First-Principles Study of the Reaction between Fluorinated Graphene and Ethylenediamine
Abstract
1. Introduction
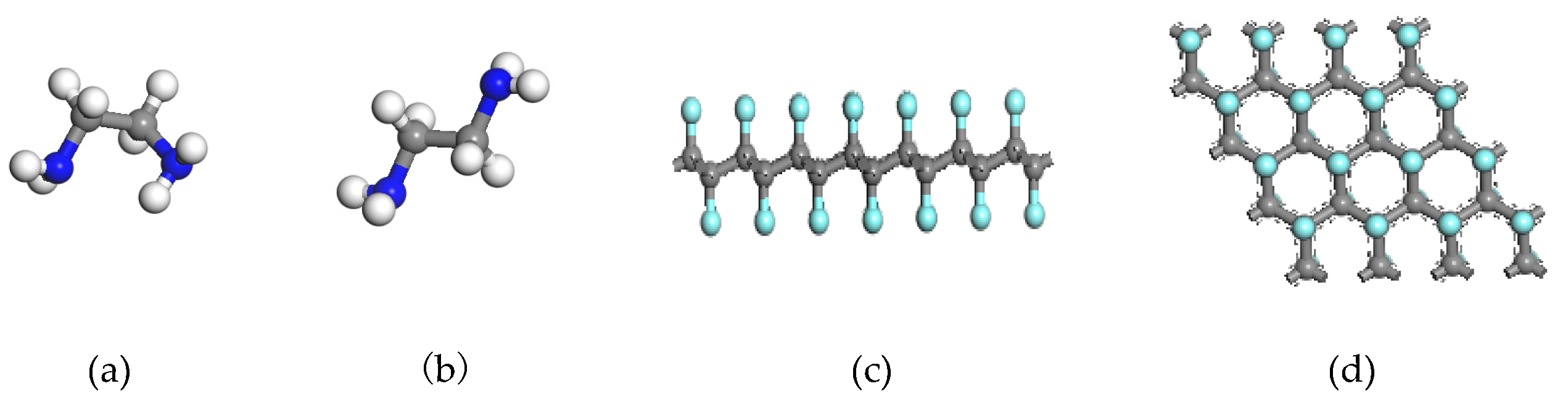
2. Materials and Methods
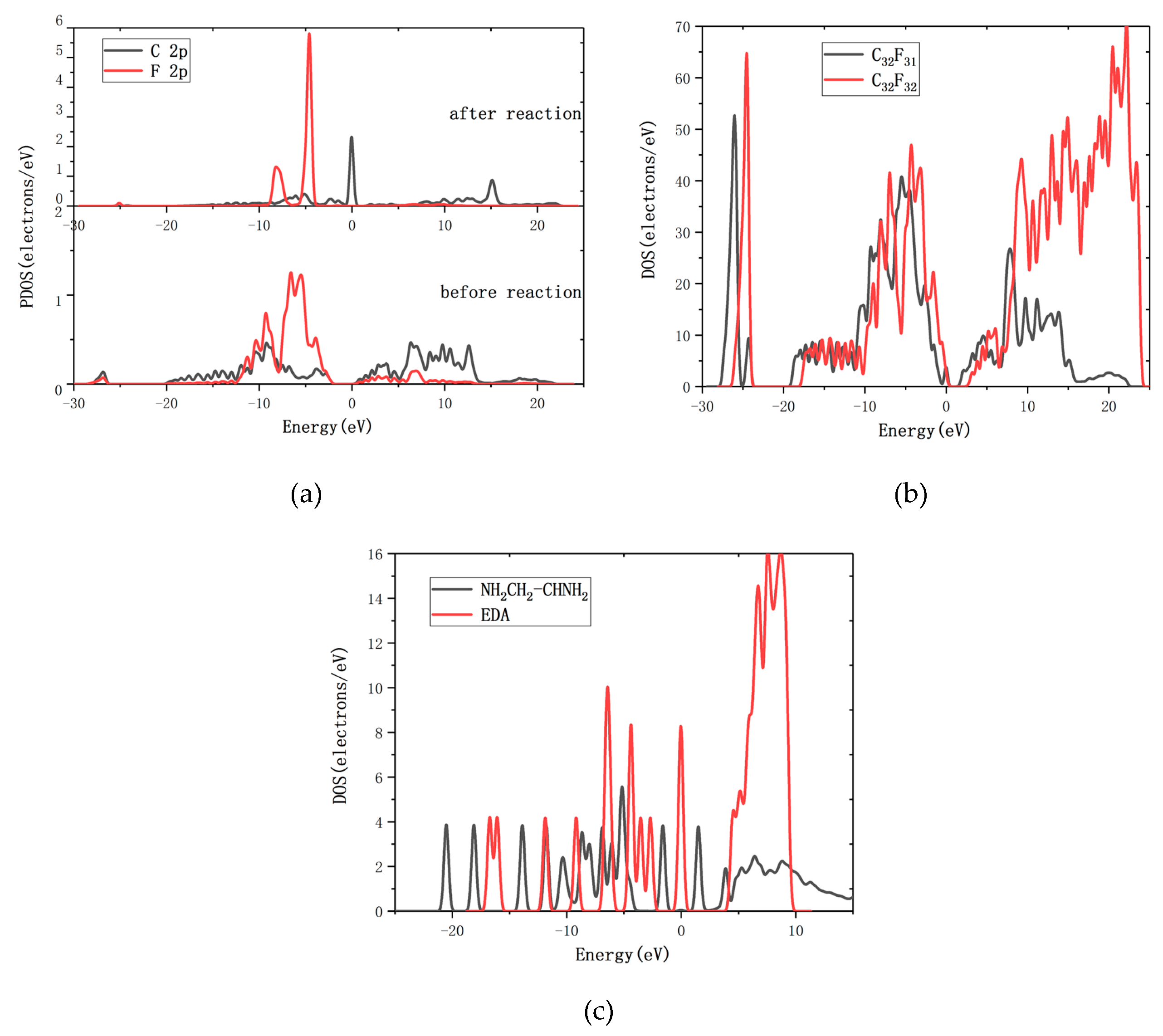
3. Results and Discussion
3.1. Reaction of Gauche-Structure EDA and CF
3.2. Reaction of Trans-Structure EDA and CF
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Treier, M.; Pignedoli, C.A.; Laino, T.; Rieger, R.; Mullen, K.; Passerone, D.; Fasel, R. Surface-assisted cyclodehydrogenation provides a synthetic route towards easily processable and chemically tailored nanographenes. Nat. Chem. 2011, 3, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.K.; Siberio-Pérez, D.Y.; Kim, J.; Go, Y.; Eddaoudi, M.; Matzger, A.J.; O’Keeffe, M.; Yaghi, O.M. A route to high surface area, porosity and inclusion of large molecules in crystals. Nature 2004, 427, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Jeroen, V.D.B. Graphene: From strength to strength. Nat. Nanotechnol. 2007, 2, 199–201. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Sutter, P.W.; Flege, J.I.; Sutter, E.A. Epitaxial graphene on ruthenium. Nat. Mater. 2008, 7, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Kim, B.H.; Song, S.H.; Kwon, J.; Kong, B.S.; Kang, K.; Jeon, S. Exfoliation of non-oxidized graphene flakes for scalable conductive film. Nano Lett. 2012, 12, 2871–2876. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.H.; Kim, P.; Choi, J.Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.S.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Che, J.F.; Shen, L.Y.; Xiao, Y.H. A new approach to fabricate graphene nanosheets in organic medium: Combination of reduction and dispersion. J. Mater. Chem. 2010, 20, 1722–1727. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Alonso, M.H.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Lagow, R.J.; Wei, H.C. Direct Fluorination of Polymers. Fluoropolymers, 1-Synthesis; Springer: New York, NY, USA, 1999; pp. 209–221. [Google Scholar] [CrossRef]
- Sahin, H.; Topsakal, M.; Ciraci, S. Structures of Fluorinated Graphenes and Their Signatures. Phys. Rev. B 2011, 83, 115432–115438. [Google Scholar] [CrossRef]
- Han, S.S.; Yu, T.H.; Merinov, B.V.; van Duin, A.C.T.; Yazami, R.; Goddard, W.A. Unraveling Structural Models of Graphite Fluorides by Density Functional Theory Calculations. Chem. Mater. 2010, 22, 2142–2154. [Google Scholar] [CrossRef]
- Bordes, É.; Szalabilnik, J.; Pádua, A. Exfoliation of graphene and fluorographene in molecular and ionic liquids. Faraday Discuss. 2017, 206, 61–75. [Google Scholar] [CrossRef]
- Zbořil, R.; Karlický, F.; Bourlinos, A.B.; Steriotis, T.A.; Stubos, A.K.; Georgakilas, V.; Safarova, K.; Jancik, D.; Trapalis, C.; Otyepka, M. Graphene Fluoride: A Stable Stoichiometric Graphene Derivative and its Chemical Conversion to Graphene. Small 2010, 6, 2885–2891. [Google Scholar] [CrossRef]
- Chen, L.; Lei, J.J.; Wang, F.H.; Wang, G.C.; Feng, H.X. Facile synthesis of graphene sheets from fluorinated graphite. RSC Adv. 2015, 5, 40148–40153. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, M.; Ma, Y.; Che, J.F. Graphene converted from graphene fluoride via interfacial chemical reduction. Mater. Lett. 2013, 112, 194–196. [Google Scholar] [CrossRef]
- Hou, K.M.; Gong, P.W.; Wang, J.Q.; Yang, Z.G.; Wang, Z.F.; Yang, S.R. Structural and tribological characterization of fluorinated graphene with various fluorine contents prepared by liquid-phase exfoliation. RSC Adv. 2014, 4, 56543–56551. [Google Scholar] [CrossRef]
- He, K.Z. Theoretical study on the Dehydrogenation Properties of Magnesium Hydride and the Reduction Mechanism of Fluorinated Graphene. Master’s Thesis, Lanzhou University of Technology, Lanzhou, China, 2017. [Google Scholar]
- Corte, D.D.; Schläpfer, C.W.; Daul, C. A density functional theory study of the conformational properties of 1,2-ethanediamine: Protonation and solvent effects. Theor. Chem. Acc. 2000, 105, 39–45. [Google Scholar] [CrossRef]
- Yokozeki, A.; Kuchitsu, K. Structure and rotational isomerism of ethylenediamine as studier by gas electron diffraction. Bull. Chem. Soc. Jpn. 1971, 44, 2926–2930. [Google Scholar] [CrossRef]
- Lanshina, L.V.; Rodnikova, M.N.; Dudnikova, K.T. Structure of liquid ethylenediamine according to data on molecular scattering of light. J. Struct. Chem. 1989, 30, 684–687. [Google Scholar] [CrossRef]
- Jamet-Delcroix, S. Détermination de la structure cristalline de l’ethylènediamine, NH2-CH2-CH2-NH2, à −60 °C. Acta Crystallogr. B 1973, 29, 977–980. [Google Scholar] [CrossRef]
- Balabaeva, N.K.; Kraevskiib, S.V.; Rodnikovab, M.N.; Solonina, I.A. Molecular dynamic study of liquid Ethylenediamine. Russ. J. Phys. Chem. A 2016, 90, 1986–1992. [Google Scholar] [CrossRef]
- Van Alsenoy, C.; Siam, K.; Ewbank, J.D.; Schäfer, L. Ab Initio studies of structural features not easily amenable to experiment Part 49. Conformational analysis and molecular structures of ethylenediamine and aminoethanol. J. Mol. Struct. Theochem. 1986, 136, 77–91. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Chen, Y.H.; Fan, J.J.; Liu, T.T.; Wang, J.; Zhang, M.L.; Zhang, C.R. Theoretical study on the effect of an O vacancy on the hydrogen storage properties of the LaFeO3 (010) surface. Int. J. Hydrogen. Energy. in press. [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef]
- Ao, Z.M.; Jiang, Q.; Li, S.; Liu, H.; Peeters, F.M.; Li, S.; Wang, G.X. Enhancement of the Stability of Fluorine Atoms on Defective Graphene and at Graphene/Fluorographene Interface. ACS Appl. Mater. Interface 2015, 7, 19659–19665. [Google Scholar] [CrossRef]
- Lazar, P.; Otyepkova, E.; Karlicky, F.; Cepe, K.; Otyepka, M. The surface and structural properties of graphite fluoride. Carbon 2015, 94, 804–809. [Google Scholar] [CrossRef]
- Jiang, Q.G.; Ao, Z.M.; Li, S.; Ze, W. Density Functional Theory Calculations on the CO Catalytic Oxidation on Al-embedded Graphene. RSC Adv. 2014, 4, 20290–20296. [Google Scholar] [CrossRef]
- MA, Y.C. Preparation of Fluorinated Graphene and Chemical Reduction Fluorinated Graphene to Prepare Graphene. Master’s Thesis, Nanjing University of Science and Technology, Nanjing, China, 2012. [Google Scholar]
- Wagner, J.P.; McDonald, D.C.; Duncan, M.A. Spectroscopy of proton Coordination with Ethylenediamine. J. Phys. Chem. A 2018, 122, 5168–5176. [Google Scholar] [CrossRef]
- Lee, Y.S. Syntheses and properties of fluorinated carbon materials. J. Fluor. Chem. 2007, 128, 392–403. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
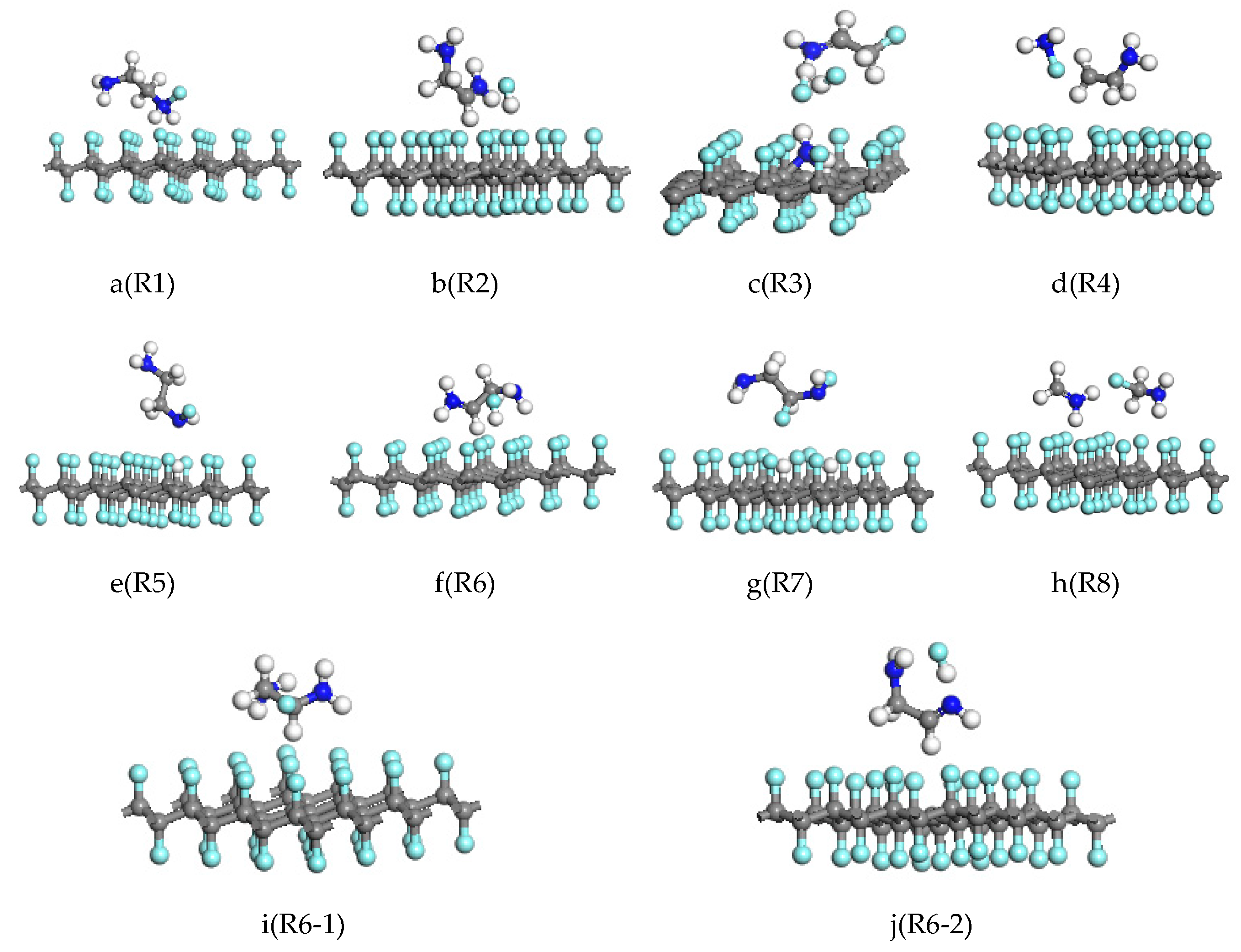

| Reactions | Barrier from Reactant (eV) | Barrier from Product (eV) | Energy of Reaction (eV) | |
|---|---|---|---|---|
| R1 | C32F32 + H2NH2C–CH2NH2 → C32F31 + H2NH2C–CHFNH2F | 4.357 | 3.038 | 1.319 |
| R2 | C32F32 + H2NH2C–CH2NH2 → C32F31 + H2NH2C–CHNH2 + HF | 5.339 | 6.246 | −0.907 |
| R3 | C32F32 + H2NH2C–CH2NH2 → C32F29NH2 + HF + FH2NHC–CH2F | 16.397 | 17.666 | −1.269 |
| R4 | C32F32 + H2NH2C–CH2NH2 → C32F31 + NH2F + H2C–CH2NH2 | 12.938 | 8.984 | 3.945 |
| R5 | C32F32 + H2NH2C–CH2NH2 → C32F31H + H2NH2C–CH2NHF | 2.358 | 2.178 | 0.220 |
| R6 | C32F32 + H2NH2C–CH2NH2 → C32F31 + H2NHC–CH2NH2 + HF | 0.810 | 1.625 | −0.815 |
| R7 | C32F32 + H2NH2C–CH2NH → C32F30H2 + H2N2HC–CHFNHF | 5.731 | 6.546 | −0.815 |
| R8 | C32F32 + H2NH2C–CH2NH2 → C32F31 + CH2NH2 + CH2FNH2 | 10.830 | 11.817 | −0.987 |
| R6-1 | C32F32 + H2NHC–CH2NH2 → C32F31 + H2NFHC–CH2NH2 | 1.899 | 2.956 | −1.057 |
| R6-2 | C32F32 + H2NHC–CH2NH2 → C32F31 + HNHC–CH2NH2 + HF | 1.945 | 2.865 | −0.920 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Chen, Y.; Wang, J.; Liu, T.; Zhang, M.; Zhang, C. First-Principles Study of the Reaction between Fluorinated Graphene and Ethylenediamine. Molecules 2019, 24, 284. https://doi.org/10.3390/molecules24020284
Tian J, Chen Y, Wang J, Liu T, Zhang M, Zhang C. First-Principles Study of the Reaction between Fluorinated Graphene and Ethylenediamine. Molecules. 2019; 24(2):284. https://doi.org/10.3390/molecules24020284
Chicago/Turabian StyleTian, Jin, Yuhong Chen, Jing Wang, Tingting Liu, Meiling Zhang, and Cairong Zhang. 2019. "First-Principles Study of the Reaction between Fluorinated Graphene and Ethylenediamine" Molecules 24, no. 2: 284. https://doi.org/10.3390/molecules24020284
APA StyleTian, J., Chen, Y., Wang, J., Liu, T., Zhang, M., & Zhang, C. (2019). First-Principles Study of the Reaction between Fluorinated Graphene and Ethylenediamine. Molecules, 24(2), 284. https://doi.org/10.3390/molecules24020284






