good heat storage and insulation properties for building energy conservation. Renew. Energ. 2017, 114, 733–743. [Google Scholar] [CrossRef]
- Konuklu, Y.; Ersoy, O.; Gokce, O. Easy and industrially applicable impregnation process for preparation of diatomite-based phase change material nanocomposites for thermal energy storage. Appl. Therm. Eng. 2015, 91, 759–766. [Google Scholar] [CrossRef]
- Jeong, S.-G.; Jeon, J.; Lee, J.-H.; Kim, S. Optimal preparation of PCM/diatomite composites for enhancing thermal properties. Int. J. Heat Mass Transf. 2013, 62, 711–717. [Google Scholar] [CrossRef]
- Nagano, K.; Ogawa, K.; Mochida, T.; Hayashi, K.; Ogoshi, H. Thermal characteristics of magnesium nitrate hexahydrate and magnesium chloride hexahydrate mixture as a phase change material for effective utilization of urban waste heat. Appl. Therm. Eng. 2004, 24, 221–232. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y. Use of nano-α-Al2O3 to improve binary eutectic hydrated salt as phase change material. Sol. Energ. Mater. Sol. Cells 2017, 160, 18–25. [Google Scholar] [CrossRef]
- Nagano, K.; Ogawa, K.; Mochida, T.; Hayashi, K.; Ogoshi, H. Performance of heat charge/discharge of magnesium nitrate hexahydrate and magnesium chloride hexahydrate mixture to a single vertical tube for a latent heat storage system. Appl. Therm. Eng. 2004, 24, 209–220. [Google Scholar] [CrossRef]
- Ding, Q.; Luo, X.; Lin, X.; Zhang, H. Study of magnesium nitrate hexahydrate and magnesium chloride hexahydrate mixture as phase change material. In Proceedings of the Asia-Pacific Power and Energy Engineering Conference (APPEEC), Sabah, Malaysia, 7–10 October 2018; 2012; pp. 1–4. [Google Scholar]
- Sole, A.; Miro, L.; Barreneche, C.; Martorell, I.; Cabeza, L.F. Review of the T-history method to determine thermophysical properties of phase change materials (PCM). Renew. Sust. Energ. Rev. 2013, 26, 425–436. [Google Scholar] [CrossRef]
- Gunther, E.; Hiebler, S.; Mehling, H.; Redlich, R. Enthalpy of Phase Change Materials as a Function of Temperature: Required Accuracy and Suitable Measurement Methods. Int. J. Thermophys. 2009, 30, 1257–1269. [Google Scholar] [CrossRef]
- Castellon, C.; Gunther, E.; Mehling, H.; Hiebler, S.; Cabeza, L.F. Determination of the enthalpy of PCM as a function of temperature using a heat-flux DSC-A study of different measurement procedures and their accuracy. Int. J. Energ. Res. 2008, 32, 1258–1265. [Google Scholar] [CrossRef]
- Chiu, J.N.W.; Martin, V. Submerged finned heat exchanger latent heat storage design and its experimental verification. Appl. Energ. 2012, 93, 507–516. [Google Scholar] [CrossRef]
- Zhou, G.B.; Xiang, Y.T. Experimental investigations on stable supercooling performance of sodium acetate trihydrate PCM for thermal storage. Sol. Energ. 2017, 155, 1261–1272. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Mixture | Compositions | |
|---|---|---|
| A | MgCl2·6H2O | NH4Al(SO4)2·12H2O |
| B | MgCl2·6H2O | KAl(SO4)2·12H2O |
| Mixture-A | Mixture-B | ||||
|---|---|---|---|---|---|
| FMgCl2·6H2O 1 (wt%) | Tmelt (°C) 2 | △H (kJ/kg) 3 | FMgCl2·6H2O (wt%) | Tmelt (°C) | △H (kJ/kg) |
| 10 | 63.54 | 141.8 | 10 | 61.00 | 165.0 |
| 20 | 63.90 | 131.9 | 20 | 61.89 | 173.6 |
| 30 | 64.15 | 192.1 | 30 | 60.93 | 198.1 |
| 40 | 65.08 | 138.4 | 40 | 64.67 | 173.3 |
| 50 | 65.97 | 83.17 | 50 | 67.92 | 145.6 |
| 60 | 53.90 | 60.13 | 60 | 60.86 | 100.0 |
| 70 | 52.23 | 46.59 | 70 | 50.42 | 92.14 |
| 80 | 51.94 | 45.72 | 80 | 45.49 | 57.30 |
| 90 | 48.00 | 7.747 | 90 | 49.00 | 24.21 |
| NH4Al(SO4)2·12H2O | KAl(SO4)2·12H2O | |||
|---|---|---|---|---|
| Tmelt (°C) | △H (kJ/kg) | Tmelt (°C) | △H (kJ/kg) | |
| 0 cycles | 93.86 | 264.0 | 90.85 | 261.3 |
| After 5 cycles 60–105 °C | 93.21 | 263.2 | 90.31 | 185.9 |
| After 10 cycles 60–105 °C | 93.50 | 251.0 | 91.83 | 107.3 |
| After 20 cycles 60–105 °C | 93.37 | 218.9 | 90.79 | 104.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Zhou, Y.; Feng, J.; Fang, X.; Ling, Z.; Zhang, Z. Compounding MgCl2·6H2O with NH4Al(SO4)2·12H2O or KAl(SO4)2·12H2O to Obtain Binary Hydrated Salts as High-Performance Phase Change Materials. Molecules 2019, 24, 363. https://doi.org/10.3390/molecules24020363
Sun W, Zhou Y, Feng J, Fang X, Ling Z, Zhang Z. Compounding MgCl2·6H2O with NH4Al(SO4)2·12H2O or KAl(SO4)2·12H2O to Obtain Binary Hydrated Salts as High-Performance Phase Change Materials. Molecules. 2019; 24(2):363. https://doi.org/10.3390/molecules24020363
Chicago/Turabian StyleSun, Wanchun, Yan Zhou, Jinxin Feng, Xiaoming Fang, Ziye Ling, and Zhengguo Zhang. 2019. "Compounding MgCl2·6H2O with NH4Al(SO4)2·12H2O or KAl(SO4)2·12H2O to Obtain Binary Hydrated Salts as High-Performance Phase Change Materials" Molecules 24, no. 2: 363. https://doi.org/10.3390/molecules24020363
APA StyleSun, W., Zhou, Y., Feng, J., Fang, X., Ling, Z., & Zhang, Z. (2019). Compounding MgCl2·6H2O with NH4Al(SO4)2·12H2O or KAl(SO4)2·12H2O to Obtain Binary Hydrated Salts as High-Performance Phase Change Materials. Molecules, 24(2), 363. https://doi.org/10.3390/molecules24020363






