Abstract
Guinep is traditionally used in the management of cardiovascular ailments. This study aims to evaluate its medicinal constituents and effects in the management of myocardial injury in an experimental isoproterenol (ISO) rat model. Sprague-Dawley rats were randomly assigned to four groups: Group 1 was the control group; Group 2 received M. bijugatus extract (100 mg/Kg; MB) for six weeks; Group 3 was given ISO (85 mg/Kg) i.p. twice during a 24-hour period; and Group 4 was given ISO (85 mg/Kg) i.p. and MB extract (100 mg/Kg) for six weeks. The MB was administered orally by gavage, daily. The blood pressure of conscious animals was measured, while ECG was performed under anesthesia. Blood and serum were collected for biochemical and hematological analysis. The ISO group treated with MB showed a significant decrease (p < 0.001) in (SBP), diastolic (DBP), mean arterial (MAP) and heart rate (HR) compared to the ISO only group. Conversely, MB treated rats that were not induced with ISO displayed a significant decreases (p < 0.001) in SBP, DBP, MAP, and HR. ISO significantly elevated the ST segment (p < 0.001) and shortened the QTc interval (p < 0.05), which were recovered after treatment with 100 mg/Kg of MB. In addition, the results showed a significant decrease (p < 0.001) in the heart to body weight ratio of the ISO group treated with MB compared to the ISO only group. Furthermore, the extract normalized the hematological values depressed by the ISO while significantly elevating the platelet count. UHPLC high-resolution orbitrap mass spectrometry analysis results revealed the presence of several antioxidants like vitamin C and related compounds, phenolic acids, flavonoid, fatty acids (oxylipins), and terpene derivatives. The results of this study indicated that Melicoccus bijugatus did display some cardio-protective effects in relation to myocardial injury.
1. Introduction
Cardiovascular disease (CVD) like acute myocardial infarction (AMI) is one of the leading causes of death; causing prolonged ischemia of the heart muscle resulting in tissue death or infarction in the myocardium []. This results in edema, a reduction in cardiac output, abnormalities in cardiac rhythm and transmission blocks that can further impair cardiac function. A reduction in cardiac output and arterial pressure may stimulate baroreceptor reflexes that lead to the activation of compensatory mechanisms, such as those of the sympathetic nerves and the renin-angiotensin-aldosterone system [], and to elevations in cardiac biomarkers [].
ISO is a potent non-selective beta-adrenergic receptor (β1 and β2) agonist that causes severe stress to the myocardium, resulting in infarct-like necrosis of the heart muscle []. Its proposed mechanism of action is through the auto-oxidation and production of free cytotoxic radicals [], as well as hyper-stimulation of beta adrenoceptors []. These actions lead to the peroxidation of the cellular membrane, a change in membrane permeability and possible derangement of calcium ion pathway signaling, hypertrophy and myocardial injury [,]. The net effect of these includes a fall in DBP and MAP while SBP may remain unchanged, rise or fall (depending on the dose). Similarly, cardiac output may increase because of the positive inotropic and chronotropic effects of the drug, due to a decrease in peripheral vascular resistance.
Melicoccus bijugatus, known colloquially in Jamaica as Guinep, is a minor member of the Sapindaceae family []. The therapeutic effects of these fruits, including the management of diarrhea, cardiovascular disease, asthma and constipation, and as an astringent [], were attributed to the combination of phenolic compounds and sugars. The phenolic content of this fruit was previously reported [,]. In the seed embryo, flavonoids, epicatechin, catechin, epigallocatechin, B-type procyanidins, naringenin, naringenin derivatives, phloretin, phloridzin, quercetin, myricetin and resveratrol, were identified in high amounts. The pulp of the fruit contains phenolic acid derivatives such as coumaric and ferulic acid derivatives, and hydroxycinnamic and sinapic acid [,]. This study aims to scientifically examine the mechanism of action of M. bijugatus in the management of cardiovascular ailments like AMI via an experimental rat model.
2. Results
2.1. Blood Pressure Changes and Electrocardiogram (ECG)
Table 1 shows that the extract group displayed a significant decrease (p < 0.001) in the MAP, SBP, and DBP when compared to the control (normotensive rats). There was also a significant decrease in the MAP, SBP, and DBP of the ISO group treated with MB compared to the ISO only group (p < 0.001).

Table 1.
Effects of Melicoccus bijugatus (MB; 100 mg/Kg) on mean arterial blood pressure (MAP), systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure (PP), and heart rate (HR) of normotensive rats and those with myocardial damage with ISO.
The MB group displayed a significantly decreased (p < 0.01) HR when compared to the normotensive animals (control). There was also a significant decrease (p < 0.001) in the HR of the ISO plus MB treatment group when compared to the ISO only treatment group (Figure 1, Table 1). In addition, there was an observable (24.5%) increase in the HR of the ISO only group when compared to the control group (normotensive). However, this increase was not statistically significant.
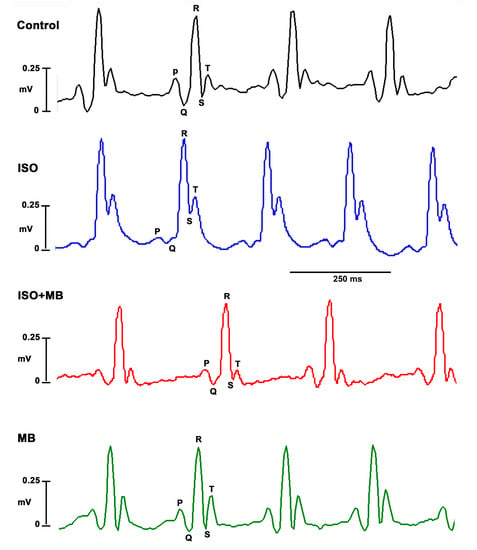
Figure 1.
Electrocardiograms (ECG) showing the bradycardic effects of M. bijugatus extract (MB, 100 mg/Kg) in myocardial injury. Control shows the normal electrocardiograph. ISO (85 mg/Kg) shows an elevated ST segment; ISO + MB shows a restored ST segment.
Although the PP significantly decreased in all groups,the causes in the MB group (p < 0.001), ISO group (p < 0.01) and ISO + MB group (p < 0.01) were different compared to that of the control. Table 1 shows that ISO significantly (p < 0.05) increased the DBP, did not increase the SBP, and decreased the PP, consistent with decreased left ventricular compliance and increased myocardial stiffness []. MB significantly decreased the blood pressure (MAP, SBP, DBP), HR, and PP, which is proportional to the stroke volume [].
In electrocardiograms from rats and mice, the beginning of the T-wave merges with the end of the QRS complex without an isoelectric ST segment. The changes, seen in the ECG, that affected the frequency of the waves can be seen to affect the HR also. ISO significantly elevated the ST segment (p < 0.001; Figure 1 and Figure 2A) and shortened the QTc interval (p < 0.05; Figure 1 and Figure 2B), which recovered after treatment with 100 mg/Kg of MB. The M. bijugatus of the plant did not, per se, cause any change.
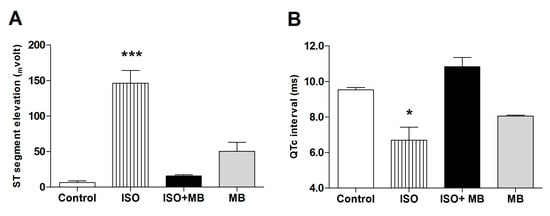
Figure 2.
Treatment, with M. bijugatus, of a myocardial injury caused by ISO. The data shows the effects of MB and ISO on ST segment elevation (A) and the duration of the QTc interval of the ECG (B). ISO (85 mg/Kg) significantly elevated the ST segment (p < 0.001) and shortened the QTc interval (p < 0.05), which recovered after treatment with 100 mg/Kg of M. bijugatus. The extract did not, per se, cause any change. * p ˂ 0.05, *** p ˂ 0.001 vs. control; n = 5.
2.2. Hematological Parameters
There were significant differences (p > 0.05) in the hematological parameters of the treatment groups when compared to the control group (Table 2 and Table 3). ISO significantly reduced the white blood count (WBC; p < 0.05), red blood count (RBC; p < 0.01), hematocrit (HCT; p < 0.01), mean cell volume (MCV; p < 0.01) and mean cell hemoglobin values (MCH; p < 0.01). M. bijugatus extract normalized the hematological values (WBC, RBC, HCT, MCV and MCH) depressed by ISO, while significantly (p < 0.01) elevating the platelet count.

Table 2.
Effect of M. bijugatus on white cell parameters in ISO-induced cardiac injury.

Table 3.
Effect of M. bijugatus on red cell and trombocytes parameters in the ISO-induced cardiac injury.
2.3. Histo-Morphological Analysis
The microscopic changes in the muscle fibers of the heart were limited to the group that was exposed to both ISO and MB. The maximum dimension of the myocardial infarction was of 1.1 mm and appeared to be in the central region of the left ventricular muscle wall, which is the area furthest away from both endocardium and epicardium and is most susceptible to infarction from vascular compromise (Figure 3C).
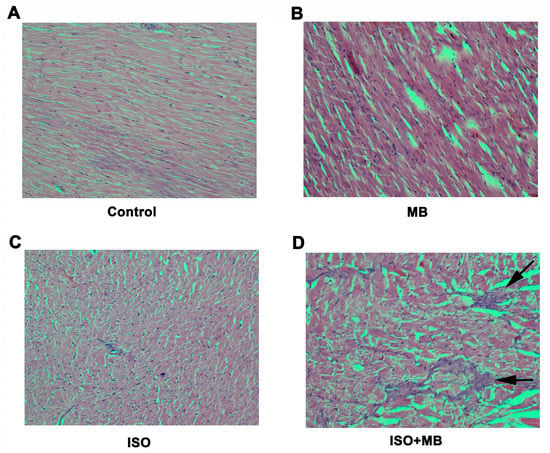
Figure 3.
Histological analysis of myocardial injury. Histomicrograph of transverse sections of the heart [×200; hematoxylin and eosin stain] taken through the ventricles, just below the atrioventricular valves of control (A), 100 mg/Kg MB alone (B), ISO treatment alone (C), ISO + MB (D). The ISO group showed myocardial infarction in the central region of the left ventricular muscle wall (C). The Control (A) and MB only group (B) displayed no features of myocardial injury. The arrows demonstrated the area of fibrosis.
Longitudinal and/or transverse sections of the large caliber abdominal blood vessels, i.e., aorta and caudal vena cava revealed no changes in the intima, media or external layer. The adventitia was composed primarily of brown fat. No inflammation was appreciated (data not shown).
2.4. Ratio of Heart Weight to Body Weight
The data does not suggest that M. bijugatus is responsible for any significant changes in body weight that could not be accounted for in the natural growth pattern of the Sprague-Dawley rat. ISO caused an elevated heart weight/body weight ratio and treatment with MB (100 mg/Kg) caused a significant decrease (Figure 4).
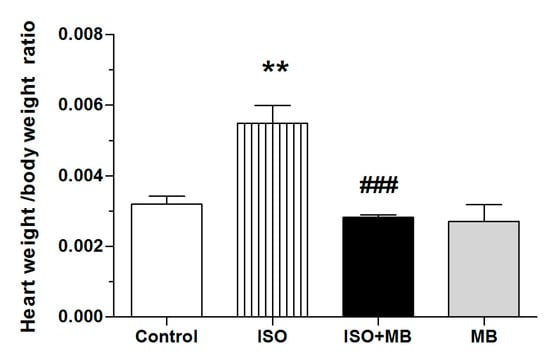
Figure 4.
Effects of ISO and treatment with M. bijugatus extract on weight/body weight ratio. Depicts the ISO (85 mg/Kg) induced myocardial damage through an elevation of the heart weight to body weight ratio. This was significantly reduced in the MB (100 mg/Kg) treated groups. ** p ˂0.01 vs. control; ### p ˂ 0.001 vs. ISO, n = 5.
2.5. Identification of the Compounds
Hyphenated UHPLC-MS experiments were employed for the identification of unknown compounds in the fruits of M. bijugatus since it provides high resolution and an accurate mass product ion spectra (Figure 5, Table 4 and Supplementary Material). Combining Q-orbitrap HRAM (high resolution accurate mass) full MS scans and MSn experiments, all compounds were tentatively identified, including simple phenolic acids, terpenes, fatty acids, and one glycosylated flavonoid. As far as we know, some of the compounds, for this species, were reported for the first time. Below is a detailed explanation of the identification.
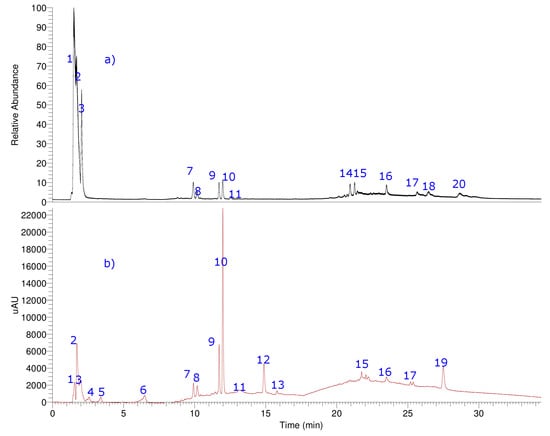
Figure 5.
UHPLC chromatogram total ion current [total ion current (a), UV at 280 nm (b)] of aqueous extract of M. bijugatus. The details of metabolites are in the Supplementary Material S1.

Table 4.
Identification of metabolites by UHPLC-PDA-OT-MS.
2.5.1. Simple Organic Acids and Sugars
Peaks 1–5 and 8 were identified as simple organic acids such as vitamin C and sucrose. Peak 1, with an [M − H]− ion at m/z: 191.01933, was identified as citric acid (C6H7O7−) and Peak 2 as the isomer isocitric acid []. Peak 3 was identified as saccharose (C12H21O11−) and Peak 4 as glucose (C6H11O6−). Sucrose and glucose were already reported as important constituents of this fruit [,]. Peak 5, with an [M − H]− ion at m/z: 111.00787, was identified as furoic acid (C3H3O3−) and Peak 16, with an [M − H]− ion at m/z: 293.17587, was assigned to the bioactive, cell permeable, 1,4-benzoquinone embelin derivative (C17H26O4−) [].
2.5.2. Flavonoids
Peak 12, with an [M − H]− ion at m/z: 477.11670, and the MS2 fragment at m/z: 314.04370 were identified as Isorhamnetin-3-O-Glucoside (C22H21O11−) [].
2.5.3. Phenolic Compounds
Peak 6 was identified as salicilic acid glucoside (C13H15O8−), the parent ion 299.07712 delivered a diagnostic salicylic acid ion at m/z: 137.02440 [], and Peak 7 was identified as the dicoumarin aflavarin (C24H21O9−). Peaks 9 and 10, with pseudomolecular ions at m/z: 325.09277 and dioagnostic MS2 fragments at m/z: 163.02910, 145.02870 and 117.03368, were identified as coumaric acid glucoside and coumaric acid galactoside, respectively (C15H17O8−). The presence of coumaric acid glucoside was already reported in this fruit pulp []. In the same manner, Peaks 11 and 13 were identified as the related compounds feruloyl glucoside and feruloyl galactoside (C16H19O9−) showing diagnostic MS2 ions at 147.04449 and 193.05056. Peak 14 was identified as chlorogenic acid: 3-O-caffeoylquinic acid (3-CQA, C16H17O9−), with a diagnostic quinic acid ion at 191.05608 [].
2.5.4. Fatty Acids
Two peaks were tentatively identified as fatty acids known as oxylipins [,]. Peak 8, with a pseudomolecular ion at m/z: 325.18443, was identified as a trihydroxy-octadecatrienoic acid (C18H29O5−) and Peak 2, with a pseudomolecular ion at m/z: 311.16876, was identified as a hydroxyheptadecatrienoic acid (C17H27O7−) [].
2.5.5. Terpenes and Related Compounds
Peak 17 was identified as the lactone sedanenolide (C12H12O2−) [] and Peak 18 was assigned to the sesquiterpene valerenic acid (C15H21O2−) []. Peak 15, with a [M − H]− ion at m/z: 221.15488, was identified as the antifungic norsesquiterpene alcohol rishitin (C14H22O2−)[]. Peak 19, with an [M − H]− ion at m/z: 209.15430, was identified as Blumenol C (C13H22O2−, 4-(3-hydroxybutyl)-3,5,5-trimethylcyclohex-2-en-1-one) [].
3. Discussion
This study, for the first time, demonstrated the hypotensive effect of the Melicoccus bijugatus Jacq when it is administrated orally in normotensive animals. This hypotensive effect was, in part, because of the decrease in HR. In addition, we demonstrated a partial recovery of ISO-induced myocardial infarction by the action of M. bijugatus.
ISO is an isopropyl analog of epinephrine that stimulates the α-1 adrenergic receptors [], oxidative stress in cardiac myocytes, cell membrane destabilization and damage. It also increased intracellular adenylyl cyclase in the myocardium, increased lipid deposition in the myocardium, and increased the heart weight to body weight ratio and myocardial infarction []. ISO also significantly elevated the ST segment and shortened the QTc interval in the experimental animals; this was reversed by treatment with M. bijugatus extracts. The chronotropic and inotropic actions of ISO also caused an increase in the SBP. The sum of these cardiovascular changes is in an increase in HR and cardiac output and a decrease in the MAP, as observed in our study. Treatment of the animals exposed to ISO with M. bijugatus extracts caused decreases in HR, and MAP. This showed the cardioprotective role of the M. bijugatus.
This increase in the ratio of heart weight to body weight was thought to be a hypertrophic response, possibly due to an increase in the amount of protein synthesis that was occurring in the damaged tissue as it attempted to repair itself, as well as an increase the number of inflammatory cells to become mobilized in response to the damage []. Other possible mechanisms include increase in the glucose uptake in cardiac myocytes along with an increase in oxidative stress with ISO administration [], accumulation of fluid in intracellular space of the tissue as well as an increase in the water content of the cells themselves []. Histo-morphological presentations of myocardial infarction to the ISO group were observed. There were no appreciable changes in the intima, media and adventitia layers of the aorta, caudal or vena cava (data not shown).
Using UHPLC high-resolution orbitrap mass spectrometry, (UHPLC-OT-HR-MS) we have identified 20 secondary metabolites in the aqueous extract of M. bijugatus, most of which, as far as we know, were reported here for the first time. Many of these compounds are simple organic acids, such as vitamin C, a flavonoid, several phenolic compounds, terpenoids, and two fatty acids. Furthermore, the results obtained in this study clearly show that the infusion can be a natural source of phenolic compounds with potential applications in the neutraceutical management of myocardial infarction.
A possible mechanism of action may be through the inhibition of the proliferation of cells of the vascular smooth muscle by caffeic acid, which is found in the fruit’s pulp tissues []. The hypotensive properties displayed may also be due to the action of coumaric acid derivatives. A derivative of the sugar of coumaric acid was confirmed to be a major peak in the HPLC fingerprint profile at 280nm []. One such derivative of coumaric acid is p-coumaric which has been known to possess antioxidant effects as well as anti-platelet activity [].
Increases in serum levels of troponin I, CK-MB, myoglobin, and high sensitivity C-reactive protein are often associated with myocardial damage [,], though with some limitations [,]. Our study showed no significant difference in the serum concentration of CK-MB in rats induced with ISO compared to the control group (data not shown), this could be due to the timeline of the analysis of these biomarkers. Our results are supported by Zhang et al. (2008), who reported a decline in these biomarkers, down to control levels, after 48 h [].
Myocardial infarction is often associated with an increase in the white blood cell count as part of an inflammatory response to the damaged cells that are present due to the necrosis of the tissue []. Other hematological features associated with myocardial infarction include an increase in whole blood viscosity and plasma viscosity, an increase in the white blood cell, leukocyte and neutrophil count [], an increase in the erythrocyte count and hemoglobin concentration [], and an increase in red cell indices, such as mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and mean corpuscular volume (MCV) []. Sangeetha and Quine, (2008) reported increases in hemoglobin, RBC, hematocrit, WBC and platelet counts with ISO induced myocardial infarction in male Wister rats [], while Lobo-Fiho et al., (2011) suggested no alterations in terms of the hemoglobin indices []. We observed significant alterations in the hematological parameters in the treatment groups when compared to the control group. This study showed that ISO significantly reduced the white blood count (WBC), red blood count (RBC), hematocrit (HTC), mean cell volume (MCV) and mean cell hemoglobin values (MCH). The extract normalized the hematological values (WBC, RBC, HCT and MCV) depressed by ISO while significantly elevating the platelet count. The increased production of platelets suggests that the extract did not present a toxic effect, such as that of Colocasia esculenta (L.) Schott [], but presented an increase in platelets similar to Carica papaya Linn. [].
4. Materials and Methods
4.1. Plant Material and Extraction
The fruit of the M. bijugatus tree was collected in September. Mr. Patrick Lewis, Department of Botany, University of the West Indies, Mona Campus (Kingston, Jamaica), made a botanical identification of the plant (voucher specimen AN 08, 10/11). The fruit pulp was separated from the seed by hand using a knife. The pulp was then blended and the juice was squeezed out through a strainer before being filtered to remove any remaining pulp fibers. Finally, the juice was placed inside a Freeze Dry machine (Freezone 4.5 L, Labconco, Kansas, MO, USA).
4.2. Experimental Animals
The study was conducted according to the Animal Scientific Procedures Act of 1986 following the receipt of approval from the UWI/FMS Ethics Committee (AN 06,15/16). Twenty male Sprague- Dawley rats (8–10 weeks old and 170 g to 230 g) were chosen, housed in plastic cages at the UWI Mona campus Animal House at room temperature (22–25 °C) with a humidity of 45–51%. Fresh tap water was available to the rats via a bottle and food was administered ad libitum. Four groups were identified, each of which consisted of five rats. The first group served as a control group and did not receive any drugs. Rats from the second group were administered, by gavage, 100 mg/Kg of M. bijugatus liquid extract only daily for six weeks. The third group of rats received only two doses of 85 mg/Kg b.w. ISO intraperitoneally within a 24-hour period. Finally, rats of Group 4 were injected intraperitoneally with two doses of 85 mg/Kg b.w. of ISO within a 24-hour period. Following this, they were given 100 mg/Kg b.w. M. bijugatus extract, by gavage, daily for six weeks. The animals were sacrificed under anesthesia seven days after the period of treatment. The heart and kidney tissues were harvested and weighed to obtain a ratio of the heart and kidney weights to body weight. In addition, a blood sample was collected to conduct biochemical and hematological assessments among the subject groups.
4.3. Blood Pressure Recordings and ECG
The tail cuff method (CODA) was used to measure the SBP and DBP of the rats once before the administration of ISO and once per week following the administration of ISO. The pulse pressure (PP) was calculated using the formula PP = (SBP − DBP). The MAP was calculated using the formula: MAP = DBP + 1/3(SBP − DBP). For ECG recordings, rats were first anesthetized with ketamine (42 mg/Kg, i.p.) and xylazine (5 mg/Kg, i.p.). The ECG electrodes (BIOPAC System Inc, California, CA, USA) were placed subcutaneously in a bipolar configuration (DII). Measurements were done using the Electrocardiogram Amplifier equipment (ECG100C, BIOPAC System Inc, California, CA, USA) and tracings were recorded using the AcqKnowledge III computer software program (3.9.1., BIOPAC System Inc, California, CA, USA). The QT interval was taken as the time from the beginning of the QRS complex to the end of the T-wave. The RR interval was taken as the time elapsed between two consecutive maxima of the R-waves. The corrected QT interval (QTc) was calculated in accordance with the formula [,]:
QTc = QT/(RR)1/2
4.4. Biochemical Estimation
Whole blood was collected and assessed using standardized ethylenediaminetetraacetic acid (EDTA) tubes to obtain a complete blood count (CBC). The Cell Dyn Emerald Hematology Analyzer (Abbott Laboratories, Chicago, IL, USA) was then used to assess hematological parameters.
4.5. Histo-Morphological Appraisal
Histo-morphologic analyses of the cardiac tissues of the Sprague-Dawley rats were done using a Nikon Eclipse Ci research microscope (Nikon Instruments Inc., New York, NY, USA). Mirco-measurements were done via integrated mechanical stages with graduated locator margins and built in slide holders, as well as X-Y translator knobs. The tissues were harvested and immediately submerged in 10% neutral buffered formalin for preservation. They were subsequently processed, embedded in wax, and serial sectioned to a thickness of 4 microns then stained with haematoxylin and eosin (H and E) stain.
Twelve (12) full-thickness transverse sections from the heart, immediately subjacent to the atrioventricular valves (three from each group), were analyzed and evaluated for edema, mononuclear cell infiltration, fibrosis, interstitial hemorrhage and myocyte degeneration. The number of foci exhibiting degenerative features was recorded and the maximum diameter of the degenerated area was measured using a stage micrometer (Nikon Instruments Inc., New York, NY, USA).
Sixteen (16) longitudinal and/or transverse sections of the large caliber abdominal blood vessels, i.e., aorta (two from each group) and caudal vena cava (two from each group), were analyzed and evaluated for evidence of endothelial injury, degeneration of the intima or elastic lamina, atherosclerotic change, or any features of adventitial cellular injury.
The Ishak systems were employed to ascribe a stage of fibrosis and to grade the degree of inflammatory changes (1). In addition to these established, semi-objective parameters, subjective evaluation was performed for sinusoidal vascular congestion and the degree of nuclear chromatin density.
4.6. UHPLC-DAD-MS Instrument
The use of the Scientific Dionex Ultimate 3000 UHPLC system (Thermo Fisher Scientific, Bremen, Germany) hyphenated with a Q exactive focus machine (Thermo Fisher Scientific, Waltham, MA, USA) was already reported []. For the analysis, 5 mg of the lyophilized material were dissolved in 2 mL of methanol, filtered (PTFE filter) and 10 µL were injected in the instrument, with all specifications set as previously reported [].
4.7. LC Parameters and MS Parameters
Liquid chromatography was performed using an UHPLC C18 column (Acclaim, 150 mm × 4.6 mm ID, 2.5 µm, Thermo Fisher Scientific, Bremen, Germany) operated at 25 °C. The detection wavelengths were 254, 280, 330 and 354 nm, and DAD was recorded from 200 to 800 nm for peak characterization. Mobile phases were 1% formic aqueous solution (A) and acetonitrile (B). The gradient program time in minutes, % (B) was: (0.00, 5); (5.00, 5); (10.00, 30); (15.00, 30); (20.00, 70); (25.00, 70); (35.00, 5) and 12 min for column equilibration before each injection. The flow rate was 1.00 mL min−1, and the injection volume was 10 µL. The standards and resin extract dissolved in methanol were kept at 10 °C during storage in the autosampler. The HESI II and Orbitrap spectrometer parameters were optimized as previously reported [,].
4.8. Statistical Analysis
The data collected were expressed as mean ±SEM (standard error of mean). The mean values from the different groups were analyzed using GraphPad Prism Version 6.0 Software (GraphPad Software, San Diego, CA, USA). One-way or two-way ANOVA were used to compare the means, followed by the Bonferroni test. A value of p < 0.05 was considered statistically significant [].
5. Conclusions
In conclusion, we confirmed that Melicoccus bijugatus partially reversed myocardial damage and injury in an experimental ISO rat model. This was indicated by changes seen in the ECGs and in the reversal of the increased heart to body weight ratio in animals with ISO induced myocardial injury and blood pressure changes associated with a normalizing or reversal of symptoms associated with cardiac injury.
Supplementary Materials
The following are available online, Figure S1: Quadrupole Orbitrap full MS spectra and structures of all detected compounds in the fruits of Melicoccus bijugatus.
Author Contributions
C.R.N. was the author of the project design, conducted and designed the experiments, and drafted the manuscript; I.W. performed some experiments; J.P. participated in the design, performed some experiments, and wrote the manuscript; F.C., A.P. and A.L. participated in the design and conducted the pharmacological assays. J.B. and M.J.S. performed the isolation and structural elucidation of the pure compounds for UHPLC-MS; M.N., S.H. and R.T. participated in the design and performed the histopatological experiments.
Funding
This research was funded in part by the World Academy of Science/UNESCO (13–108 RG/BIO/LA) Grant and UWI Grants to C.R. Nwokocha. Mario Simirgiotis and Jorge Bórquez acknowledge funds from Fondecyt 1180059 Universidad Austral de Chile.
Acknowledgments
The authors wish to express their gratitude to the Rectoria y Vicerrectoria de Investigacion, Innovacion y Postgrado Universidad de Antofagasta for their financial and technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lobo Filho, H.G.; Ferreira, N.L.; Sousa, R.B.; Carvalho, E.R.; Lobo, P.L.; Lobo Filho, J.G. Experimental model of myocardial infarction induced by isoproterenol in rats. Rev. Bras. Cir. Cardiovasc. 2011, 26, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Górecki, A.; Bednarz, B.; Jaxa-Chamiec, T.; Maciejewski, P.; Łukaszewicz, R.; Ceremuzyński, L.; Dyduszyński, A. Lipid profile during the first 24 hours after myocardial infarction has significant prognostic value. Kardiol. Pol. 2004, 60, 229–236. [Google Scholar] [PubMed]
- Thippeswamy, B.; Thakker, S.; Tubachi, S.; Kalyani, G.; Netra, M.; Patil, U.; Desai, S.; Gavimath, C.; Veerapur, V. Cardioprotective effect of Cucumis trigonus Roxb on Isoproterenol-induced myocardial infarction in rat. Am. J. Pharmaco. Toxico. 2009, 4, 29–37. [Google Scholar] [CrossRef]
- Nwokocha, C.; Palacios, J.; Simirgiotis, M.J.; Thomas, J.; Nwokocha, M.; Young, L.; Thompson, R.; Cifuentes, F.; Paredes, A.; Delgoda, R. Aqueous extract from leaf of Artocarpus altilis provides cardio-protection from isoproterenol induced myocardial damage in rats: Negative chronotropic and inotropic effects. J. Ethnopharmacol. 2017, 203, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Singal, P.K.; Beamish, R.E.; Dhalla, N.S. Potential oxidative pathways of catecholamines in the formation of lipid peroxides and genesis of heart disease. Adv. Exp. Med. Biol. 1983, 161, 391–401. [Google Scholar] [PubMed]
- Haenen, G.R.; Veerman, M.; Bast, A. Reduction of beta-adrenoceptor function by oxidative stress in the heart. Free Radic Biol. Med. 1990, 9, 279–288. [Google Scholar] [CrossRef]
- Tappia, P.S.; Hata, T.; Hozaima, L.; Sandhu, M.S.; Panagia, V.; Dhalla, N.S. Role of oxidative stress in catecholamine-induced changes in cardiac sarcolemmal Ca2+ transport. Arch. Biochem. Biophys. 2001, 387, 85–92. [Google Scholar] [CrossRef]
- Jagadeesh, G.S.; Nagoor Meeran, M.F.; Selvaraj, P. Activation of β1-adrenoceptor triggers oxidative stress mediated myocardial membrane destabilization in isoproterenol induced myocardial infarcted rats: 7-hydroxycoumarin and its counter action. Eur. J. Pharmacol. 2016, 777, 70–77. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Lewis, B.A.; Brown, D.L.; Rodriguez, E.; Obendorf, R.L. Phenolics, Sugars, Antimicrobial and Free-Radical-Scavenging Activities of Melicoccus bijugatus Jacq. Fruits from the Dominican Republic and Florida. Plant Food. Hum. Nutr. 2009, 64, 160–166. [Google Scholar] [CrossRef]
- Bystrom, L.M. The potential health effects of Melicoccus bijugatus Jacq. fruits: Phytochemical, chemotaxonomic and ethnobotanical investigations. Fitoterapia 2012, 83, 266–271. [Google Scholar] [CrossRef]
- Padilla, F.C.; Rincon, A.M.; Bou-Rached, L. Polyphenol content and antioxidant activity of several seeds and nuts. Arch. Latinoam. Nutr. 2008, 58, 303–308. [Google Scholar] [PubMed]
- Bystrom, L.M.; Lewis, B.A.; Brown, D.L.; Rodriguez, E.; Obendorf, R.L. Characterisation of phenolics by LC–UV/Vis, LC–MS/MS and sugars by GC in Melicoccus bijugatus Jacq. ‘Montgomery’ fruits. Food Chem. 2008, 111, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Brooks, W.W.; Conrad, C.H. Isoproterenol-induced myocardial injury and diastolic dysfunction in mice: Structural and functional correlates. Comp. Med. 2009, 59, 339–343. [Google Scholar] [PubMed]
- Cifuentes, F.; Bravo, J.; Norambuena, M.; Stegen, S.; Ayavire, A.; Palacios, J. Chronic exposure to arsenic in tap water reduces acetylcholine-induced relaxation in the aorta and increases oxidative stress in female rats. Int. J. Toxicol. 2009, 28, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Deshmukh, R. Embelin Attenuates Intracerebroventricular Streptozotocin-Induced Behavioral, Biochemical, and Neurochemical Abnormalities in Rats. Mol. Neurobiol. 2017, 54, 6670–6680. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Quispe, C.; Mocan, A.; Villatoro, J.M.; Areche, C.; Bórquez, J.; Sepúlveda, B.; Echiburu-Chau, C. UHPLC high resolution orbitrap metabolomic fingerprinting of the unique species Ophryosporus triangularis Meyen from the Atacama Desert, Northern Chile. Rev. Bras. Farmacogn. 2017. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Hirun, S.; Phillips, P.A.; Chuen, T.L.K.; Bowyer, M.C.; Goldsmith, C.D.; Scarlett, C.J. Fruit-derived phenolic compounds and pancreatic cancer: Perspectives from Australian native fruits. J. Ethnopharmacol. 2014, 152, 227–242. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Areche, C.; Sepúlveda, B.X. Fast Detection of Phenolic Compounds in Extracts of Easter Pears (Pyrus communis) from the Atacama Desert by Ultrahigh-Performance Liquid Chromatography and Mass Spectrometry (UHPLC-Q/Orbitrap/MS/MS). Molecules 2016, 21, 92. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Rodríguez-Pérez, C.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comprehensive, untargeted, and qualitative RP-HPLC-ESI-QTOF/MS2 metabolite profiling of green asparagus (Asparagus officinalis). J. Food Compos. Anal. 2016, 46, 78–87. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Ramirez, J.E.; Hirschmann, G.S.; Kennelly, E.J. Bioactive coumarins and HPLC-PDA-ESI-ToF-MS metabolic profiling of edible queule fruits (Gomortega keule), an endangered endemic Chilean species. Food Res. Int. 2013, 54, 532–543. [Google Scholar] [CrossRef]
- Dong, M.; Oda, Y.; Hirota, M. (10E,12Z,15Z)-9-hydroxy-10,12,15-octadecatrienoic acid methyl ester as an anti-inflammatory compound from Ehretia dicksonii. Biosci. Biotechnol. Biochem. 2000, 64, 882–886. [Google Scholar] [CrossRef]
- Oguro, D.; Watanabe, H. Asymmetric Synthesis and Sensory Evaluation of Sedanenolide. Biosci. Biotech. Bioch. 2011, 75, 1502–1505. [Google Scholar] [CrossRef]
- Torkamani, M.R.D.; Abbaspour, N.; Jafari, M.; Samadi, A. Elicitation of Valerenic Acid in the Hairy Root Cultures of Valeriana officinalis L (Valerianaceae). Trop. J. Pharm. Res. 2014, 13, 943–949. [Google Scholar] [CrossRef]
- Ishizaka, N.; Tomiyama, K.; Katsui, N.; Murai, A.; Masamune, T. Biological activities of rishitin, an antifungal compound isolated from diseased potato tubers, and its derivatives1. Plant Cell Physiol. 1969, 10, 183–192. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Gao, Y.; Lai, P.X. Chemical Composition, Antioxidant, Antimicrobial and Cytotoxic Activities of Essential Oil from Premna microphylla Turczaninow. Molecules 2017, 22, 381. [Google Scholar] [CrossRef]
- Subash, D.; Kapoor, N.; Nityanand, S. Effect of isoprenaline on lipid profil and cardiac enzymes in rats. Ind. J. Exp. Biol. 1978, 16, 376–378. [Google Scholar]
- Nirmala, C.; Puvanakrishnan, R. Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol. Cell Biochem. 1996, 159, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Montessuit, C.; Thorburn, A. Transcriptional activation of the glucose transporter GLUT1 in ventricular cardiac myocytes by hypertrophic agonists. J. Biol. Chem. 1999, 274, 9006–9012. [Google Scholar] [CrossRef]
- Patel, V.; Upaganlawar, A.; Zalawadia, R.; Balaraman, R. Cardioprotective effect of melatonin against isoproterenol induced myocardial infarction in rats: A biochemical, electrocardiographic and histoarchitectural evaluation. Eur. J. Pharmacol. 2010, 644, 160–168. [Google Scholar] [CrossRef]
- Li, P.G.; Xu, J.W.; Ikeda, K.; Kobayakawa, A.; Kayano, Y.; Mitani, T.; Ikami, T.; Yamori, Y. Caffeic acid inhibits vascular smooth muscle cell proliferation induced by angiotensin II in stroke-prone spontaneously hypertensive rats. Hypertens. Res. 2005, 28, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Luceri, C.; Giannini, L.; Lodovici, M.; Antonucci, E.; Abbate, R.; Masini, E.; Dolara, P. p-Coumaric acid, a common dietary phenol, inhibits platelet activity in vitro and in vivo. Br. J. Nutr. 2007, 97, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, M.A.; Hassan, A.I.; Mahmoud, M.G.; Asker, M.S. Protective Effect of Adansonia digitata against Isoproterenol-Induced Myocardial Injury in Rats. Anim. Biotechnol. 2016, 27, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Dianita, R.; Jantan, I.; Amran, A.Z.; Jalil, J. Protective effects of Labisia pumila var. alata on biochemical and histopathological alterations of cardiac muscle cells in isoproterenol-induced myocardial infarction rats. Molecules 2015, 20, 4746–4763. [Google Scholar] [CrossRef] [PubMed]
- Sabeena Farvin, K.H.; Anandan, R.; Kumar, S.H.; Shiny, K.S.; Sankar, T.V.; Thankappan, T.K. Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmacol. Res. 2004, 50, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Knapton, A.; Lipshultz, S.E.; Weaver, J.L.; Herman, E.H. Isoproterenol-induced cardiotoxicity in sprague-dawley rats: Correlation of reversible and irreversible myocardial injury with release of cardiac troponin T and roles of iNOS in myocardial injury. Toxicol. Pathol. 2008, 36, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Mahmoud, A.; Khaled, R.; Sami, A.; Karim, A.; Iyad, A.; Mohamed, K.; Samir, K.; Moaath, J.; Ahmad, A. Effects of experimental acute myocardial infarction on blood cell counts and plasma biochemical values in a nude rat model (Crl:NIH-Fox1RNU). Comp. Clin. Pathol. 2009, 18, 433–437. [Google Scholar] [CrossRef]
- Sangeetha, T.; Quine, S.D. Protective effect of S-allyl cysteine sulphoxide (alliin) on glycoproteins and hematology in isoproterenol induced myocardial infarction in male Wistar rats. J. Appl. Toxicol. 2008, 28, 710–716. [Google Scholar] [CrossRef]
- Nyonseu Nzebang, D.C.; Ngaha Njila, M.I.; Bend, E.F.; Oundoum Oundoum, P.C.; Koloko, B.L.; Bogning Zangueu, C.; Belle Ekedi, P.; Sameza, M.; Massoma Lembè, D. Evaluation of the toxicity of Colocasia esculenta (Aracaceae): Preliminary study of leaves infected by Phytophthora colocasiae on wistar albinos rats. Biomed. Pharmacother. 2018, 99, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Zunjar, V.; Dash, R.P.; Jivrajani, M.; Trivedi, B.; Nivsarkar, M. Antithrombocytopenic activity of carpaine and alkaloidal extract of Carica papaya Linn. leaves in busulfan induced thrombocytopenic Wistar rats. J. Ethnopharmacol. 2016, 181, 20–25. [Google Scholar] [CrossRef]
- Cifuentes, F.; Paredes, A.; Palacios, J.; Muñoz, F.; Carvajal, L.; Nwokocha, C.R.; Morales, G. Hypotensive and antihypertensive effects of a hydroalcoholic extract from Senecio nutans Sch. Bip. (Compositae) in mice: Chronotropic and negative inotropic effect, a nifedipine-like action. J. Ethnopharmacol. 2016, 179, 367–374. [Google Scholar] [CrossRef]
- Cifuentes, F.; Palacios, J.; Nwokocha, C.R. Synchronization in the Heart Rate and the Vasomotion in Rat Aorta: Effect of Arsenic Trioxide. Cardiovasc. Toxicol. 2016, 16, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Schmeda-Hirschmann, G.; Avendaño, M.; Sepúlveda, B.; Winterhalter, P. Fast high resolution Orbitrap MS fingerprinting of the resin of Heliotropium taltalense Phil. from the Atacama Desert. Ind. Crops Prod. 2016, 85, 159–166. [Google Scholar] [CrossRef]
- Garneau, F.X.; Collin, G.J.; Jean, F.I.; Gagnon, H.; Lopez Arze, J.B. Essential oils from Bolivia. XII. Asteraceae: Ophryosporus piquerioides (DC) Benth. ex Baker. J. Essent. Oil Res. 2013, 25, 388–393. [Google Scholar] [CrossRef]
- Cifuentes, F.; Palacios, J.; Paredes, A.; Nwokocha, C.R.; Paz, C. 8-Oxo-9-Dihydromakomakine Isolated from Aristotelia chilensis Induces Vasodilation in Rat Aorta: Role of the Extracellular Calcium Influx. Molecules 2018, 23, 3050. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of Melicoccus bijugatus is available from the corresponding author. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).