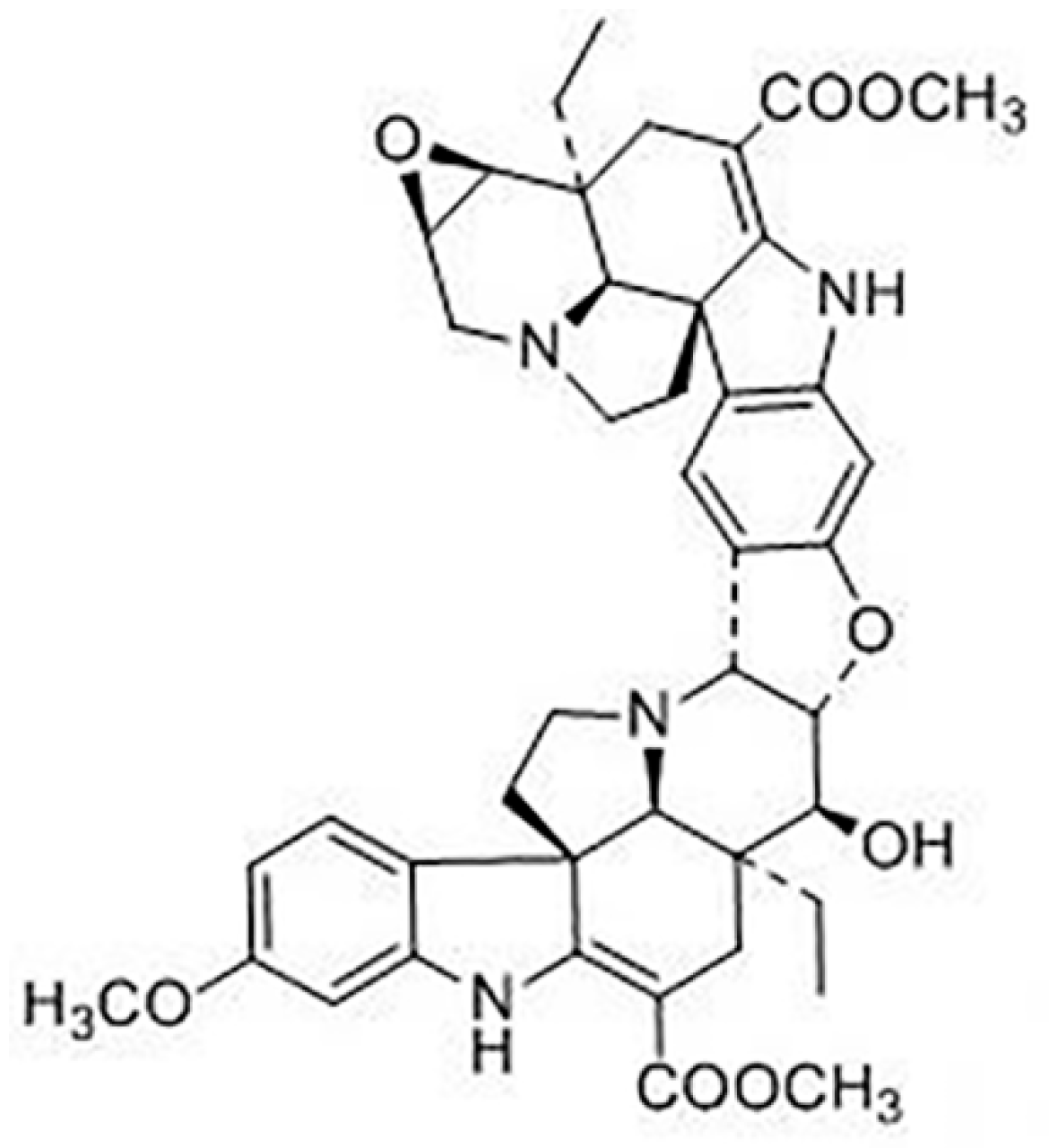
Pharmacokinetics, Tissue Distribution, Plasma Protein Binding Studies of 10-Dehydroxyl-12-Demethoxy-Conophylline, a Novel Anti-Tumor Candidate, in Rats
Abstract
1. Introduction
2. Results and Discussion
2.1. Validation of Analytical Method
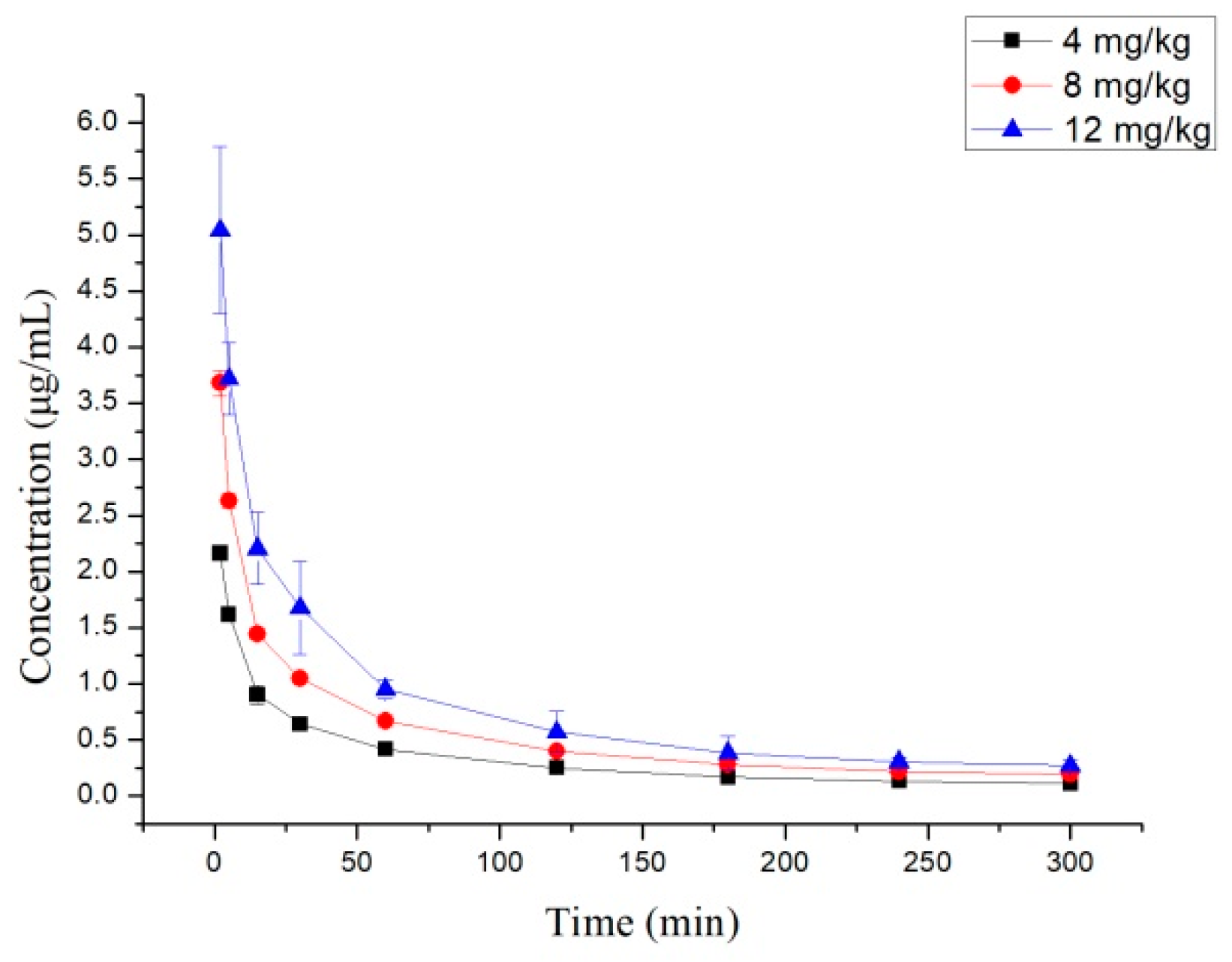
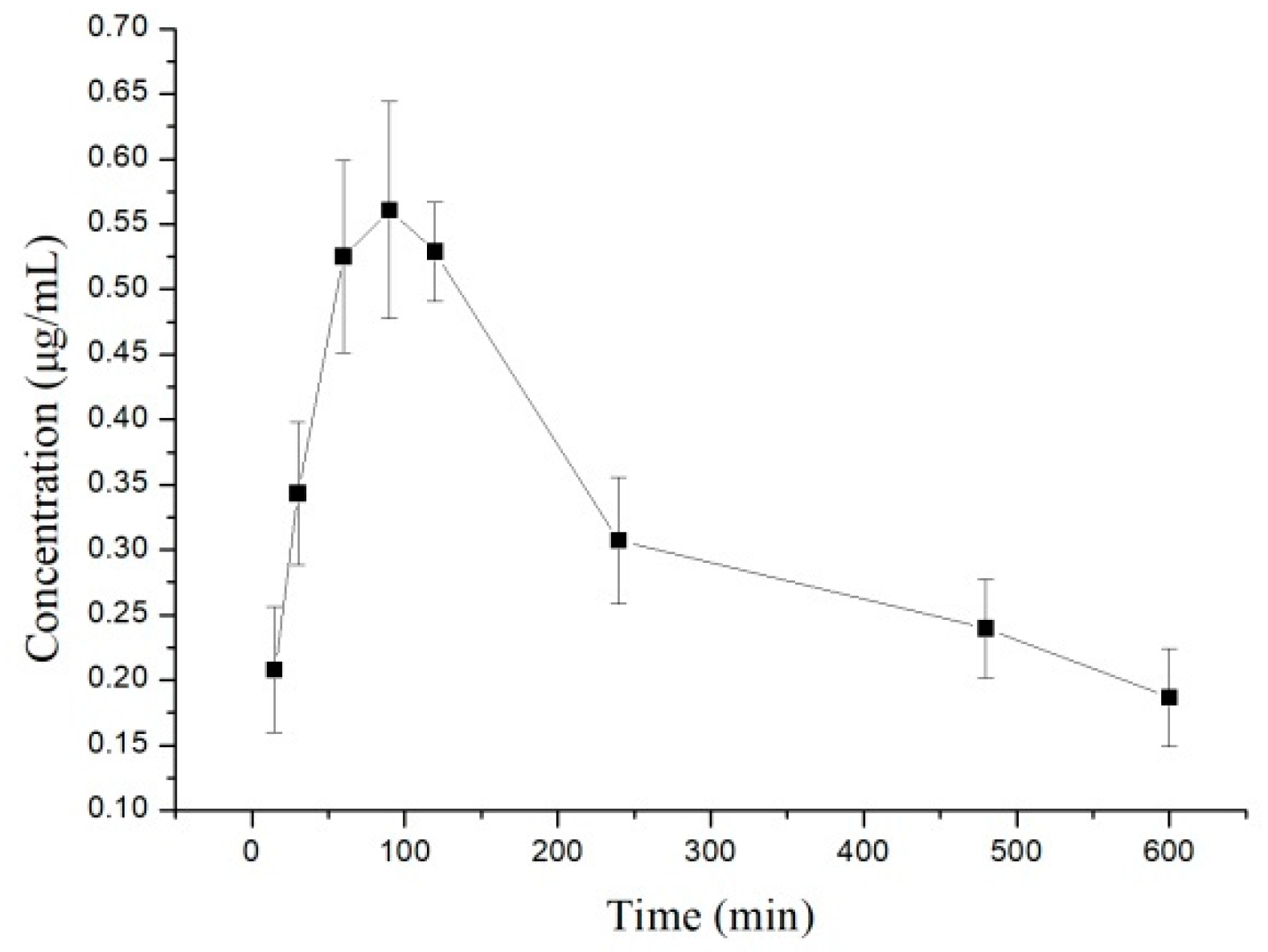
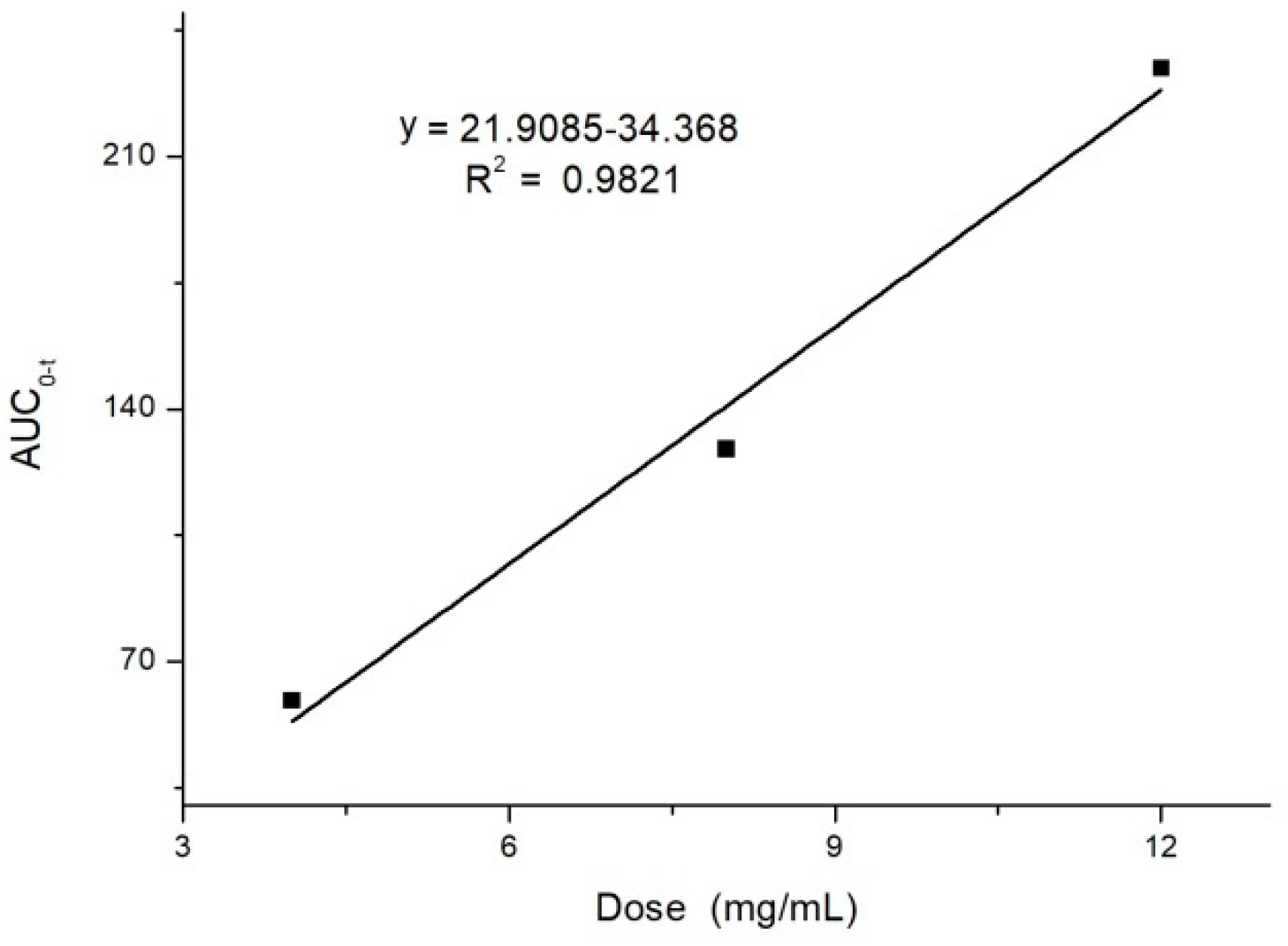
2.2. Pharmacokinetics Study
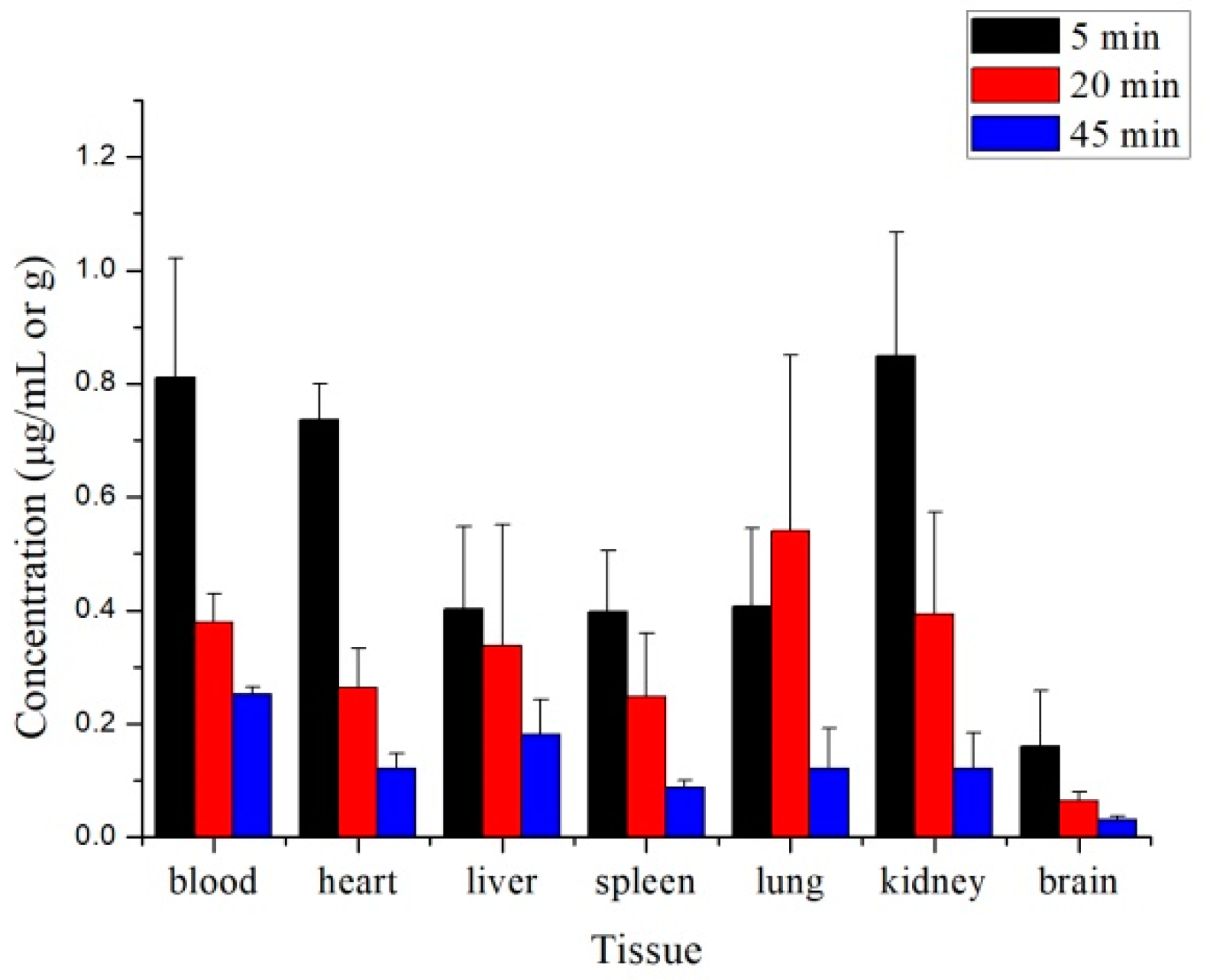
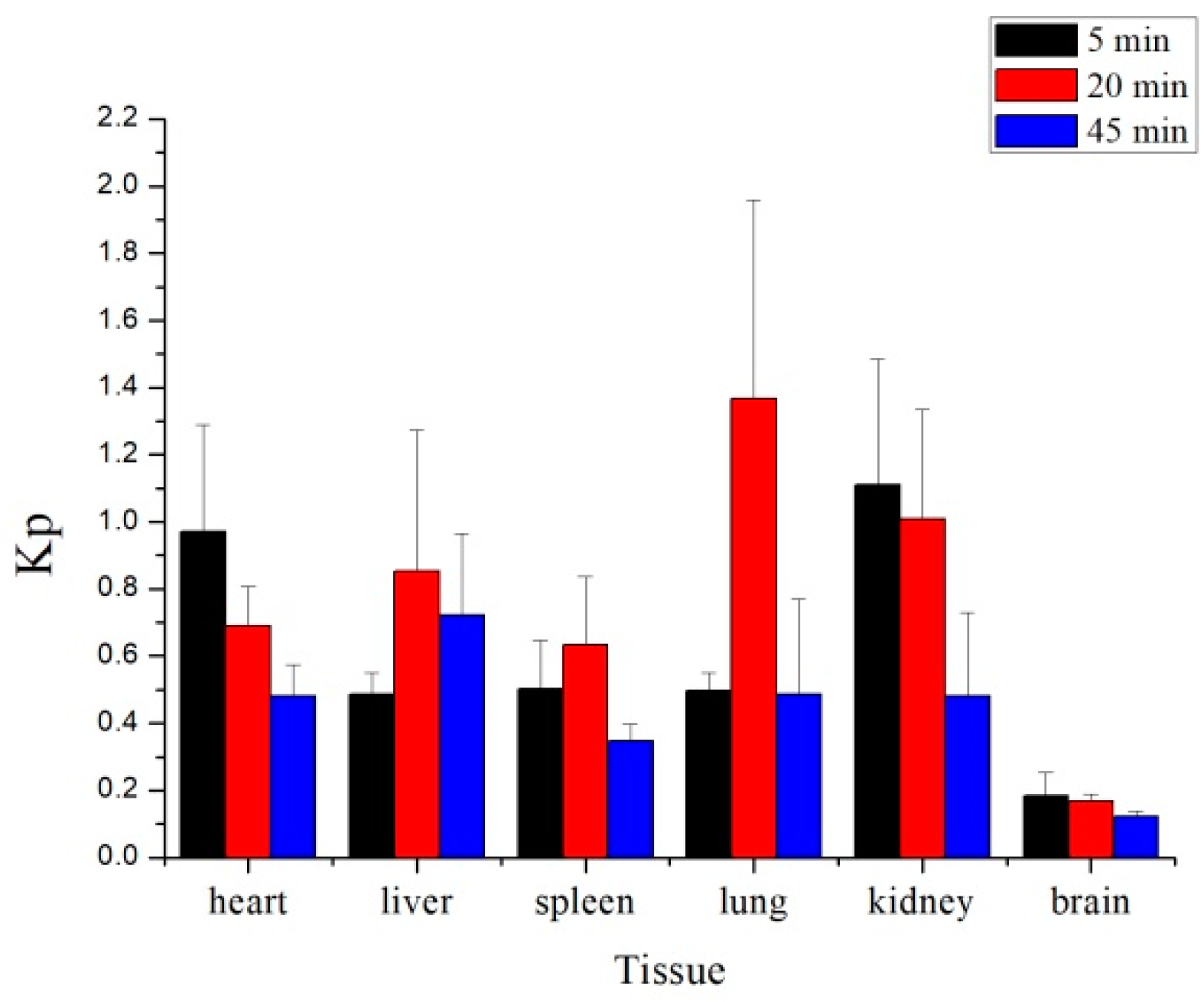
2.3. Tissue Distribution Study
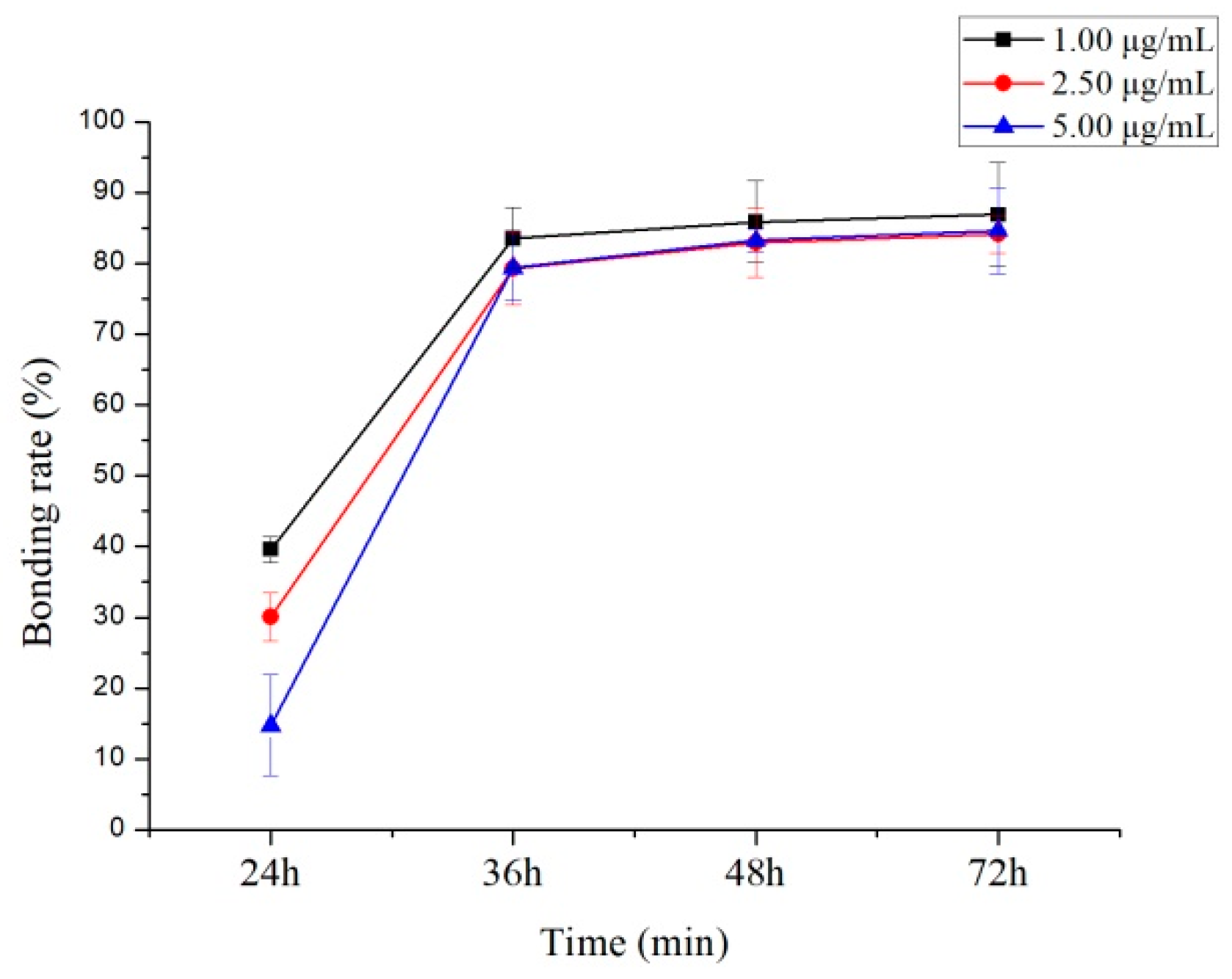
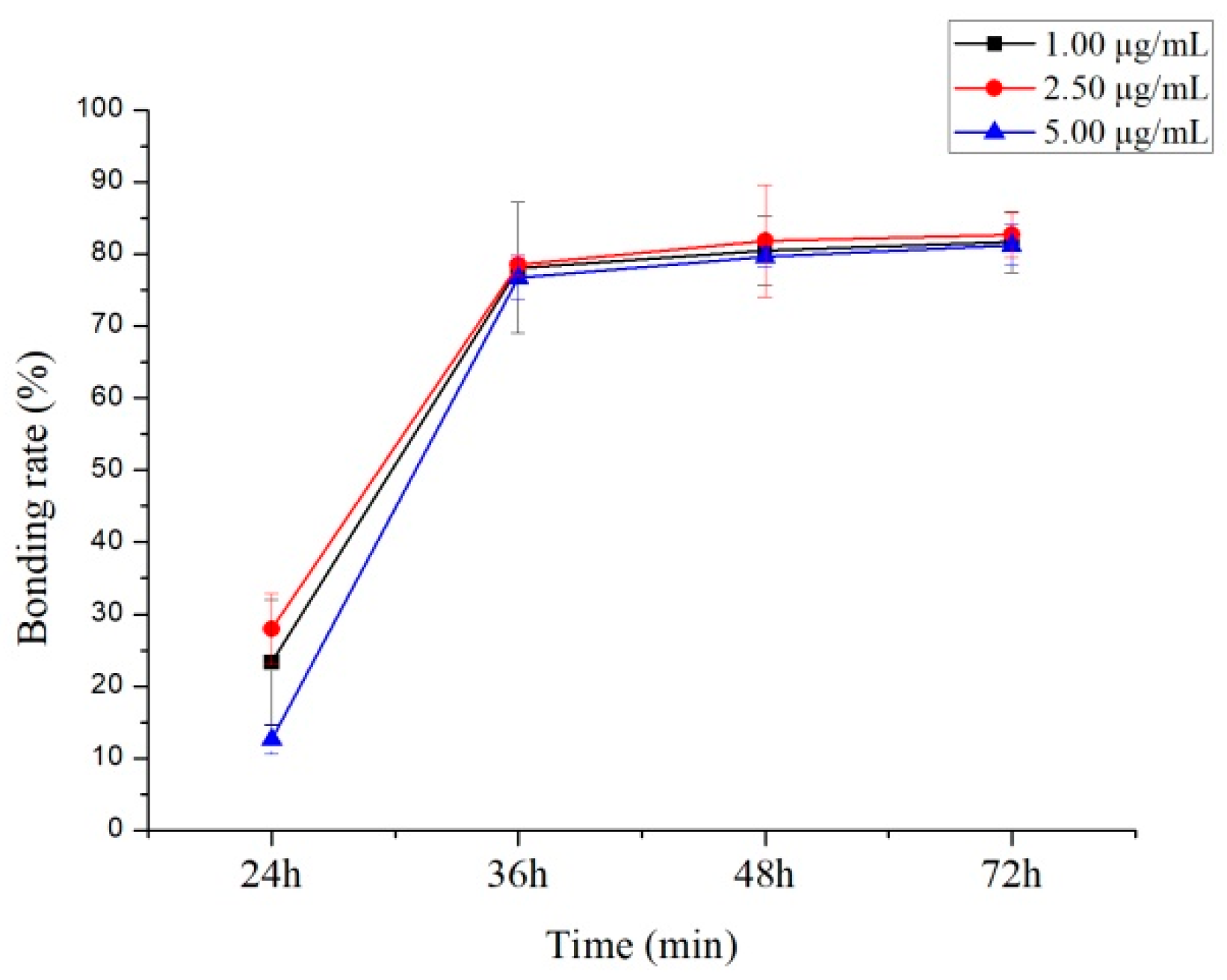
2.4. Protein Binding Study
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Animal Experimentation
3.3. UPLC-FLR Analysis
3.4. Pharmacokinetic Studies
3.5. Tissue Distribution Study
3.6. Plasma Protein Binding Test
3.7. Pharmacokinetic and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ma, H.; Xie, X.; Jing, P.; Zhang, W.; She, X. Concise total synthesis of (+/−)-aspidospermidine. Org. Biomol. Chem. 2015, 13, 5255–5259. [Google Scholar] [CrossRef]
- Qu, Y.; Easson, M.; Simionescu, R.; Hajicek, J.; Thamm, A.M.K.; Salim, V.; De Luca, V. Solution of the multistep pathway for assembly of corynanthean, strychnos, iboga, and aspidosperma monoterpenoid indole alkaloids from 19E-geissoschizine. Proc. Natl Acad. Sci. USA. 2018, 115, 3180–3185. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.R.; Shilpashree, H.B.; Nagegowda, D.A. Terpene moiety enhancement by overexpression of geranyl(geranyl) diphosphate synthase and geraniol synthase elevates monomeric and dimeric monoterpene indole alkaloids in transgenic catharanthus roseus. Front. Plant Sci. 2018, 9, 942. [Google Scholar] [CrossRef] [PubMed]
- Saxton, J.E. Recent progress in the chemistry of the monoterpenoid indole alkaloids. Nat. Prod. Rep. 1995, 12, 385–411. [Google Scholar] [CrossRef] [PubMed]
- Coatti, G.C.; Marcarini, J.C.; Sartori, D.; Fidelis, Q.C.; Ferreira, D.T.; Mantovani, M.S. Cytotoxicity, genotoxicity and mechanism of action (via gene expression analysis) of the indole alkaloid aspidospermine (antiparasitic) extracted from Aspidosperma polyneuron in HepG2 cells. Cytotechnology 2016, 68, 1161–1170. [Google Scholar] [CrossRef]
- Liu, Y.P.; Li, Y.; Cai, X.H.; Li, X.Y.; Kong, L.M.; Cheng, G.G.; Luo, X.D. Melodinines M-U, cytotoxic alkaloids from Melodinus suaveolens. J. Nat. Prod. 2012, 75, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Cai, X.H.; Liu, Y.P.; Li, Y.; Wang, Y.Y.; Luo, X.D. Melodinines A-G, monoterpenoid indole alkaloids from Melodinus henryi. J. Nat. Prod. 2010, 73, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Perez, F.; Almagro, L.; Pedreno, M.A.; Gomez Ros, L.V. Synergistic and cytotoxic action of indole alkaloids produced from elicited cell cultures of Catharanthus roseus. Pharm. Biol. 2013, 51, 304–310. [Google Scholar] [CrossRef]
- Yan, K.; Hong, S.; Feng, X. Demethyltenuicausine, a new bisindole alkaloid from Melodinus hemsleyanus. Yao Xue Xue Bao—Acta Pharm. Sin. 1998, 33, 597–599. [Google Scholar]
- Yi, W.F.; Chen, D.Z.; Ding, X.; Li, X.N.; Li, S.L.; Di, Y.T.; Zhang, Y.; Hao, X.J. Cytotoxic indole alkaloids from Melodinus khasianus and Melodinus tenuicaudatus. Fitoterapia 2018, 128, 162–168. [Google Scholar] [CrossRef]
- Huelsken, J.; Birchmeier, W. New aspects of Wnt signaling pathways in higher vertebrates. Curr. Opin. Genet. Dev. 2001, 11, 547–553. [Google Scholar] [CrossRef]
- Bienz, M.; Clevers, H. Linking colorectal cancer to Wnt signaling. Cell. 2000, 103, 311–320. [Google Scholar] [CrossRef]
- Krupnik, V.E.; Sharp, J.D.; Jiang, C.; Robison, K.; Chickering, T.W.; Amaravadi, L.; Brown, D.E.; Guyot, D.; Mays, G.; Leiby, K.; et al. Functional and structural diversity of the human Dickkopf gene family. Gene 1999, 238, 301–313. [Google Scholar] [CrossRef]
- Feng, T.; Li, Y.; Liu, Y.P.; Cai, X.H.; Wang, Y.Y.; Luo, X.D. Melotenine A, a cytotoxic monoterpenoid indole alkaloid from Melodinus tenuicaudatus. Org. Lett. 2010, 12, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Van Amsterdam, P.; Companjen, A.; Brudny-Kloeppel, M.; Golob, M.; Luedtke, S.; Timmerman, P. The European Bioanalysis Forum community’s evaluation, interpretation and implementation of the European Medicines Agency guideline on Bioanalytical Method Validation. Bioanalysis 2013, 5, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Poulin, P.; Ekins, S.; Theil, F.P. A hybrid approach to advancing quantitative prediction of tissue distribution of basic drugs in human. Toxic. Appl. Pharmacol. 2011, 250, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.J.; Desai-Krieger, D.; Shum, L. Simultaneous determination of glipizide and rosiglitazone unbound drug concentrations in plasma by equilibrium dialysis and liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 801, 265–272. [Google Scholar] [CrossRef]
- Di, L.; Umland, J.P.; Trapa, P.E.; Maurer, T.S. Impact of recovery on fraction unbound using equilibrium dialysis. J. Pharm. Sci. 2012, 101, 1327–1335. [Google Scholar] [CrossRef]
- Talbi, A.; Zhao, D.; Liu, Q.; Li, J.; Fan, A.; Yang, W.; Han, X.; Chen, X. Pharmacokinetics, tissue distribution, excretion and plasma protein binding studies of wogonin in rats. Molecules 2014, 19, 5538–5549. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef]
- Jones, Q.R.; Warford, J.; Rupasinghe, H.P.; Robertson, G.S. Target-based selection of flavonoids for neurodegenerative disorders. Trends Pharmacol. Sci. 2012, 33, 602–610. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Sample | Calibration Curve | R2 | Linear Range (μg/mL) |
|---|---|---|---|
| Plasma | y = 1.3661 + 0.008 | R2 = 0.9973 | 0.039–6.000 |
| Heart | y = 8577.8x − 1701.100 | R2 = 0.9977 | 0.29–18.700 |
| Liver | y = 7997.6x − 3192.600 | R2 = 0.9956 | 0.292–9.375 |
| Spleen | y = 8203.4x − 164.790 | R2 = 0.9992 | 0.292–18.780 |
| Lung | y = 7602.5x + 911.940 | R2 = 0.9910 | 0.292–9.375 |
| Kidney | y = 8413.3x − 194.040 | R2 = 0.9970 | 0.292–18.700 |
| Brain | y = 9592.8x − 2367.300 | R2 = 0.9943 | 0.292–9.375 |
| Parameter | 4 mg/kg | 8 mg/kg | 12 mg/kg | 20 mg/kg |
|---|---|---|---|---|
| (i.v.) | (i.v.) | (i.v.) | (i.g.) | |
| t1/2α (min) | 6.983 ± 2.299 | 7.108 ± 1.908 | 7.046 ± 2.050 | 52.415 ± 1.920 |
| t1/2β (min) | 68.529 ± 3.290 | 66.465 ± 4.551 | 69.315 ± 4.324 | 69.942 ± 2.680 |
| Tmax (min) | 2 ± 0 | 2 ± 0 | 2 ± 0 | 90 ± 0 |
| CL (mL/min) | 0.041 ± 0.070 | 0.039 ± 0.120 | 0.041 ± 0.050 | 0.046 ± 0.013 |
| V (L/kg) | 2.096 ± 0.0465 | 1.906 ± 0.012 | 2.014 ± 0.019 | 4.881 ± 2.390 |
| MRT (min) | 91.444 ± 4.428 | 85.105 ± 1.882 | 81.871 ± 5.157 | 256.76 ± 3.510 |
| AUC0–t (μg·min/mL) | 59.256 ± 6.910 | 128.938 ± 8.871 | 234.524 ± 12.070 | 52.048 ± 4.671 |
| AUC0–∞ (μg·min/mL) | 68.478 ± 8.167 | 131.438 ± 9.392 | 305.616 ± 17.432 | 74.307 ± 16.548 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, C.; Li, J.; Cai, X.; Li, N.; Guo, Y.; Wang, D. Pharmacokinetics, Tissue Distribution, Plasma Protein Binding Studies of 10-Dehydroxyl-12-Demethoxy-Conophylline, a Novel Anti-Tumor Candidate, in Rats. Molecules 2019, 24, 283. https://doi.org/10.3390/molecules24020283
Jiang C, Li J, Cai X, Li N, Guo Y, Wang D. Pharmacokinetics, Tissue Distribution, Plasma Protein Binding Studies of 10-Dehydroxyl-12-Demethoxy-Conophylline, a Novel Anti-Tumor Candidate, in Rats. Molecules. 2019; 24(2):283. https://doi.org/10.3390/molecules24020283
Chicago/Turabian StyleJiang, Chengjun, Jie Li, Xianghai Cai, Nini Li, Yan Guo, and Dianlei Wang. 2019. "Pharmacokinetics, Tissue Distribution, Plasma Protein Binding Studies of 10-Dehydroxyl-12-Demethoxy-Conophylline, a Novel Anti-Tumor Candidate, in Rats" Molecules 24, no. 2: 283. https://doi.org/10.3390/molecules24020283
APA StyleJiang, C., Li, J., Cai, X., Li, N., Guo, Y., & Wang, D. (2019). Pharmacokinetics, Tissue Distribution, Plasma Protein Binding Studies of 10-Dehydroxyl-12-Demethoxy-Conophylline, a Novel Anti-Tumor Candidate, in Rats. Molecules, 24(2), 283. https://doi.org/10.3390/molecules24020283




