A Microbial Transformation Model for Simulating Mammal Metabolism of Artemisinin
Abstract
1. Introduction
2. Results
2.1. Optimization of Ultra-Performance Liquid Chromatography (UPLC) Conditions
2.2. Optimization of Sample Preparation
2.3. Identification of Reference Substances
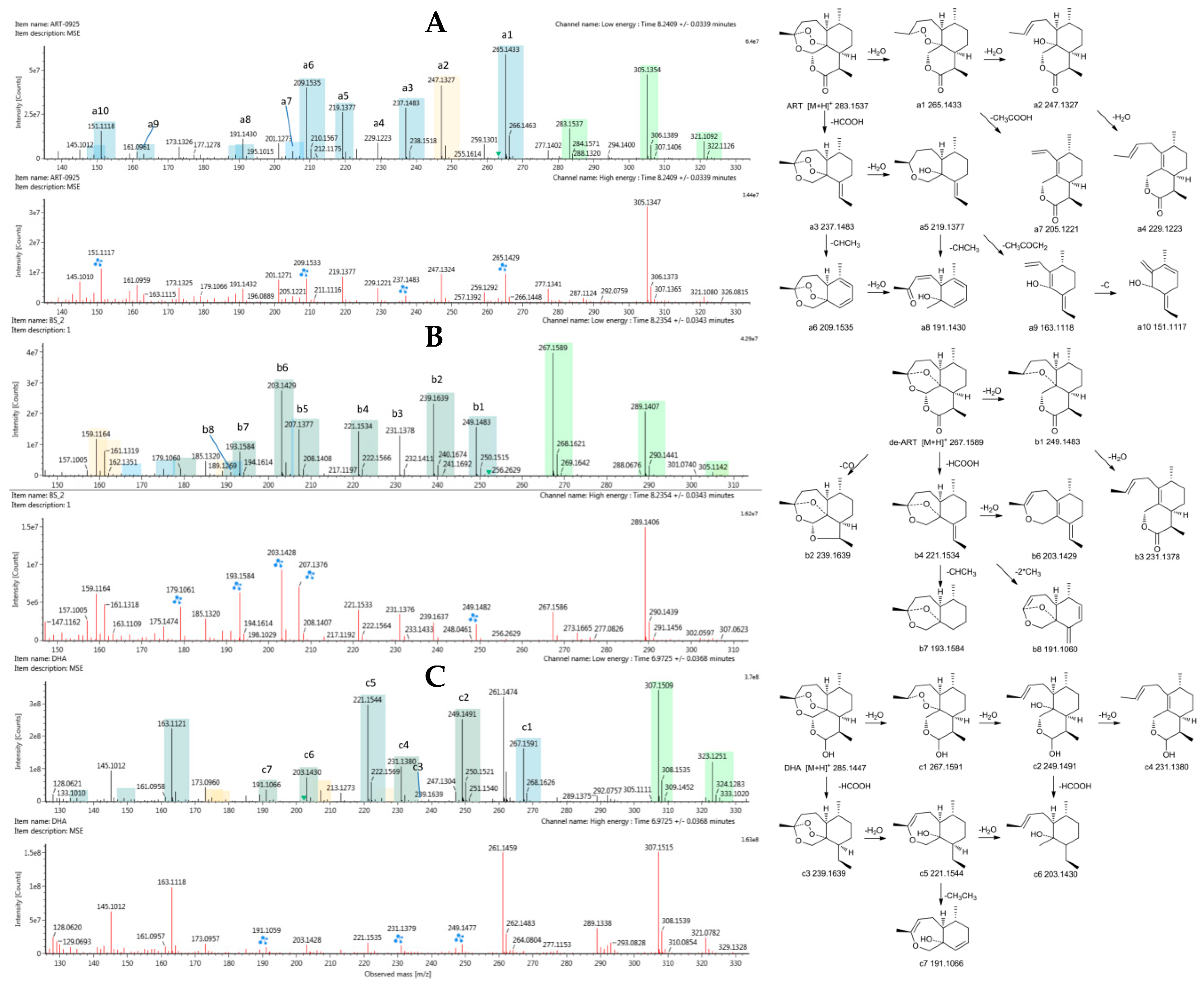
2.4. Identification of Metabolites
2.4.1. Structural Elucidation of ART Metabolites
2.4.2. Comparison of Results
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Instruments and LC–MS/MS Conditions
4.3. MS Data Processing
4.4. Animal Handling and Sample Preparation
4.5. Culture and Biotransformation Procedures
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, C.Z.; Zhao, Y.; Wang, Y.C. Artemisinin: Current state and perspectives for biotechnological production of an antimalarial drug. Appl. Microbiol. Biotechnol. 2006, 72, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Katarzyna, B.; Wanda, B.D. Searching for artemisinin production improvement in plants and microorganisms. Curr. Pharm. Biotechnol. 2011, 12, 1743–1751. [Google Scholar]
- World Health Organization. World Malaria Report 2017; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-156552-3. [Google Scholar]
- Shi, C.C.; Li, H.P.; Yang, Y.F.; Hou, L.F. Anti-inflammatory and immunoregulatory functions of artemisinin and its derivatives. Mediat. Inflamm. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Li, C.C.; Yin, K.; Yang, G. Advance in research on the pharmacological action of the antimalarial artemisinin and its derivates. J. Pathog. Biol. 2016, 11, 185–188. [Google Scholar]
- Wang, J.; Zhang, C.-J.; Chia, W.N.; Loh, C.C.Y.; Li, Z.; Lee, Y.M.; He, Y.; Yuan, L.-X.; Lim, T.K.; Liu, M.; et al. Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Pawluk, S.A.; Wilby, K.J.; Ensom, M.H.H. Pharmacokinetic profile of artemisinin derivatives and companion drugs used in artemisinin-based combination therapies for the treatment of plasmodium falciparum malaria in children. Clin. Pharmacokinet. 2013, 52, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.M. Pharmacokinetic studies of antimalarials; recent developments. Expert Rev. Clin. Pharmacol. 2016, 9, 341–343. [Google Scholar] [CrossRef]
- Gordi, T.; Xie, R.; Huong, N.V. A semiphysiological pharmacokinetic model for artemisinin in healthy subjects incorporating autoinduction of metabolism and saturable first-pass hepatic extraction. Br. J. Clin. Pharmacol. 2005, 59, 189–198. [Google Scholar] [CrossRef]
- Feng, Y.L. Establishment of the Microbial Model with the Activity of CYP3A4. Master’s Thesis, Shenyang Pharmaceutical University, Shenyang, China, May 2007. [Google Scholar]
- Yang, X.W. Establish a Microbial Model with the Activity of Drug-Metabolizing Enzyme CYP2C9. Master’s Thesis, Jilin University, Changchun, China, May 2005. [Google Scholar]
- Lin, L.; Huang, H.; Zhang, P.; Qi, X.; Zhong, D. Microbial transformation of dextromethorphan by cunninghamella blakesleeana as 3.153. Chem. Pharm. Bull. 2007, 55, 658–661. [Google Scholar] [CrossRef]
- Zhang, P.; Lin, L.H.; Huang, H.H.; Xu, H.Y.; Zhong, D.F. Biotransformation of indomethacin by the fungus Cunninghamella blakesleeana. Acta Pharmacol. Sin. 2006, 27, 1097–1102. [Google Scholar] [CrossRef]
- Amadio, J.; Gordon, K.; Murphy, C.D. Biotransformation of flurbiprofen by Cunninghamella Species. Appl. Environ. Microb. 2010, 76, 6299–6303. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.Y.; Huang, B.S.; Chen, Z.L.; Yin, M.L.; Yang, Y.M.; Dai, M.L.; Wang, B.D.; Huang, Z.H. Isolation and identification of the metabolite of artemisinin in human. Zhongguo Yao Li Xue Bao 1983, 4, 194–197. [Google Scholar] [PubMed]
- Liu, T.; Du, F.; Wan, Y.; Zhu, F.; Xing, J. Rapid identification of phase i and ii metabolites of artemisinin antimalarials using ltq-orbitrap hybrid mass spectrometer in combination with online hydrogen/deuterium exchange technique. J. Mass Spectrom. 2011, 46, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Liu, T.; Shen, T.; Zhu, F.; Xing, J. Qualitative-(semi)quantitative data acquisition of artemisinin and its metabolites in rat plasma using an ltq/orbitrap mass spectrometer. J. Mass Spectrom. 2012, 47, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Louw, S.; Njoroge, M.; Chigorimbo-Murefu, N.; Chibale, K. Comparison of electrospray ionisation, atmospheric pressure chemical ionisation and atmospheric pressure photoionisation for the identification of metabolites from labile artemisinin-based anti-malarial drugs using a qtrap® mass spectrometer. Rapid Commun. Mass Spectrom. 2012, 26, 2431–2442. [Google Scholar] [CrossRef]
- Svensson, U.S.H.; Mäki-Jouppila, M.; Hoffmann, K.-J.; Ashton, M. Characterisation of the human liver in vitro metabolic pattern of artemisinin and auto-induction in the rat by use of nonlinear mixed effects modelling. Biopharm. Drug Dispos. 2003, 24, 71–85. [Google Scholar] [CrossRef]
- Svensson, U.S.H.; Ashton, M. Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. J. Clin. Pharmacol. 1999, 48, 528–535. [Google Scholar] [CrossRef]
- Azerad, R. Microbial models for drug metabolism. Biochem. Eng. Biotechnol. 1999, 63, 169–218. [Google Scholar]
- Prasad, G.S.; Govardhan, P.; Girisham, S. Fungal mediated generation of mammalian metabolites of fenofibrate and enhanced pharmacological activity of the main metabolite fenofibric acid. Drug Metab. Lett. 2014, 8, 88–95. [Google Scholar] [CrossRef]
- Hufford, C.D.; Capiton, G.A.; Clark, A.M.; Baker, J.K. Metabolism of imipramine by microorganisms. J. Pharm. Sci. 1981, 70, 151–155. [Google Scholar] [CrossRef]
- Rocha, B.A.; Assis, M.D.; Peti, A.P.; Moraes, L.A.; Moreira, F.L.; Lopes, N.P.; Pospíšil, S.; Gates, P.J.; de Oliveira, A.R. In vitro metabolism of monensin A: Microbial and human liver microsomes models. Xenobiotica 2014, 44, 326–335. [Google Scholar] [CrossRef]
- Qiao, L.; Zhou, Y.Z.; Qi, X.L.; Lin, L.H.; Chen, H.; Pang, L.Y.; Pei, Y.H. Biotransformation of cinobufagin by Cunninghamella elegans. J. Antibiot. 2007, 60, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Parshikov, I.A.; Netrusov, A.I.; Sutherland, J.B. Microbial transformation of antimalarial terpenoids. Biotechnol. Adv. 2012, 30, 1516–1523. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.S.; Parshikov, I.A.; Avery, M.A. Microbial transformations of artemisinin. RPMP Phytochem. Pharmacol. 2007, 17, 115–138. [Google Scholar] [CrossRef]
- Liu, J.H.; Chen, Y.G.; Yu, B.Y.; Chen, Y.J. A novel ketone derivative of artemisinin biotransformed by Streptomyces griseus ATCC 13273. Bioorg. Med. Chem. Lett. 2006, 16, 1909–1912. [Google Scholar] [CrossRef] [PubMed]
- Parshikov, I.A.; Miriyala, B.; Muraleedharan, K.M.; Illendula, A.; Avery, M.A.; Williamson, J.S. Biocatalysis of the Antimalarial Artemisinin by Mucor ramannianus Strains. Pharm. Biol. 2005, 43, 579–582. [Google Scholar] [CrossRef]
- Zhan, J.; Guo, H.; Dai, J.; Zhang, Y.; Guo, D. Microbial transformations of artemisinin by cunninghamella echinulata and aspergillus niger. Tetrahedron Lett. 2002, 43, 4519–4521. [Google Scholar] [CrossRef]
- Parshikov, I.A.; Muraleedharan, K.M.; Avery, M.A. Transformation of artemisinin by Cunninghamella elegans. Appl. Microbiol. Biotechnol. 2004, 64, 782–786. [Google Scholar] [CrossRef]
- Gaur, R.; Darokar, M.P.; Ajayakumar, P.V.; Shukla, R.S.; Bhakuni, R.S. In vitro antimalarial studies of novel artemisinin biotransformed products and its derivatives. Phytochemistry 2014, 107, 135–140. [Google Scholar] [CrossRef]
- Goswami, A.; Saikia, P.P.; Barua, N.C.; Bordoloi, M.; Yadav, A.; Bora, T.C.; Gogoi, B.K.; Saxena, A.K.; Suri, N.; Sharma, M. Bio-transformation of artemisinin using soil microbe: Direct c-acetoxylation of artemisinin at c-9 by penicillium simplissimum. Bioorg. Med. Chem. Lett. 2010, 20, 359–361. [Google Scholar] [CrossRef]
- Srivastava, S.; Luqman, S.; Fatima, A.; Darokar, M.P.; Negi, A.S.; Kumar, J.K.; Shanker, K.; Chanotiya, C.S.; Tandon, S.; Khanuja, S.P.S. Biotransformation of artemisinin mediated through fungal strains for obtaining derivatives with novel activities. Sci. Pharm. 2009, 77, 87–95. [Google Scholar] [CrossRef]
- Zhan, Y.; Liu, H.; Wu, Y.; Wei, P.; Chen, Z.; Williamson, J.S. Biotransformation of artemisinin by Aspergillus niger. Appl. Microbiol. Biotechnol. 2015, 99, 3443–3446. [Google Scholar] [CrossRef]
- Lee, I.-S.; ElSohly, H.N.; Croom, E.M.; Hufford, C.D. Microbial metabolism studies of the antimalarial sesquiterpene artemisinin. J. Nat. Prod. 1989, 52, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Parshikov, I.A.; Miriyala, B.; Muraleedharan, K.M.; Avery, M.A.; Williamson, J.S. Microbial transformation of artemisinin to 5-hydroxyartemisinin by eurotium amstelodami and aspergillus niger. J. Ind. Microbiol. Biotechnol. 2006, 33, 349–352. [Google Scholar] [CrossRef]
- Zhan, Y.; Wu, Y.; Xu, F.; Bai, Y.; Guan, Y.; Williamson, J.S.; Liu, B. A novel dihydroxylated derivative of artemisinin from microbial transformation. Fitoterapia 2017, 120, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Ponnapalli, M.G.; Sura, M.B.; Sudhakar, R.; Govindarajalu, G.; Sijwali, P.S. Biotransformation of Artemisinin to 14-hydroxydeoxyartemisinin: C-14 hydroxylation by Aspergillus flavus. J. Agric. Food Chem. 2018, 66, 10490–10495. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat. Med. 2011, 17, 1217–1220. [Google Scholar] [CrossRef]
- Tu, Y.Y. Artemisinin and Artemisinin Drugs; Chemical Industry Press: Beijing, China, 2009; pp. 34–56. ISBN 978-7-122-00857-2. [Google Scholar]
- Meshnick, S.R. Artemisinin: Mechanisms of action, resistance and toxicity. Int. J. Parasitol. 2002, 32, 1655–1660. [Google Scholar] [CrossRef]
- Meunier, B.; Robert, A. Heme as trigger and target for trioxane-containing antimalarial drugs. Acc. Chem. Res. 2010, 43, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Asha, S.; Vidyavathi, M. Cunninghamella—A microbial model for drug metabolism studies—A review. Biotechnol. Adv. 2009, 27, 16–29. [Google Scholar] [CrossRef]
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biotransformations and biological activities of hop flavonoids. Biotechnol. Adv. 2015, 33, 1063–1090. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds artemisinin, dihydroartemisinin, and deoxyartemisinin are available from the authors. |
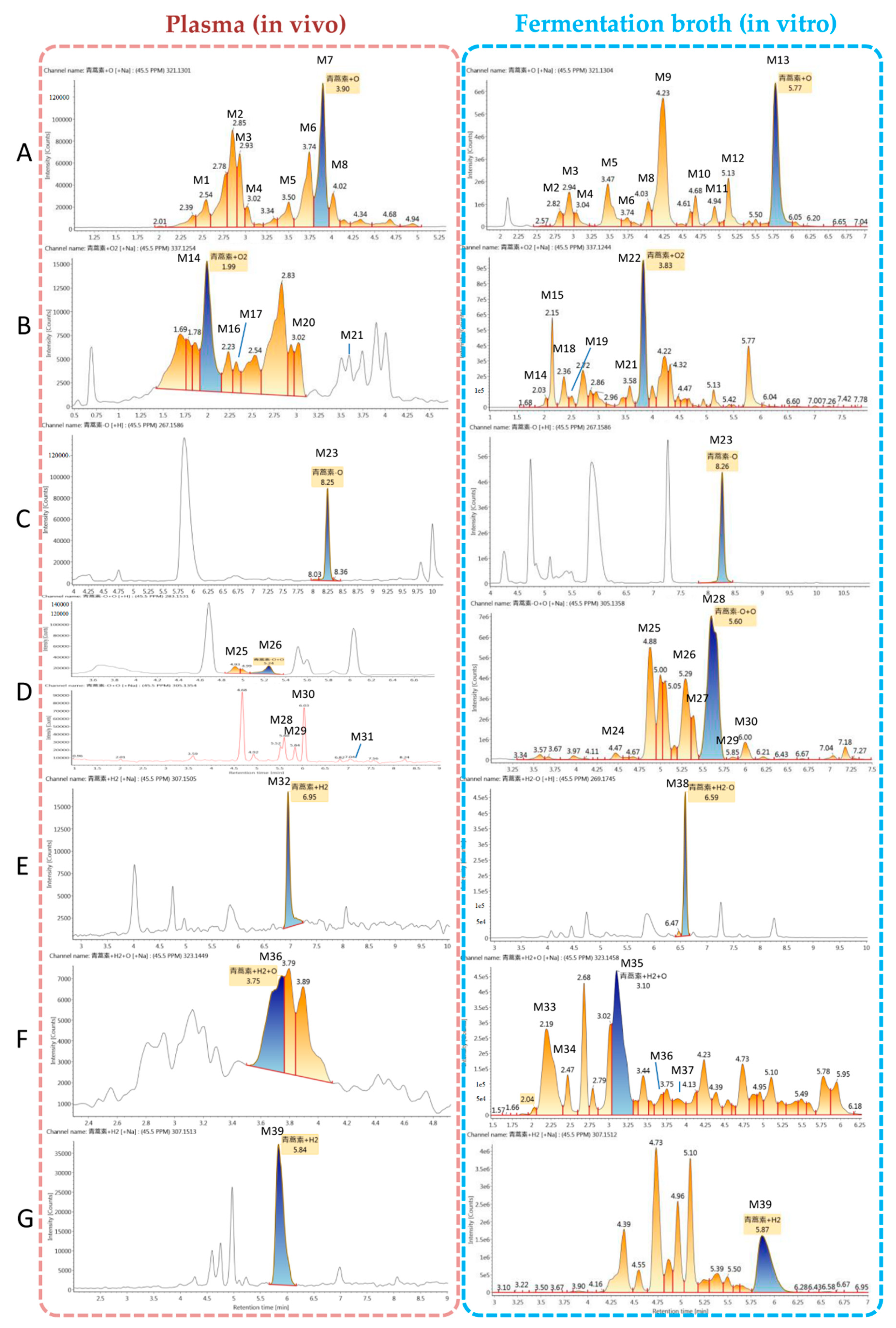
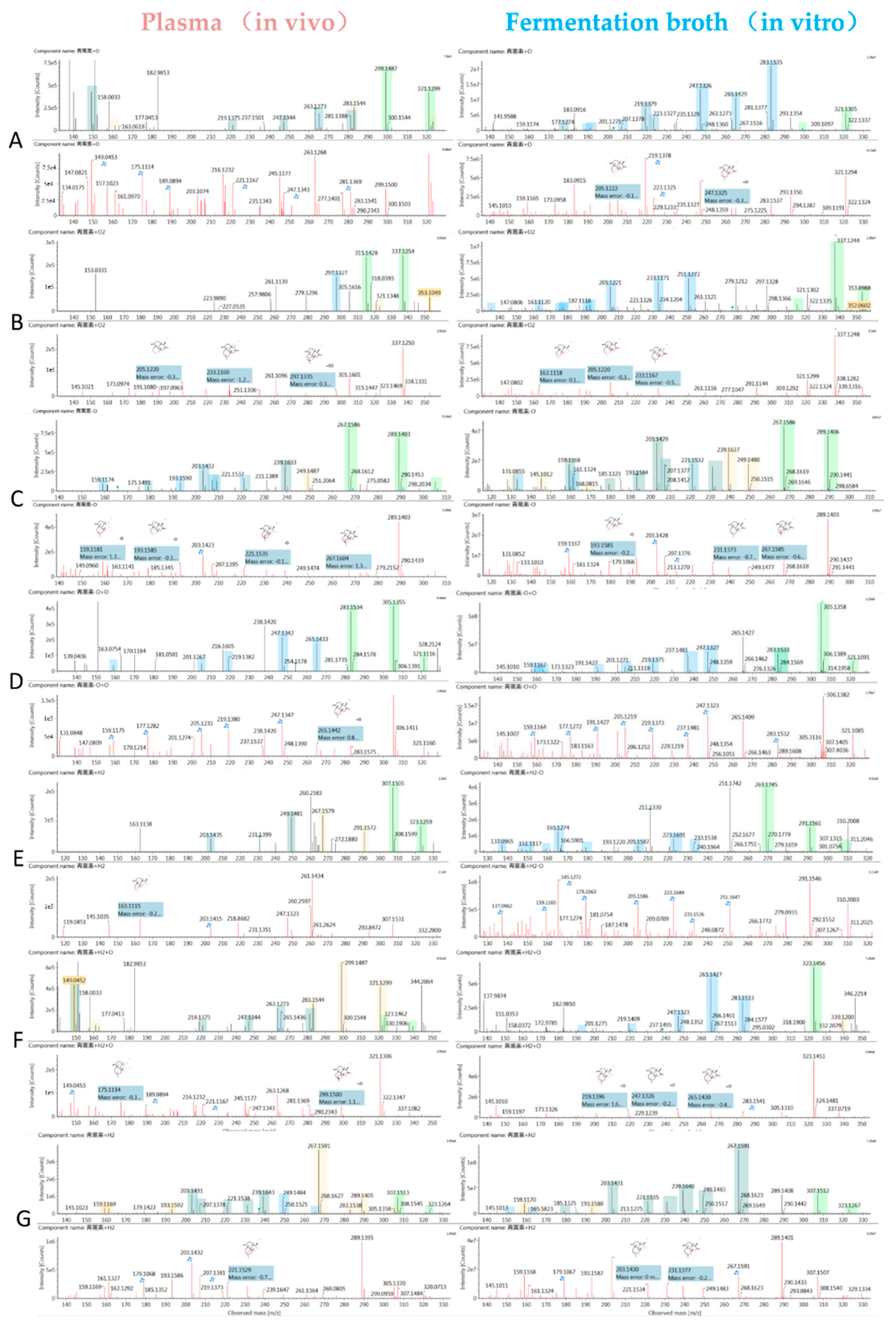
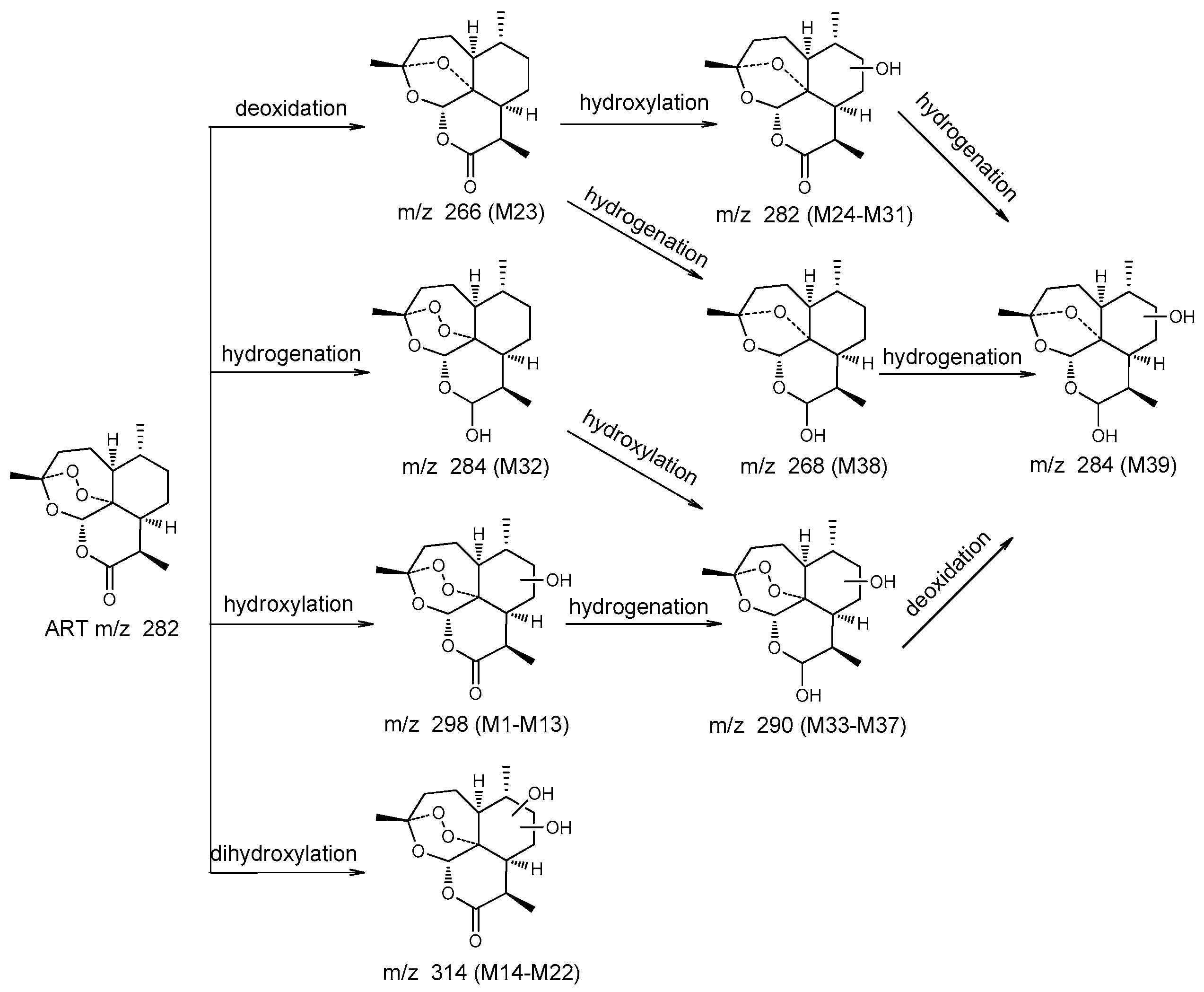
| No. | Component | Formula | Observed Retention Time (min) | Plasma (In Vivo) | Fermentation Broth (In Vitro) | Major Fragments | ||
|---|---|---|---|---|---|---|---|---|
| Observed m/z | Mass Error (mDa) | Observed m/z | Mass Error (mDa) | |||||
| M1 | ART + O | C15H22O6 + H+ | 2.6 | 299.1487 | −0.2 | - | - | 321, 299, 337, 281, 263, 253, 235, 217, 207 |
| M2 | ART + O | C15H22O6 + Na+ | 2.8 | 321.1301 | −0.5 | 321.1312 | 0.3 | 321, 337, 299, 281, 263, 253, 235, 221, 207, 189 |
| M3 | ART + O | C15H22O6 + Na+ | 2.9 | 321.1301 | −0.4 | 321.1309 | 0.1 | 321, 337, 299, 281, 263, 245, 235, 221, 217, 189, 175 |
| M4 | ART + O | C15H22O6 + Na+ | 3.0 | 321.1304 | −0.4 | 321.1305 | −0.3 | 321, 337, 299, 283, 265, 235, 223, 219, 207, 189, 147 |
| M5 | ART + O | C15H22O6 + Na+ | 3.5 | 321.1307 | −0.1 | 321.1304 | −0.5 | 321, 337, 281, 263, 245, 235, 217, 205, 189 |
| M6 | ART + O | C15H22O6 + Na+ | 3.7 | 321.1304 | −0.4 | 321.1302 | 1.8 | 321, 337, 299, 281, 263, 235, 221, 207, 193, 189, 175 |
| M7 | ART + O | C15H22O6 + Na+ | 3.9 | 321.1301 | −0.7 | - | - | 321, 299, 337, 281, 253, 245, 235, 223, 217, 177, 149 |
| M8 | ART + O | C15H22O6 + H+ | 4.0 | 299.1481 | −0.8 | 321.1301 | −0.8 | 321, 337, 283, 263, 253, 235, 221, 207, 189, 175 |
| M9 | ART + O | C15H22O6 + Na+ | 4.2 | - | - | 321.1306 | −0.3 | 321, 337, 307, 267, 253, 249, 235, 207, 189, 175, 165 |
| M10 | ART + O | C15H22O6 + Na+ | 4.7 | - | - | 321.1301 | −0.7 | 321, 299, 337, 283, 261, 237, 235, 219, 205, 177, 165 |
| M11 | ART + O | C15H22O6 + Na+ | 4.9 | 337.1038 | −0.6 | 337.1039 | −0.9 | 321, 299, 337, 281, 253, 235, 207, 189, 179 |
| M12 | ART + O | C15H22O6 + Na+ | 5.1 | - | - | 321.1296 | −1.3 | 321, 337, 299, 281, 263, 253, 235, 207, 189, 175, 149 |
| M13 | ART + O | C15H22O6 + Na+ | 5.8 | - | - | 321.1304 | −0.4 | 321, 337, 299, 281, 263, 235, 217, 207, 189, 175, 149 |
| M14 | ART + O2 | C15H22O7 + Na+ | 2.0 | 337.1254 | −0.4 | 337.1275 | 1.8 | 337, 353, 315, 297, 279, 261, 251, 233, 205 |
| M15 | ART + O2 | C15H22O7 + Na+ | 2.2 | - | - | 337.1246 | −1.1 | 337, 353, 277, 255, 237, 219, 209, 191, 163, 135 |
| M16 | ART + O2 | C15H22O7 + Na+ | 2.2 | 337.1255 | −0.2 | - | - | 337, 315, 267, 252, 226, 199,176 |
| M17 | ART + O2 | C15H22O7 + Na+ | 2.3 | 337.1259 | 0.1 | - | - | 337, 297, 279, 265, 247, 233, 217, 201, 189 |
| M18 | ART + O2 | C15H22O7 + Na+ | 2.4 | - | - | 337.1256 | −0.1 | 337, 353, 299, 299, 281, 277, 263, 255 |
| M19 | ART + O2 | C15H22O7 + Na+ | 2.5 | - | - | 337.1247 | −1.0 | 337, 353, 277, 253, 235, 219, 209, 207, 163, 135 |
| M20 | ART + O2 | C15H22O7 + Na+ | 3.0 | 337.1274 | 1.7 | - | - | 337, 321, 299, 281, 279, 263, 252, 235, 207, 189, 147 |
| M21 | ART + O2 | C15H22O7 + Na+ | 3.6 | 337.1281 | −0.4 | 337.1068 | −1.1 | 337, 353, 305, 297, 279, 233, 221, 177 |
| M22 | ART + O2 | C15H22O7 + Na+ | 3.8 | 337.1244 | −1.3 | 337, 353, 315, 297, 279, 261, 251, 233, 205, 191, 175 | ||
| M23 | de-ART | C15H22O4 + H+ | 8.3 | 267.1586 | −0.5 | 267.1586 | −0.5 | 289, 267, 305, 249, 239, 231, 221, 207, 203, 193, 179 |
| M24 | de-ART + O | C15H22O5 + Na+ | 4.5 | - | - | 305.1355 | −0.5 | 305, 321, 283, 265, 247, 237, 219, 201, 189 |
| M25 | de-ART + O | C15H22O5 + H+ | 4.9 | 283.1536 | −0.4 | 283.1535 | −0.3 | 283, 305, 321, 265, 247, 239, 191 |
| M26 | de-ART + O | C15H22O5 + Na+ | 5.2 | 283.1531 | −0.9 | 283.1355 | −0.5 | 283, 305, 265, 247, 237, 219, 191, 177, |
| M27 | de-ART + O | C15H22O5 + Na+ | 5.4 | - | - | 305.1354 | −0.6 | 305, 321, 267, 247, 219, 205, 177, 159 |
| M28 | de-ART + O | C15H22O5 + Na+ | 5.6 | 305.1355 | −0.4 | 305.1358 | −0.1 | 283, 305, 321, 265, 247, 219, 205, 177, 159 |
| M29 | de-ART + O | C15H22O5 + Na+ | 5.8 | 305.1358 | −0.1 | - | - | 305, 283, 276, 249, 239, 231, 221, 207, 203, 193, 179 |
| M30 | de-ART + O | C15H22O5 + Na+ | 6.0 | 305.1353 | −0.8 | 305.1353 | −0.6 | 305, 321, 265, 247, 219, 205, 193, 149 |
| M31 | de-ART + O | C15H22O5 + Na+ | 7.2 | - | - | 305.1355 | −0.5 | 305, 321, 283, 247, 223, 195, 167 |
| M32 | DHA | C15H24O5 + Na+ | 7.0 | 307.1505 | −1.1 | - | - | 307, 323, 267, 249, 239, 231, 221, 203, 163 |
| M33 | DHA + O | C15H24O6 + Na+ | 2.2 | - | - | 323.1456 | −1.0 | 323, 339, 283, 265, 247, 219, 189 |
| M34 | DHA + O | C15H24O6 + Na+ | 2.5 | - | - | 323.1458 | −0.7 | 323, 339, 283, 265, 247, 219, 191 |
| M35 | DHA + O | C15H24O6 + Na+ | 3.1 | - | - | 323.1458 | −0.8 | 323, 339, 285, 267, 249, 219, 191, 177 |
| M36 | DHA + O | C15H24O6 + Na+ | 3.7 | 323.1449 | −1.6 | 323.1484 | 1.8 | 323, 283, 265, 253, 247, 237, 219, 209 |
| M37 | DHA + O | C15H24O6 + Na+ | 3.9 | - | - | 323.1456 | −1.0 | 323, 285, 267, 249, 239, 235, 231, 221, 203 |
| M38 | DHA-O | C15H24O6 + Na+ | 6.6 | - | - | 291.1561 | −0.3 | 291, 307, 279, 269, 251, 233, 223, 215, 205, 179 |
| M39 | DHA-O + O | C15H24O5 + Na+ | 5.8 | 307.1513 | −0.4 | 307.1512 | −0.5 | 307, 323, 267, 249, 239, 231, 221, 203, 179 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Sun, P.; Zhao, Y.; Wang, K.; Chang, X.; Bai, Y.; Zhang, D.; Yang, L. A Microbial Transformation Model for Simulating Mammal Metabolism of Artemisinin. Molecules 2019, 24, 315. https://doi.org/10.3390/molecules24020315
Ma Y, Sun P, Zhao Y, Wang K, Chang X, Bai Y, Zhang D, Yang L. A Microbial Transformation Model for Simulating Mammal Metabolism of Artemisinin. Molecules. 2019; 24(2):315. https://doi.org/10.3390/molecules24020315
Chicago/Turabian StyleMa, Yue, Peng Sun, Yifan Zhao, Kun Wang, Xiaoqiang Chang, Yue Bai, Dong Zhang, and Lan Yang. 2019. "A Microbial Transformation Model for Simulating Mammal Metabolism of Artemisinin" Molecules 24, no. 2: 315. https://doi.org/10.3390/molecules24020315
APA StyleMa, Y., Sun, P., Zhao, Y., Wang, K., Chang, X., Bai, Y., Zhang, D., & Yang, L. (2019). A Microbial Transformation Model for Simulating Mammal Metabolism of Artemisinin. Molecules, 24(2), 315. https://doi.org/10.3390/molecules24020315







