Evaluation of a Nondestructive NMR and MRI Method for Monitoring the Drying Process of Gastrodia elata Blume
Abstract
:1. Introduction
2. Results and discussion
2.1. Effects of Steaming on Water States and Distribution
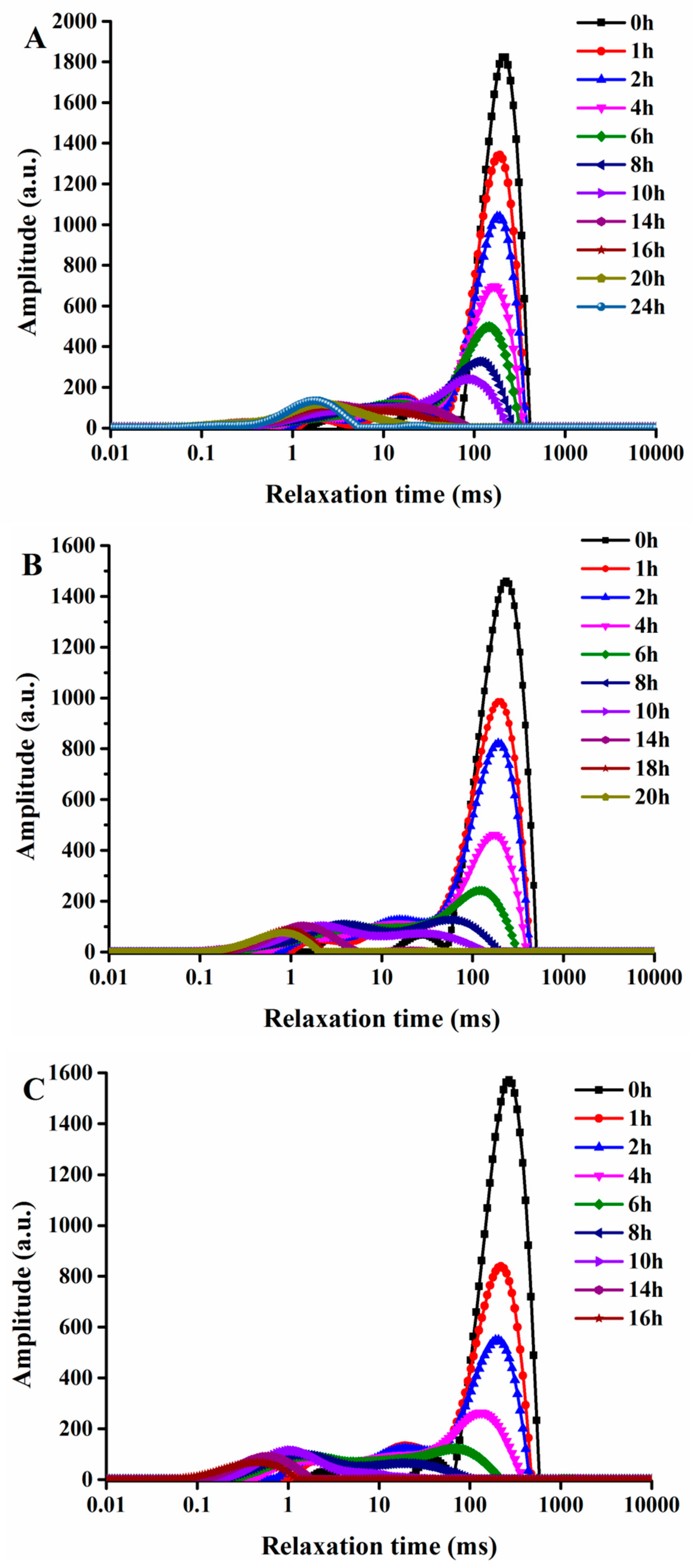
2.2. Transverse Relaxation Time Analysis of G. elata after Steaming at Different Drying Temperatures
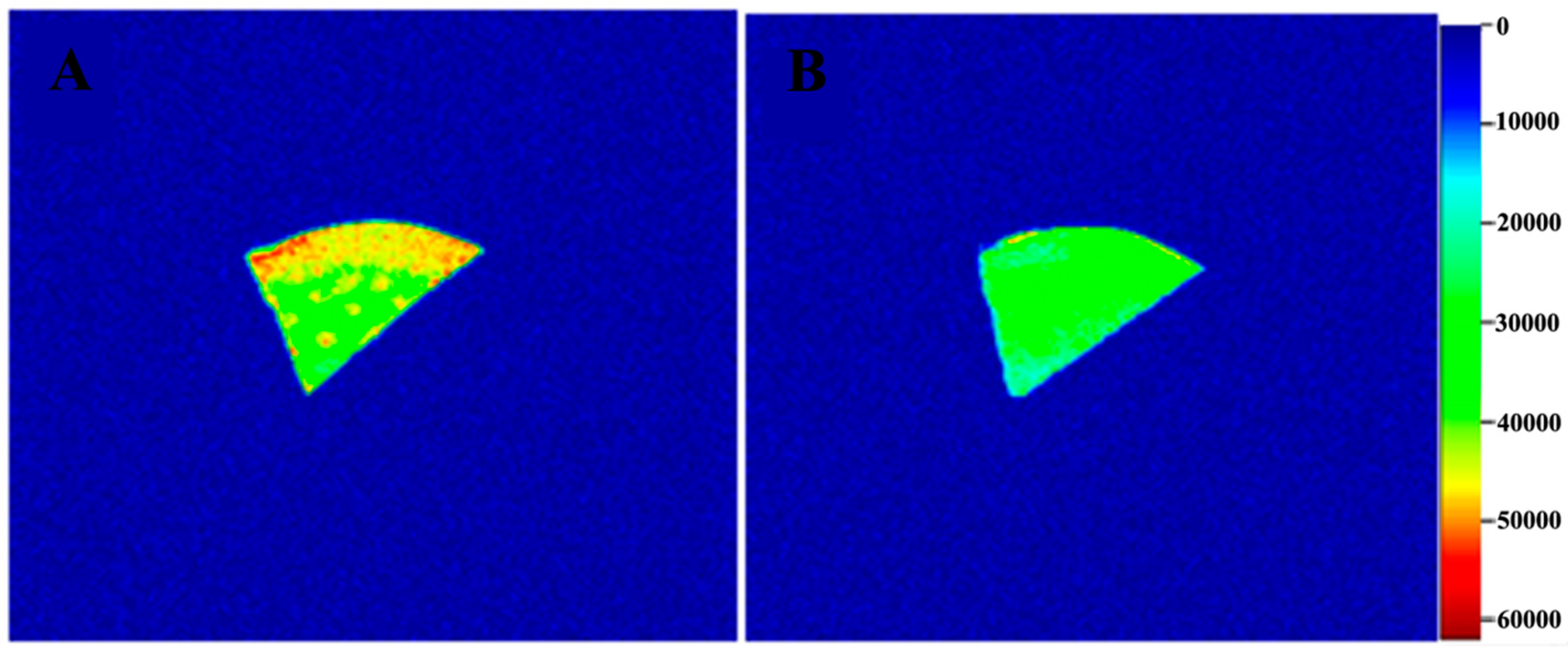
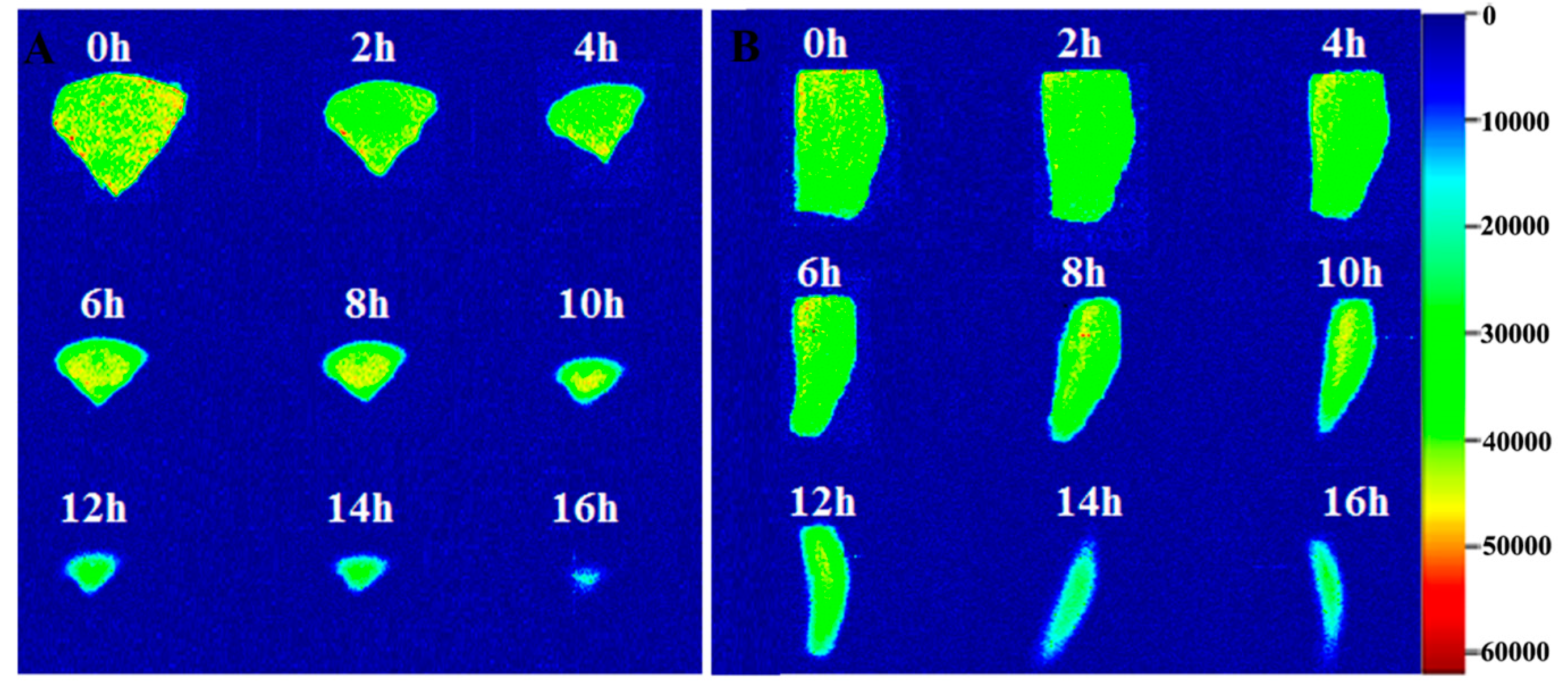
2.3. MRI Analysis of G. Elata during Drying
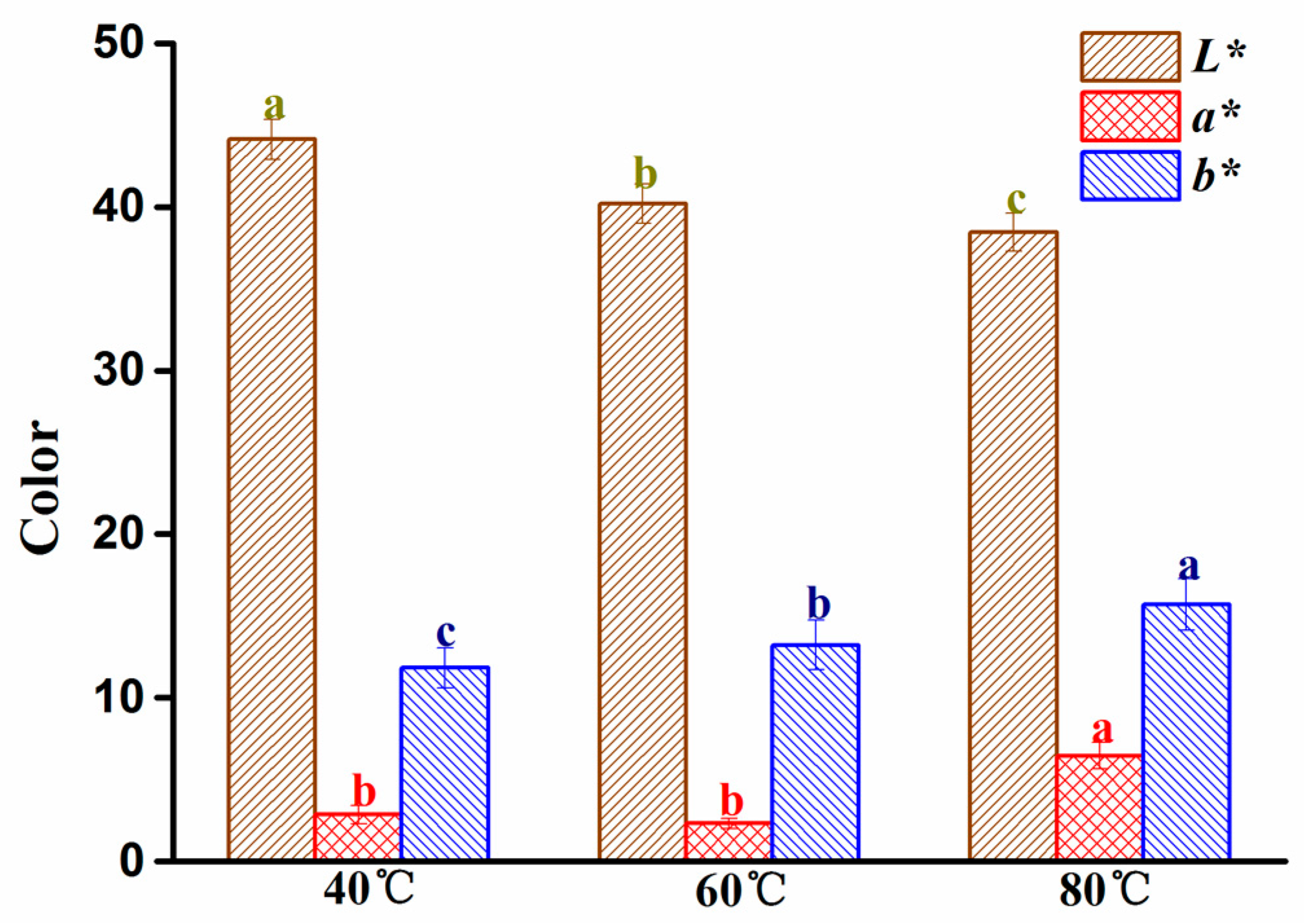
2.4. Change in the Color of G. elata at Different Drying Temperatures
2.5. Correlation Analysis
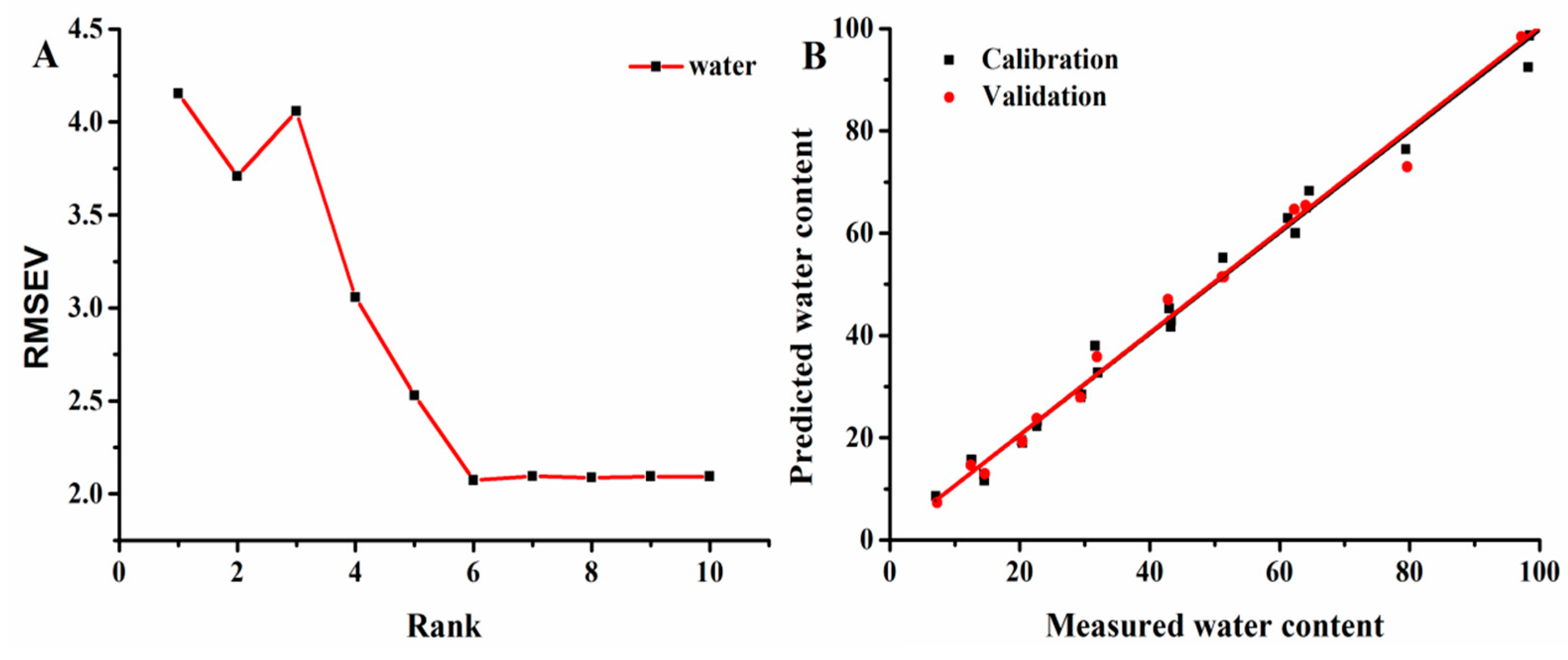
2.6. Establishment of the PLSR Model
3. Experimental
3.1. Sample Preparation
3.2. Moisture Content Measurement
3.3. Drying Process
3.4. LF-1H NMR Measurements
3.5. MRI Detection
3.6. Color Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, W.; Li, J.; Song, F.; Li, Z.; Zhang, Z. Research Process of the Drying Technology of Chinese Herbal Medicine. Med. Plant 2014, 5, 8–11. [Google Scholar]
- Yan, D.; Yuan, X.; Xie, D.; Hu, M.; Li, M.; Liu, Y.; Wu, C. Research status and thoughts on sterilization of Chinese herbal medicine pieces. Tradit. Herb. Drugs 2016, 47, 1425–1428. [Google Scholar]
- Segtnan, V.H.; Sasic, S.; Isaksson, T.; Ozaki, Y. Studies on the Structure of Water Using Two-Dimensional Near-Infrared Correlation Spectroscopy and Principal Component Analysis. Anal. Chem. 2001, 73, 3153–3161. [Google Scholar] [CrossRef]
- Lee, D.J. Measurement of Bound Water Content in Sludge: The Use of Differential Scanning Calorimetry (DSC). J. Chem. Technol. Biotechnol 1995, 62, 359–365. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Li, W.; Li, H.K.; Chen, X.H.; Jiang, M.; Dong, M.S. Low-field nuclear magnetic resonance for online determination of water content during sausage fermentation. J. Food Eng. 2017, 212, 291–297. [Google Scholar] [CrossRef]
- Li, T.; Tu, C.; Ru, X.; Gao, Y.; Li, W.; Wang, K.; Xiao, Y.; Dong, M. Study of Water Dynamics in the Soaking, Steaming, and Solid-State Fermentation of Glutinous Rice by LF-NMR: A Novel Monitoring Approach. J. Agric. Food Chem. 2015, 63, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Li, Y. Application of Low-Field NMR to Analyze Water Characteristics and Predict Unfrozen Water in Blanched Sweet Corn. Food Bioprocess Technol. 2013, 6, 1593–1599. [Google Scholar] [CrossRef]
- Yang, S.; Liu, X.; Jin, Y.; Li, X.; Chen, F.; Zhang, M.; Lin, S. Water Dynamics in Egg White Peptide, Asp-His-Thr-Lys-Glu, Powder Monitored by Dynamic Vapor Sorption and LF-NMR. J. Agric. Food Chem. 2016, 64, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.; Wang, Z.; Shen, Q.; Li, G.; Song, X.; Zhang, D. LF NMR to explore water migration and water protein interaction of lamb meat being air dried at 35 °C. Drying Technol. 2018, 36, 366–373. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Moreno, P.; Careche, M. Low field nuclear magnetic resonance (LF-NMR) relaxometry in hake (Merluccius merluccius, L.) muscle after different freezing and storage conditions. Food Chem. 2014, 153, 250–257. [Google Scholar] [CrossRef]
- Ghosha, P.K.; Jayasa, D.S.; Gruwel, M.L.H.; White, N.D.G. A magnetic resonance imaging study of wheat drying kinetics. Biosyst. Eng. 2007, 97, 189–199. [Google Scholar] [CrossRef]
- Geng, S.; Wang, H.; Wang, X.; Ma, X.; Xiao, S.; Wanga, J.; Tan, M. A non-invasive NMR and MRI method to analyze the rehydration of dried sea cucumber. Anal. Methods 2015, 7, 2413–2419. [Google Scholar] [CrossRef]
- Wu, J.; Li, Y.; Gao, X. Monitoring a typical fermentation process of natto by low-field nuclear magnetic resonance (LF-NMR) and magnetic resonance imaging (MRI) techniques. Anal. Methods 2016, 8, 7134–7140. [Google Scholar] [CrossRef]
- Cheng, S.; Tang, Y.; Zhang, T.; Song, Y.; Wang, X.; Wang, H.; Wang, H.; Tan, M. Approach for monitoring the dynamic states of water in shrimp during drying process with LF-NMR and MRI. Drying Technol. 2018, 36, 841–848. [Google Scholar] [CrossRef]
- Xu, F.; Jin, X.; Zhang, L.; Chen, X.D. Investigation on water status and distribution in broccoli and the effects of drying on water status using NMR and MRI methods. Food Res. Int. 2017, 96, 191–197. [Google Scholar] [CrossRef]
- Matias, M.; Silvestre, S.; Falcão, A.; Alves, G. Gastrodia elata and epilepsy: Rationale and therapeutic-potential. Phytomedicine 2016, 26, 1511–1526. [Google Scholar] [CrossRef] [PubMed]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Evaluation of the extraction efficiency of thermally labile bioactive compounds in Gastrodia elata Blume by pressurized hot water extraction and microwave-assisted extraction. J. Chromatogr. A 2008, 1182, 34–40. [Google Scholar] [CrossRef]
- Chen, W.C.; Lai, Y.S.; Lin, S.H.; Lu, K.H.; Lin, Y.E.; Panyod, S.; Ho, C.T.; Sheen, L.Y. Anti-depressant effects of Gastrodia elata Blume and its compounds gastrodin and 4-hydroxybenzyl alcohol, via the monoaminergic system and neuronal cytoskeletal remodeling. J. Ethnopharmacol. 2016, 182, 190–199. [Google Scholar] [CrossRef]
- Wong, H.Y.; Hu, B.; So, P.K.; Chan, C.O.; Mok, D.K.W.; Xin, G.Z.; Li, P.; Yao, Z.P. Rapid authentication of Gastrodiae rhizoma by direct ionization mass spectrometry. Anal. Chim. Acta 2016, 938, 90–97. [Google Scholar] [CrossRef]
- Shan, M.; Qian, Y.; Yu, S.; Zhang, L.; Ding, A. Study on integrative technology of primary processing for Gastrodiae Rhizoma based on response surface methodology. Chin. Tradit. Herb. Drugs 2016, 47, 420–424. [Google Scholar]
- Tian, Z.; Wang, J.; Liu, J.; Dai, K.; Liu, X.; Yu, X.; Ma, C.; Liu, D. Effects of Different Processsing Methods and Steamed Time on Quality of Zhaotong Gastrodiae rhizoma. Southwest Chin. J. Agric. Sci. 2016, 29, 1701–1706. [Google Scholar]
- Raffo, A.; Gianferri, R.; Barbieri, R.; Brosio, E. Ripening of banana fruit monitored by water relaxation and diffusion 1H-NMR measurements. Food Chem. 2005, 89, 149–158. [Google Scholar] [CrossRef]
- Chen, L.; Tian, Y.; Sun, B.; Wang, J.; Tong, Q.; Jin, Z. Rapid, accurate, and simultaneous measurement of water and oil contents in the fried starchy system using low-field NMR. Food Chem. 2017, 233, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Wang, R.Y.; Song, Y.K.; Kamal, T.; Lv, Y.; Zhu, B.W.; Tao, X.H.; Tan, M.Q. A fast and non-destructive LF-NMR and MRI method to discriminate adulterated shrimp. J. Food Meas. Charact. 2018, 12, 1340–1349. [Google Scholar] [CrossRef]
- Tan, M.; Lin, Z.; Zu, Y.; Zhu, B.; Cheng, S. Effect of multiple freeze-thaw cycles on the quality of instant sea cucumber: Emphatically on water status of by LF-NMR and MRI. Food Res. Int. 2018, 109, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Tian, B.Q.; Jia, H.F.; Zhang, H.Y.; He, F.; Song, Z. Investigation on water distribution and state in tobacco leaves with stalks during curing by LFNMR and MRI. Drying Technol. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Mothibe, K.J.; Zhang, M.; Mujumdar, A.S.; Wang, Y.C.; Cheng, X.F. Effects of Ultrasound and Microwave Pretreatments of Apple Before Spouted Bed Drying on Rate of Dehydration and Physical Properties. Drying Technol. 2014, 32, 1848–1856. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Zhao, G.; Qiao, M.; Li, M.; Sun, L.; Gao, X.; Zhang, J. Determining the drying degree and quality of chicken jerky by LF-NMR. J. Food Eng. 2014, 139, 43–49. [Google Scholar] [CrossRef]
- Adiletta, G.; Iannone, G.; Russo, P.; Patimo, G.; Pasquale, S.D.; Matteo, M.D. Moisture migration by magnetic resonance imaging during eggplant drying: A preliminary study. Int. J. Food Sci. Technol. 2014, 49, 2602–2609. [Google Scholar] [CrossRef]
- Michalska, A.; Honke, J.; Łysiak, G.; Andlauer, W. Effect of drying parameters on the formation of early and intermediate stage products of the Maillard reaction in different plum (Prunus domestica L.) cultivars. LWT Food Sci. Technol. 2016, 65, 932–938. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.; Bhandari, B.; Zhou, L. LF-NMR online detection of water dynamics in apple cubes during microwave vacuum drying. Drying Technol. 2018, 1–10. [Google Scholar] [CrossRef]
- Zheng, H.; Lu, H. Use of kinetic, Weibull and PLSR models to predict the retention of ascorbic acid, total phenols and antioxidant activity during storage of pasteurized pineapple juice. LWT Food Sci. Technol. 2011, 44, 1273–1281. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the authors. |
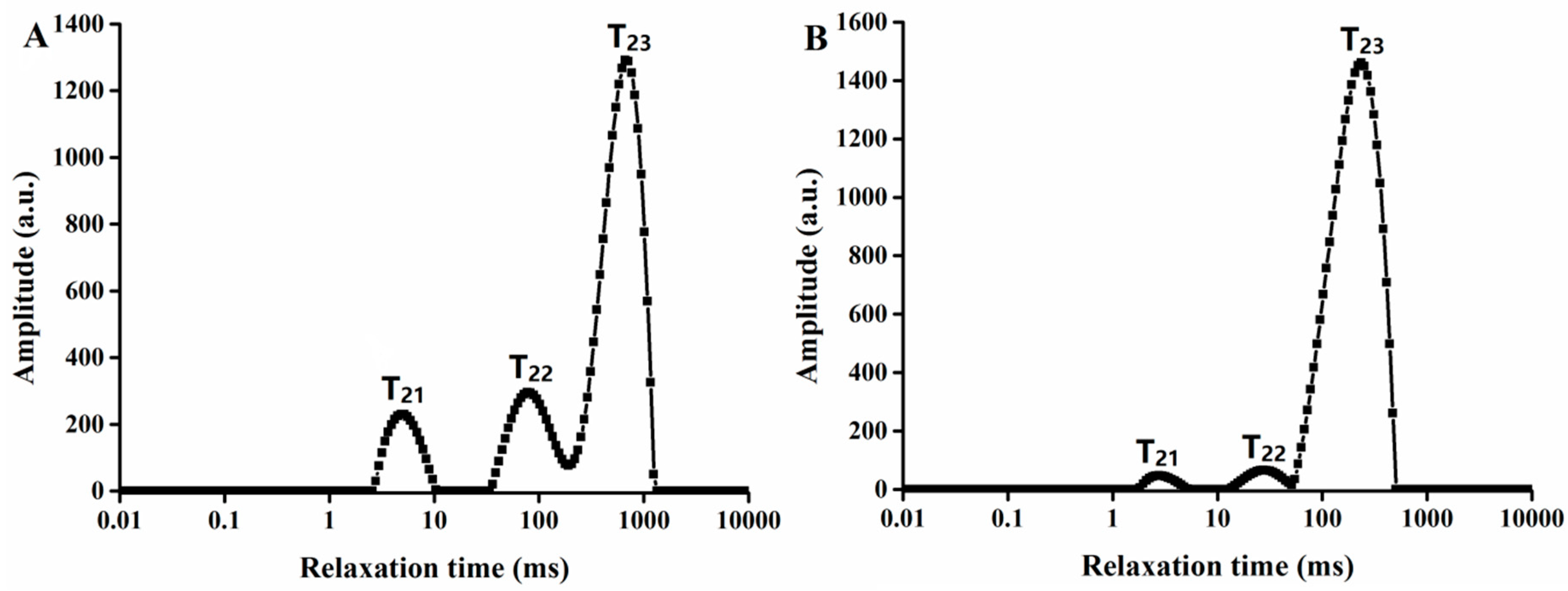
| A21 (%) | A22 (%) | A2 3 (%) | T21 (ms) | T22 (ms) | T23 (ms) | |
|---|---|---|---|---|---|---|
| Before steaming | 11.11 ± 0.3a | 18.01 ± 0.5a | 70.88 ± 0.4a | 5.18 ± 0.5a | 96.81 ± 0.4a | 584.94 ± 0.4a |
| After steaming | 1.62 ± 0.2b | 2.93 ± 0.6b | 95.45 ± 0.5b | 5.39 ± 0.3a | 89.59 ± 0.5b | 274.18 ± 0.6b |
| Drying Time (min) | T21 (ms) 1,2 | T22 (ms) 1,2 | T23 (ms) 1,2 | A21/g 1,2 | A22/g 1,2 | A23/g 1,2 | ATotal/g 1,2 |
|---|---|---|---|---|---|---|---|
| 2 | 5.03 ± 0.15a | 83.66 ± 4.66a | 248.94 ± 3.65a | 48.01 ± 1.91f | 86.75 ± 3.22e | 2210.86 ± 4.25a | 2345.62 ± 6.23a |
| 4 | 4.47 ± 0.19b | 75.14 ± 5.31b | 234.98 ± 4.60b | 55.03 ± 3.56f | 99.04 ± 2.45d | 2015.86 ± 14.60b | 2169.93 ± 4.26b |
| 6 | 3.99 ± 0.21c | 62.12 ± 4.57c | 215.61 ± 3.73c | 70.58 ± 4.04e | 110.32 ± 4.08c | 1878.33 ± 9.48c | 2059.23 ± 3.58c |
| 8 | 3.27 ± 0.20d | 53.42 ± 2.61d | 200.47 ± 4.26d | 86.37 ± 4.41d | 125.50 ± 3.97b | 1650.33 ± 13.51d | 1862.20 ± 3.22d |
| 10 | 2.82 ± 0.14e | 44.71 ± 3.07e | 186.46 ± 2.99e | 100.57 ± 4.08c | 142.13 ± 2.78a | 1433.37 ± 9.11e | 1676.08 ± 8.21e |
| 12 | 2.26 ± 0.26f | 36.79 ± 2.86f | 172.90 ± 6.29f | 118.61 ± 6.25b | 124.58 ± 4.20b | 1116.68 ± 10.21f | 1359.87 ± 6.58f |
| 14 | 1.63 ± 0.17g | 30.83 ± 1.33fg | 155.35 ± 5.23g | 135.29 ± 4.12a | 95.40 ± 4.06d | 882.68 ± 6.94g | 1113.37 ± 7.66g |
| 16 | 1.21 ± 0.22h | 25.23 ± 1.64gh | 136.09 ± 3.15h | 108.95 ± 4.15c | 104.71 ± 4.69f | 544.84 ± 9.52h | 758.50 ± 6.23h |
| 18 | 0.96 ± 0.08hi | 21.03 ± 1.55hi | 121.28 ± 1.56i | 73.95 ± 4.56e | 56.61 ± 3.17g | 212.35 ± 7.67i | 342.91 ± 5.02i |
| 20 | 0.76 ± 0.06ij | 18.05 ± 1.36i | - | 51.66 ± 2.63f | 32.85 ± 1.86h | - | 84.51 ± 2.18j |
| 22 | 0.53 ± 0.08j | - | - | 33.81 ± 3.33g | - | - | 33.81 ± 1.47k |
| Drying Time (min) | T21 (ms) 1,2 | T22 (ms) 1,2 | T23 (ms) 1,2 | A21/g 1,2 | A22/g 1,2 | A23/g 1,2 | ATotal/g 1,2 |
|---|---|---|---|---|---|---|---|
| 2 | 4.69 ± 0.35a | 79.83 ± 5.47a | 243.61 ± 4.48a | 50.35 ± 4.09f | 89.75 ± 4.03d | 2129.84 ± 4.88a | 2269.94 ± 12.43a |
| 4 | 4.13 ± 0.60ab | 70.13 ± 6.28b | 226.65 ± 3.32b | 58.03 ± 3.91f | 102.37 ± 2.34c | 1734.84 ± 8.75b | 1895.25 ± 7.83b |
| 6 | 3.69 ± 0.48b | 59.77 ± 3.09c | 208.94 ± 2.63c | 75.253.40e | 115.4 ± 4.17b | 1417.91 ± 11.16c | 1608.55 ± 6.25c |
| 8 | 2.91 ± 0.16c | 50.00 ± 4.05d | 195.80 ± 6.31d | 93.37 ± 4.21d | 133.6 ± 5.11a | 1088.21 ± 4.19d | 1315.18 ± 7.56d |
| 10 | 2.48 ± 0.34cd | 41.83 ± 2.92e | 186.46 ± 2.99d | 105.25 ± 3.22c | 114.71 ± 3.42b | 829.41 ± 4.81e | 1049.37 ± 5.28e |
| 12 | 1.95 ± 0.13d | 33.57 ± 1.51f | 163.23 ± 7.05e | 127.61 ± 5.27a | 89.74 ± 2.40d | 635.05 ± 8.62f | 852.4 ± 4.33f |
| 14 | 1.23 ± 0.28e | 27.92 ± 0.97fg | 145.35 ± 6.31f | 115.29 ± 4.12b | 92.35 ± 2.11e | 415.83 ± 4.36g | 623.48 ± 5.25g |
| 16 | 1.07 ± 0.18e | 22.73 ± 1.38gh | 126.09 ± 3.15g | 96.06 ± 5.44d | 50.98 ± 1.17f | 291.71 ± 6.90h | 438.74 ± 3.28h |
| 18 | 0.86 ± 0.12e | 18.58 ± 2.15h | - | 70.61 ± 5.37e | 32.73 ± 4.25g | - | 103.35 ± 3.22i |
| 20 | 0.69 ± 0.05e | - | - | 40.99 ± 3.30g | - | - | 40.99 ± 2.13j |
| Drying Time (min) | T21 (ms) 1,2 | T22 (ms) 1,2 | T23 (ms) 1,2 | A21/g 1,2 | A22/g 1,2 | A23/g 1,2 | ATotal/g 1,2 |
|---|---|---|---|---|---|---|---|
| 2 | 4.20 ± 0.14a | 73.84 ± 4.06a | 226.27 ± 5.32a | 57.68 ± 1.93d | 91.08 ± 4.04c | 2026.95 ± 6.34a | 2175.71 ± 9.82a |
| 4 | 3.70 ± 0.49a | 65.46 ± 3.67b | 209.65 ± 4.18b | 72.70 ± 4.75c | 114.61 ± 3.13b | 1546.24 ± 10.91b | 1733.55 ± 8.43b |
| 6 | 2.97 ± 0.22b | 55.10 ± 3.72c | 198.94 ± 5.81b | 90.58 ± 7.13b | 125.40 ± 3.99a | 1136.94 ± 11.04c | 1352.92 ± 7.26c |
| 8 | 2.47 ± 0.24bc | 46.34 ± 4.36d | 185.32 ± 4.90c | 113.37 ± 5.79a | 116.93 ± 4.75b | 774.60 ± 6.61d | 1004.9 ± 5.25d |
| 10 | 1.98 ± 0.18cd | 37.17 ± 1.93e | 166.81 ± 3.20d | 96.92 ± 4.59b | 88.04 ± 2.04c | 570.56 ± 5.58e | 755.52 ± 6.47e |
| 12 | 1.64 ± 0.24d | 28.57 ± 1.38f | 143.23 ± 7.05e | 79.28 ± 2.89c | 63.07 ± 2.58d | 269.21 ± 4.21f | 411.56 ± 5.23f |
| 14 | 1.03 ± 0.15e | 21.58 ± 3.70f | - | 55.96 ± 4.15d | 35.67 ± 3.69e | - | 91.62 ± 4.22g |
| 16 | 0.93 ± 0.22e | - | - | 35.39 ± 3.51e | - | - | 35.39 ± 3.21h |
| T21 | T22 | T23 | A21/g | A22/g | A23/g | A Total/g | |
|---|---|---|---|---|---|---|---|
| MC | 0.982 * | 0.980 ** | 0.972 * | 0.233 | 0.131 | 0.991 ** | 0.996 ** |
| L* | −0.162 | −0.628 | −0.729 | 0.527 | 0.521 | 0.904 ** | 0.913 ** |
| a* | 0.017 | −0.219 | 0.228 | 0.509 | 0.432 | 0.613 | 0.722 |
| b* | 0.728 | 0.823 * | 0.845 * | 0.749 | 0.071 | 0.769 | 0.776 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Dong, H.; Li, J.; Guo, L.; Wang, X. Evaluation of a Nondestructive NMR and MRI Method for Monitoring the Drying Process of Gastrodia elata Blume. Molecules 2019, 24, 236. https://doi.org/10.3390/molecules24020236
Chen Y, Dong H, Li J, Guo L, Wang X. Evaluation of a Nondestructive NMR and MRI Method for Monitoring the Drying Process of Gastrodia elata Blume. Molecules. 2019; 24(2):236. https://doi.org/10.3390/molecules24020236
Chicago/Turabian StyleChen, Yannan, Hongjing Dong, Jingkun Li, Lanping Guo, and Xiao Wang. 2019. "Evaluation of a Nondestructive NMR and MRI Method for Monitoring the Drying Process of Gastrodia elata Blume" Molecules 24, no. 2: 236. https://doi.org/10.3390/molecules24020236
APA StyleChen, Y., Dong, H., Li, J., Guo, L., & Wang, X. (2019). Evaluation of a Nondestructive NMR and MRI Method for Monitoring the Drying Process of Gastrodia elata Blume. Molecules, 24(2), 236. https://doi.org/10.3390/molecules24020236




