Structure of Langmuir Monolayers of Perfluorinated Fatty Acids: Evidence of a New 2D Smectic C Phase
Abstract
1. Introduction
2. Results
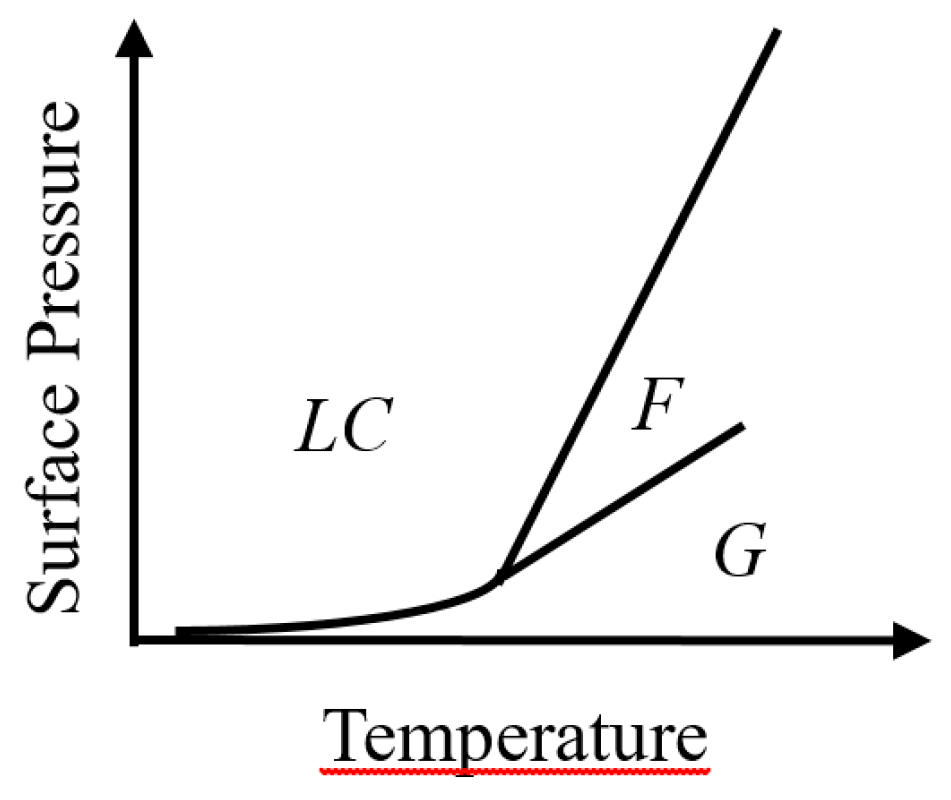
2.1. Thermodynamic Study
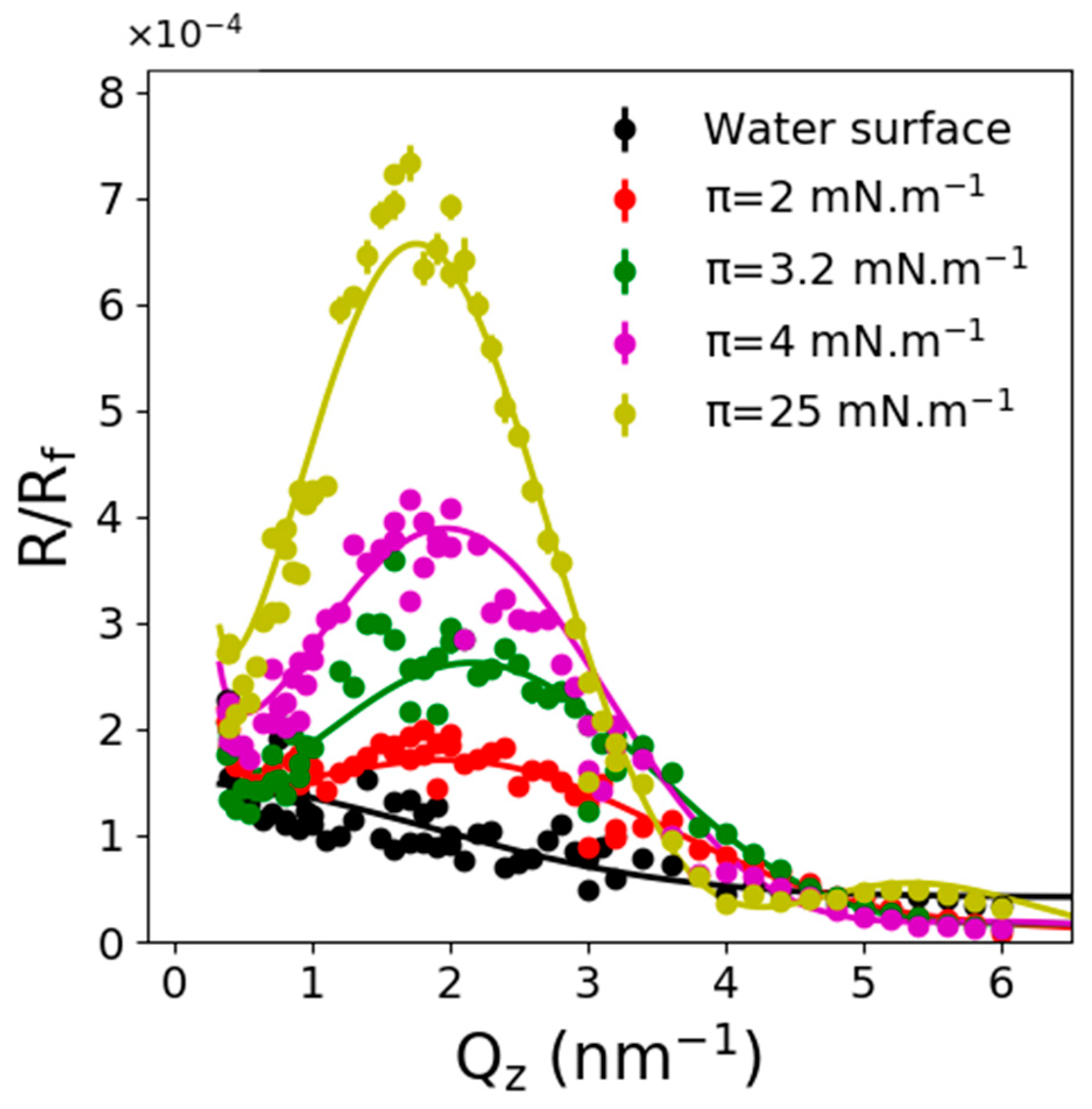
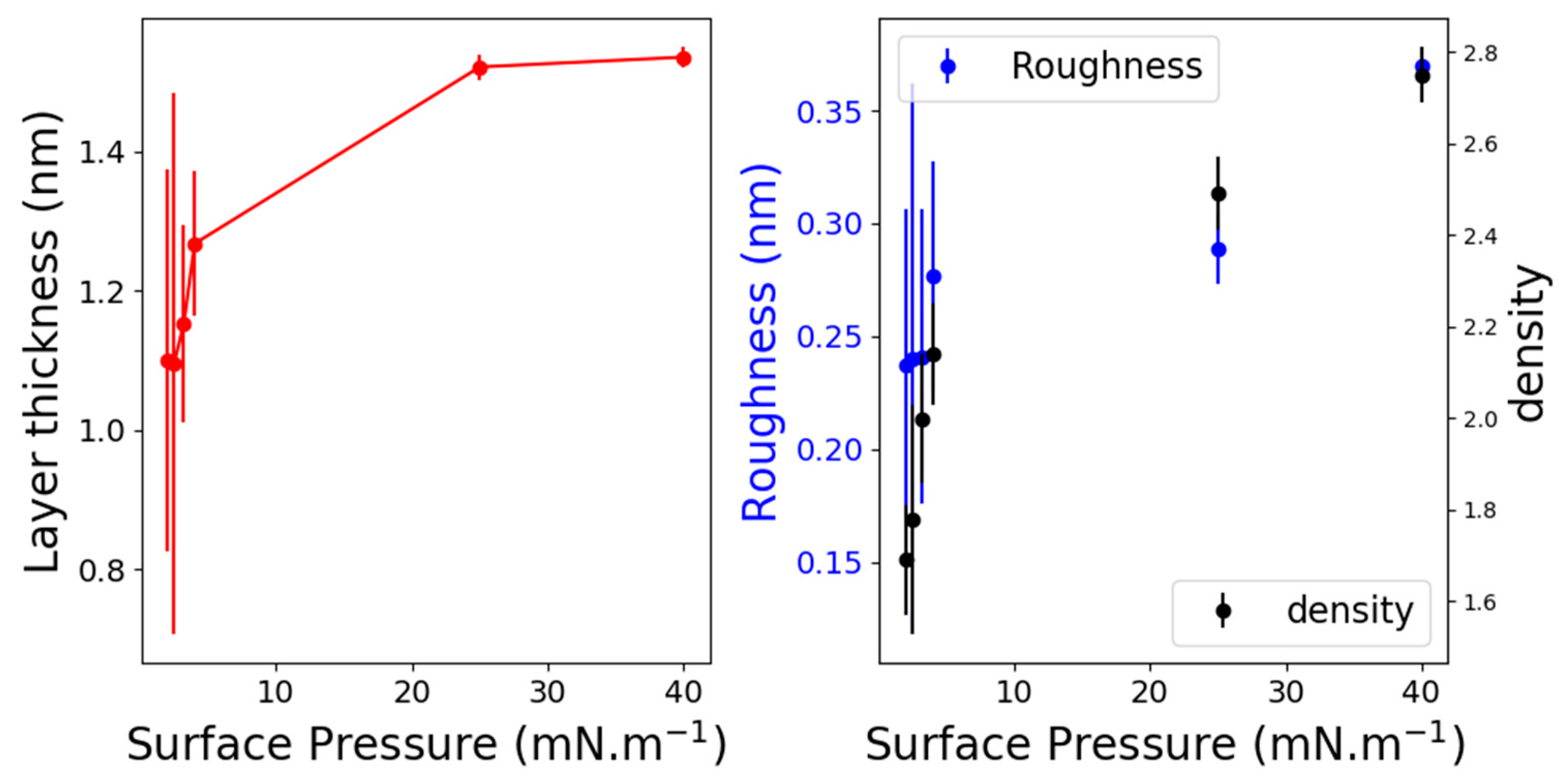
2.2. X-ray Reflectivity
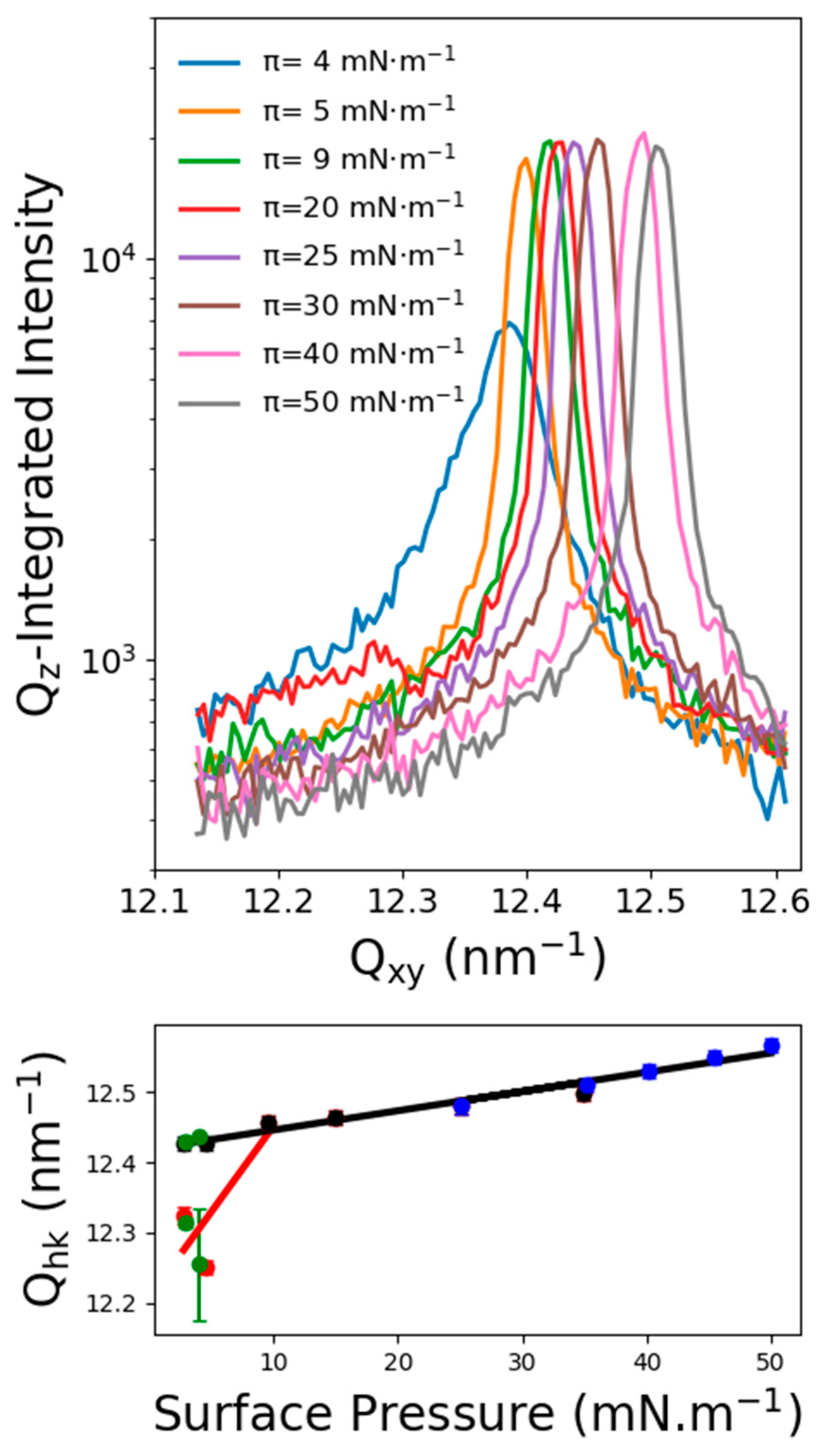
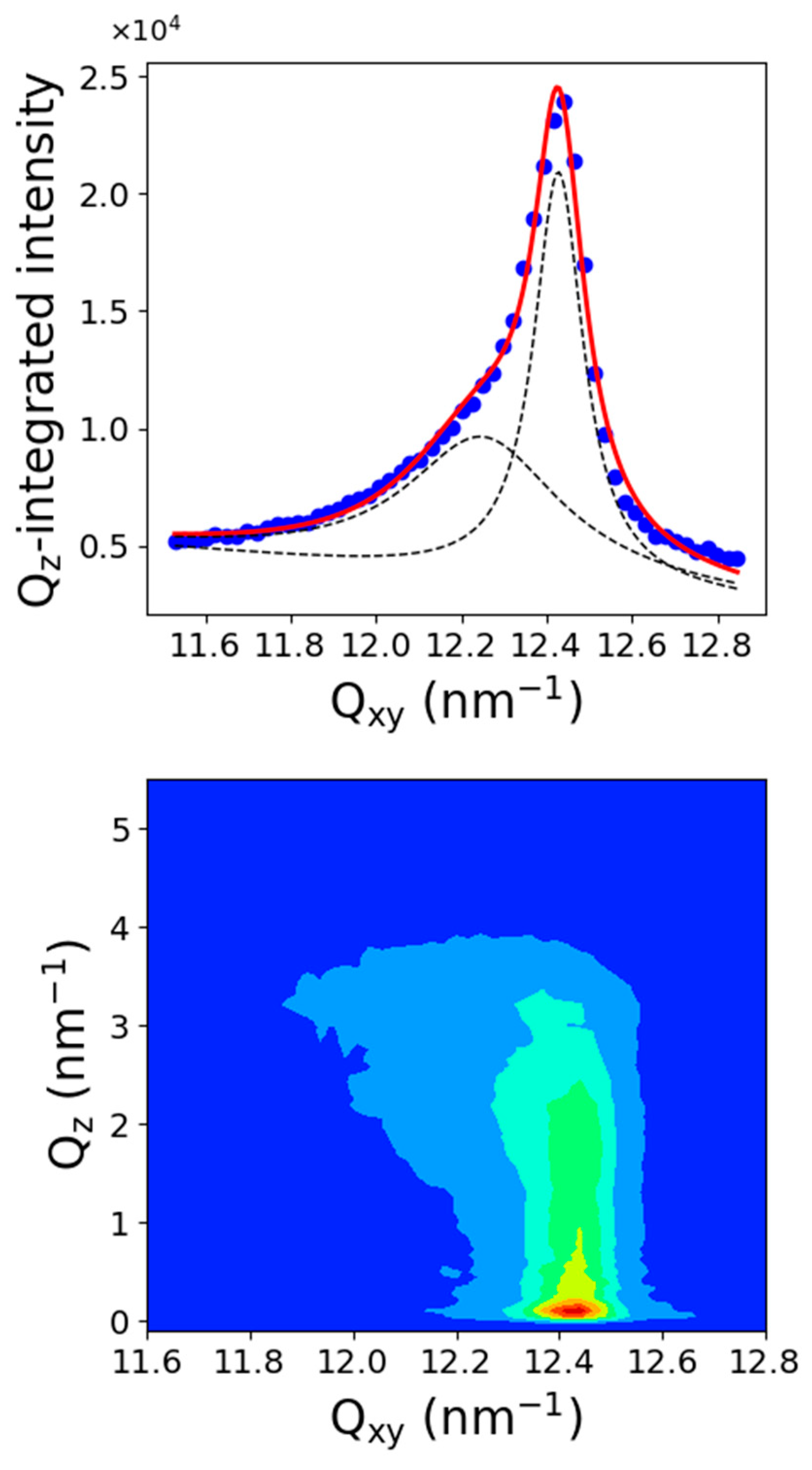
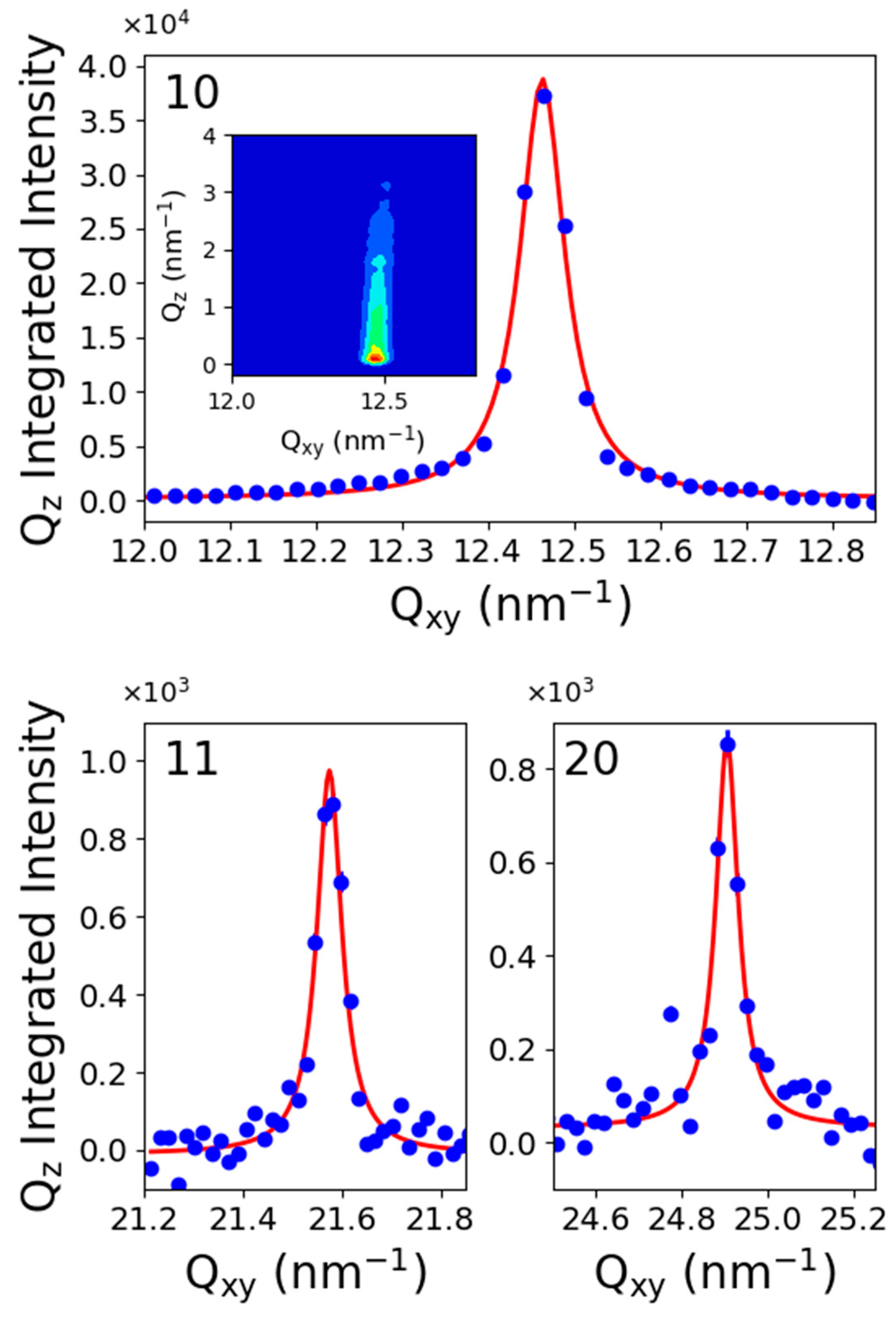
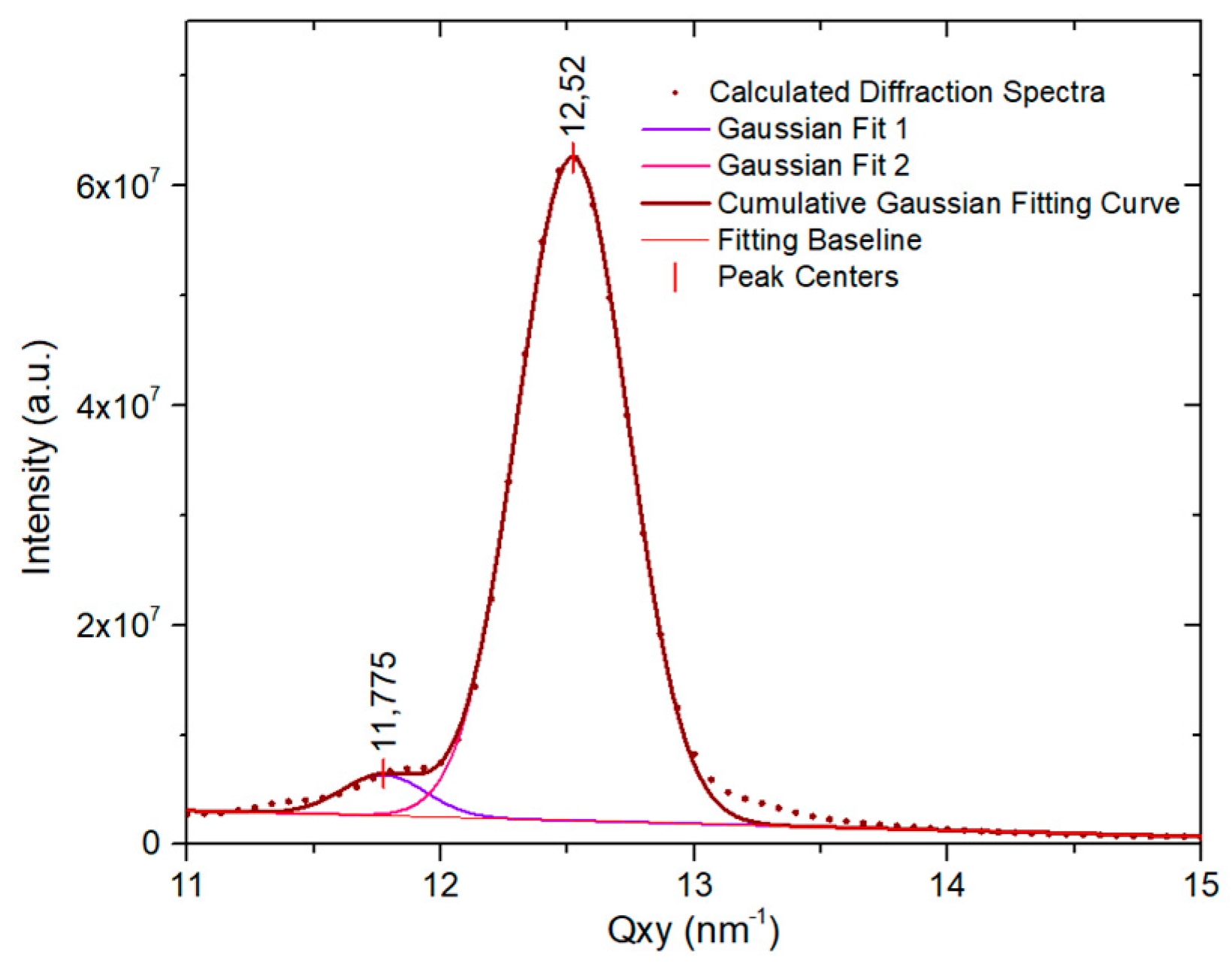
2.3. Grazing Incidence X-ray Diffraction
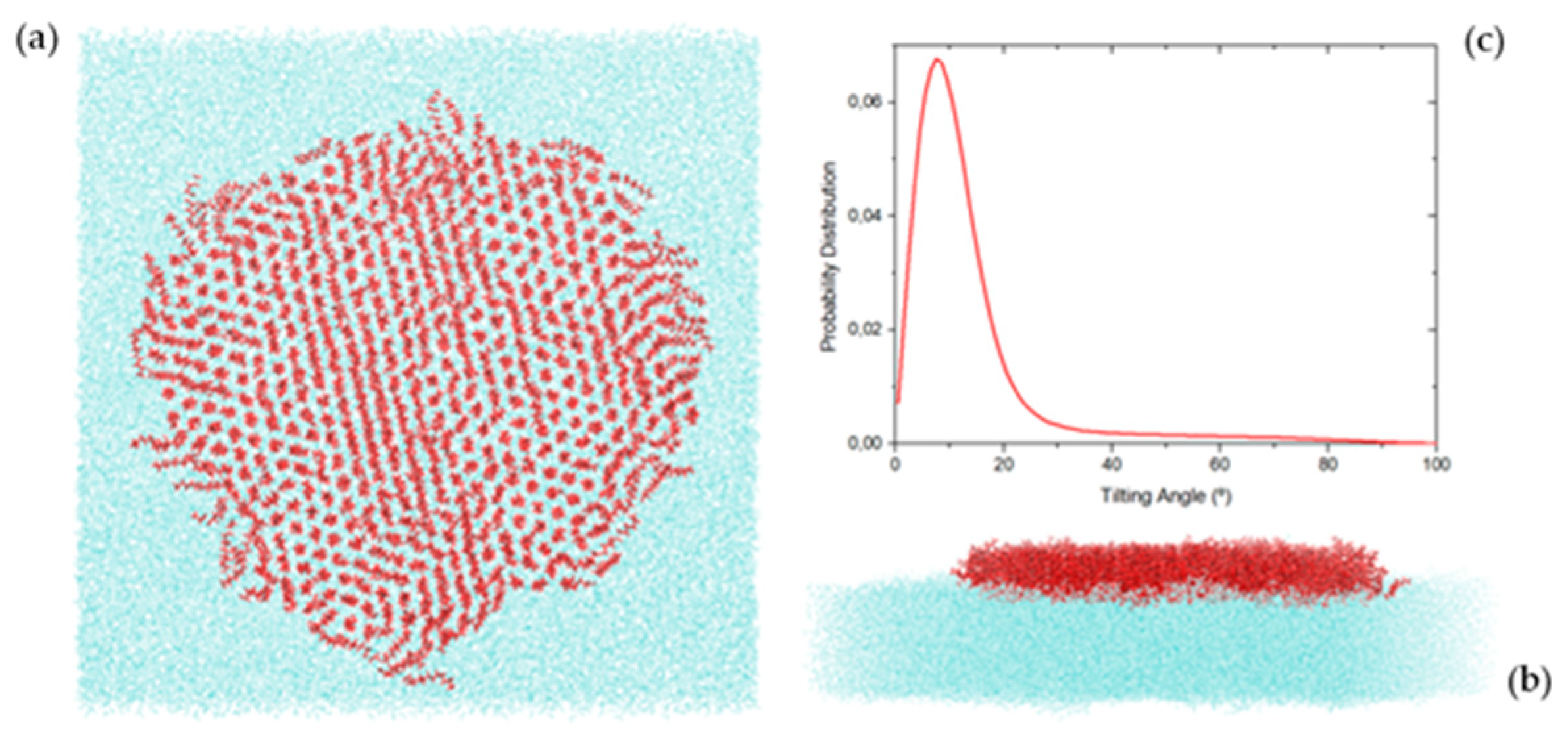
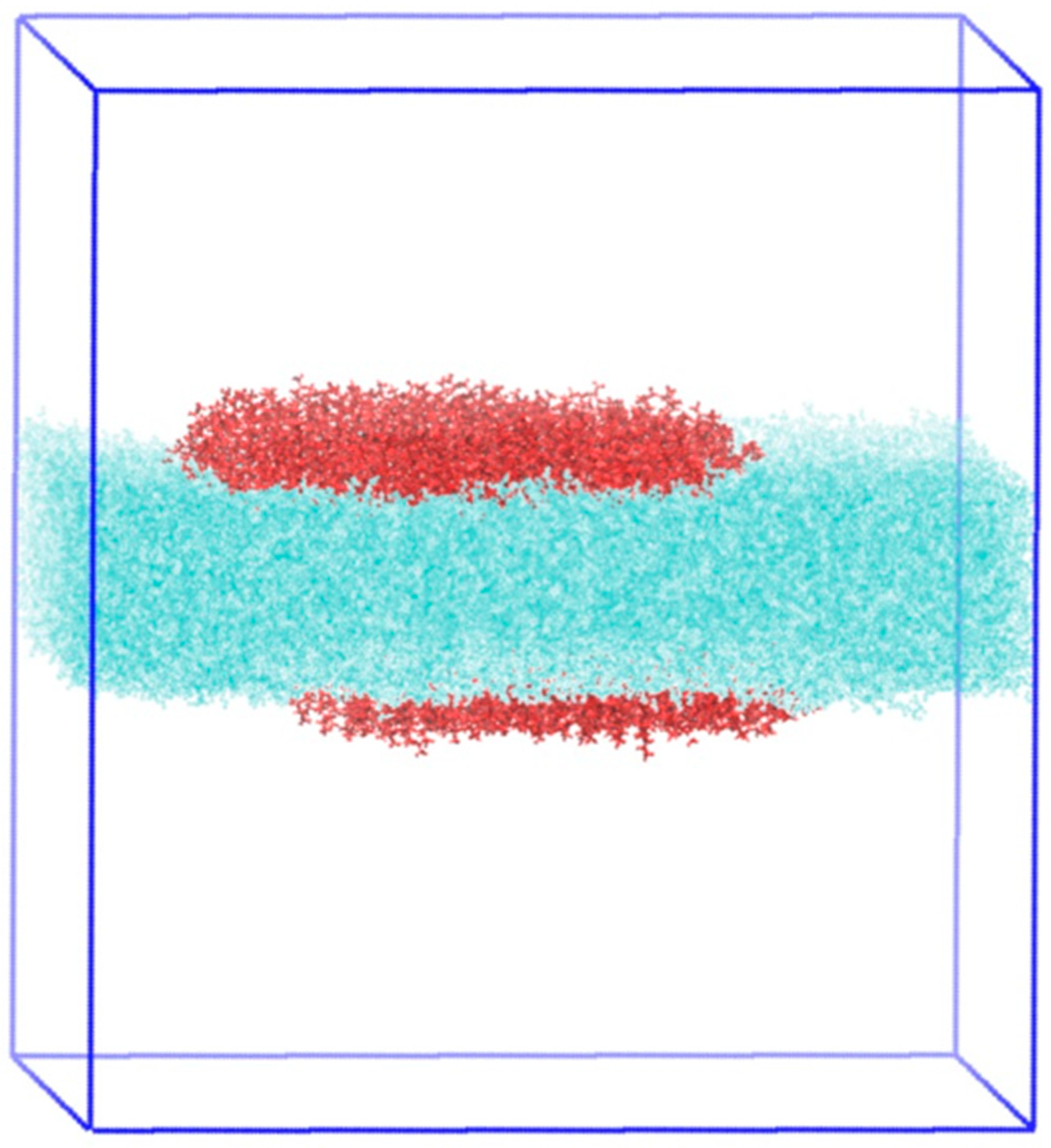
2.4. Molecular Dynamics Simulation Results
3. Discussion
4. Materials and Methods
4.1. Molecules and Sample Preparation
4.2. X-ray Reflectivity (XRR)
4.3. Grazing Incidence X-ray Diffraction (GIXD)
4.4. Simulation Detail
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karsa, D.R. Fluorinated Surfactants: Synthesis Properties Applications, by Erik Kissa; Marcel Dekker Inc.: New York, NY, USA, 1994; p. vii+469. [Google Scholar]
- Cappelletti, G.; Ardizzone, S.; Meroni, D.; Soliveri, G.; Ceotto, M.; Biaggi, C.; Benagia, M.; Raimond, L. Wettability of bare and fluorinated silanes: A combined approach based on surface free energy evaluations and dipole moment calculations. J. Colloid Interface Sci. 2013, 389, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Szwajca, A.; Koroniak, H. Fluorinated SAMs on Si(001) surface; Surface electronic properties and structural aspects. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 502–506. [Google Scholar] [CrossRef]
- Riess, J.G. Oxygen carriers (blood substitutes)—Raison d’être, chemistry and some physiology. Chem. Rev. 2001, 101, 2797–2919. [Google Scholar] [CrossRef] [PubMed]
- Riess, J.G. Highly fluorinated amphiphilic molecules and self-assemblies with biomedical potential. Curr. Opin. Colloid Interface Sci. 2009, 14, 294–304. [Google Scholar] [CrossRef]
- Gerber, F.; Krafft, M.P.; Vandamme, T.F.; Goldmann, M.; Fontaine, P. Potential use of fluorocarbons in lung surfactant therapy. Artif. Cells Blood Substit. Biotechnol. 2007, 35, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Schnutt, E.G.; Klein, D.H.; Mattrey, R.M.; Riess, J.G. Injectable microbubbles as contrast agents for diagnostic ultrasound imaging: The key role of perfluoro chemicals. Angew. Chem. 2003, 42, 3218–3235. [Google Scholar] [CrossRef] [PubMed]
- Steven, P.; Scherer, D.; Krösser, S.; Beckert, M.; Cursiefent, C.; Kaercher, T. Semifluorinated alkane eye drops for treatment of dry eye disease: A prospective, multicenter, non-interventional study. J. Ocul. Pharmacol. Ther. 2015, 31, 498–502. [Google Scholar]
- Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Bunn, C.W.; Lowells, E.R. Structure of molecules and crystals of fluorocarbons. Nature 1954, 4429, 549–551. [Google Scholar] [CrossRef]
- Jang, S.S.; Blanco, M.; Goddard, W.A.; Caldwell, G.; Ross, R.B. The Source of Helicity in Perfluorinated N-Alkanes. Macromolecules 2003, 36, 5331. [Google Scholar] [CrossRef]
- Costa, J.C.; Lima, C.F.; Mendes, A.; Santos, L.M. Fluorination effect on the thermodynamic properties of long-chain hydrocarbons and alcohols. J. Chem. Thermodyn. 2016, 102, 378–385. [Google Scholar] [CrossRef]
- Silva, G.M.; Morgado, P.; Haley, J.D.; Montoya, V.M.T.; McCabe, C.; Martins, L.F.; Filipe, E.J. Vapor pressure and liquid density of fluorinated alcohols: Experimental, simulation and GC-SAFT-VR predictions. Fluid Phase Equilibria 2016, 425, 297–304. [Google Scholar] [CrossRef]
- Gaines, G.L., Jr. Insoluble Monolayers at Liquid-Gas Interfaces; John Wiley & Sons: New York, NY, USA, 1966. [Google Scholar]
- Kaganer, V.M.; Möhwald, H.; Dutta, P. Structure and phase transitions in Langmuir monolayers. Rev. Mod. Phys. 1999, 71, 779. [Google Scholar] [CrossRef]
- Acero, A.A.; Li, M.; Lin, B.; Rice, S.A.; Goldmann, M.; Ben Azouz, I.; Goudot, A.; Rondelez, F. Molecular packing in water supported monolayers of F(CF2)11COOH and F(CF2)10CH2COOH. J. Chem. Phys. 1993, 99, 7214. [Google Scholar] [CrossRef]
- Goldmann, M.; Nassoy, P.; Rondelez, F.; Renault, A.; Shin, S.; Rice, S.A. In-plane X-ray diffraction from monolayers of perfluorinated fatty acids: Evidence for azimuthal ordering in the condensed phase. J. Phys. II 1994, 4, 773–785. [Google Scholar] [CrossRef][Green Version]
- Schmidt, M.E.; Shin, S.; Rice, S. Molecular dynamics studies of Langmuir monolayers of F(CF2)11COOH. J. Chem. Phys. 1996, 104, 2101. [Google Scholar] [CrossRef]
- Fontaine, P.; Ciatto, G.; Aubert, N.; Goldmann, M. Soft Interfaces and Resonant Investigation on Undulator Source: A Surface X-ray Scattering Beamline to Study Organic Molecular Films at the SOLEIL Synchrotron. Sci. Adv. Mater. 2014, 6, 2312–2316. [Google Scholar] [CrossRef]
- Braslau, A.; Deutsch, M.; Pershan, P.S.; Weiss, A.H. Surface Roughness of Water Measured by X-ray Reflectivity. Phys. Rev. Lett. 1985, 54, 114–117. [Google Scholar] [CrossRef]
- Braslau, A.; Pershan, P.S.; Swislow, G.; Ocko, B.M.; Als Nielsen, J. Capillary waves on the surface of simple liquid measured by X-ray reflectivity. Phys. Rev. A 1988, 38, 2457–2470. [Google Scholar] [CrossRef]
- Yu, Z.W.; Jin, J.; Cao, Y. Characterization of the liquid-expanded to liquid-condensed phase transition of monolayers by means of compressibility. Langmuir 2002, 18, 4530–4531. [Google Scholar] [CrossRef]
- Atkins, P.W.; De Paula, J.; Keeler, J. Physical Chemistry; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Chen, Z.-Y.; Talbot, J.; Gelbart, W.; Ben-Shaul, A. Phase Transitions in Systems of Grafted Rods. Phys. Rev. Lett. 1998, 61, 1376. [Google Scholar] [CrossRef]
- Kramer, D.; Ben-Shaul, A.; Chen, Z.-Y.; Gelbart, W.M. Monte-Carlo and Mean-Field Studies of Successive Phase Transitions in Rod Monolayers. J. Chem. Phys. 1992, 96, 2236. [Google Scholar] [CrossRef]
- Onsager, L. The Effects of Shape on the Interaction of Colloidal Particles. Ann. N. Y. Acad. Sci. 1949, 51, 627. [Google Scholar] [CrossRef]
- Zwanzig, R. First-Order Transition in a Gas of Long Thin rods. J. Chem. Phys. 1963, 39, 1714. [Google Scholar] [CrossRef]
- Szabolcs, V.; Gurin, P.; Armas-Pérez, C.J.; Quintana, H.J. Nematic and smectic ordering in a system of two dimensional hard zigzag particles. J. Chem. Phys. 2009, 131, 184901. [Google Scholar]
- Landau, L.D.; Lifschitz, E.M. Statistical Physics; Pergamon Press: London, UK, 1959. [Google Scholar]
- Lajzerowicz, J.; Sivadière, J. Spin-1 lattice gas model I. Condensation and solidification of a simple fluid. Phys. Rev. A 1974, 11, 2079. [Google Scholar] [CrossRef]
- Arnold, T.; Nicklin, C.; Rawle, J.; Sutter, J.; Bates, T.; Nutter, B.; McIntyre, G.; Burt, M. Implementation of a beam deflection system for studies of liquid interfaces on beamline I07 at Diamond. J. Synchrotron Rad. 2012, 19, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, Flexible, and Free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Watkins, E.K.; Jorgensen, W.L. Perfluoroalkanes: Conformational Analysis and Liquid-State Properties from Ab Initio and Monte Carlo Calculations. J. Phys. Chem. A 2001, 105, 4118–4125. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. Packmol: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical Sampling through Velocity-Rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are only commercially available. |

| Temperature (°C) | Fluid Phase (m/N) | LC Phase (m/N) |
|---|---|---|
| 24 | 130–300 | 8 ± 0.2 |
| 20 | 160–300 | 8.5 ± 0.2 |
| 18 | 190–300 | 7.3 ± 0.2 |
| 12 | 200–300 | 13 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontaine, P.; Filipe, E.J.M.; Fauré, M.-C.; Rego, T.; Taßler, S.; Alves, A.C.; Silva, G.M.C.; Morgado, P.; Goldmann, M. Structure of Langmuir Monolayers of Perfluorinated Fatty Acids: Evidence of a New 2D Smectic C Phase. Molecules 2019, 24, 3590. https://doi.org/10.3390/molecules24193590
Fontaine P, Filipe EJM, Fauré M-C, Rego T, Taßler S, Alves AC, Silva GMC, Morgado P, Goldmann M. Structure of Langmuir Monolayers of Perfluorinated Fatty Acids: Evidence of a New 2D Smectic C Phase. Molecules. 2019; 24(19):3590. https://doi.org/10.3390/molecules24193590
Chicago/Turabian StyleFontaine, Philippe, Eduardo J. M. Filipe, Marie-Claude Fauré, Tomas Rego, Stephanie Taßler, Ana Carolina Alves, Gonçalo M. C. Silva, Pedro Morgado, and Michel Goldmann. 2019. "Structure of Langmuir Monolayers of Perfluorinated Fatty Acids: Evidence of a New 2D Smectic C Phase" Molecules 24, no. 19: 3590. https://doi.org/10.3390/molecules24193590
APA StyleFontaine, P., Filipe, E. J. M., Fauré, M.-C., Rego, T., Taßler, S., Alves, A. C., Silva, G. M. C., Morgado, P., & Goldmann, M. (2019). Structure of Langmuir Monolayers of Perfluorinated Fatty Acids: Evidence of a New 2D Smectic C Phase. Molecules, 24(19), 3590. https://doi.org/10.3390/molecules24193590





