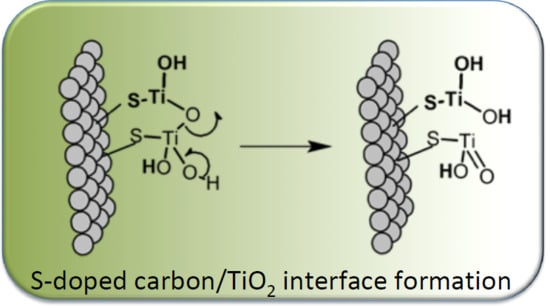
TiO2/S-Doped Carbons Hybrids: Analysis of Their Interfacial and Surface Features
Abstract
1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Surface Characterization
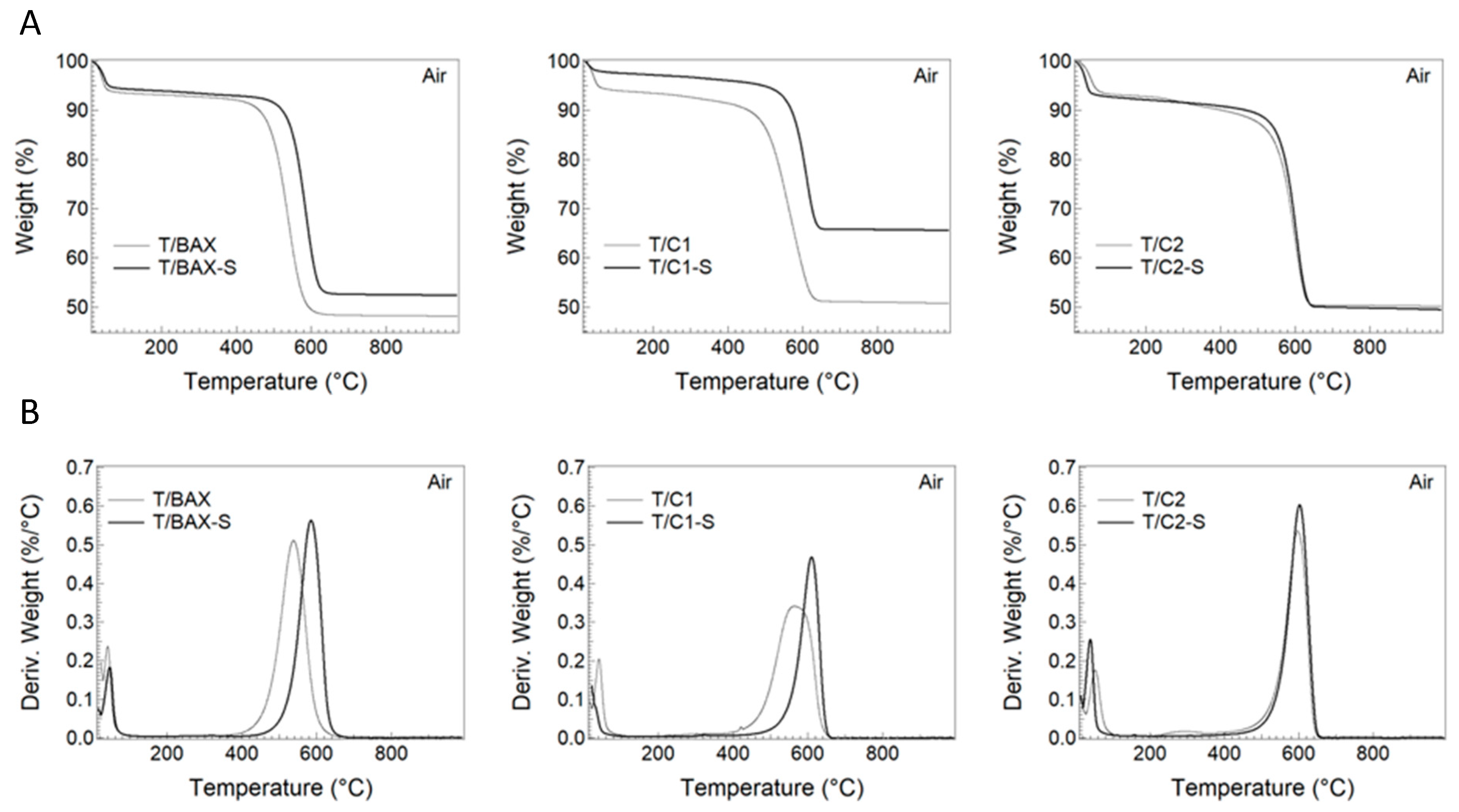
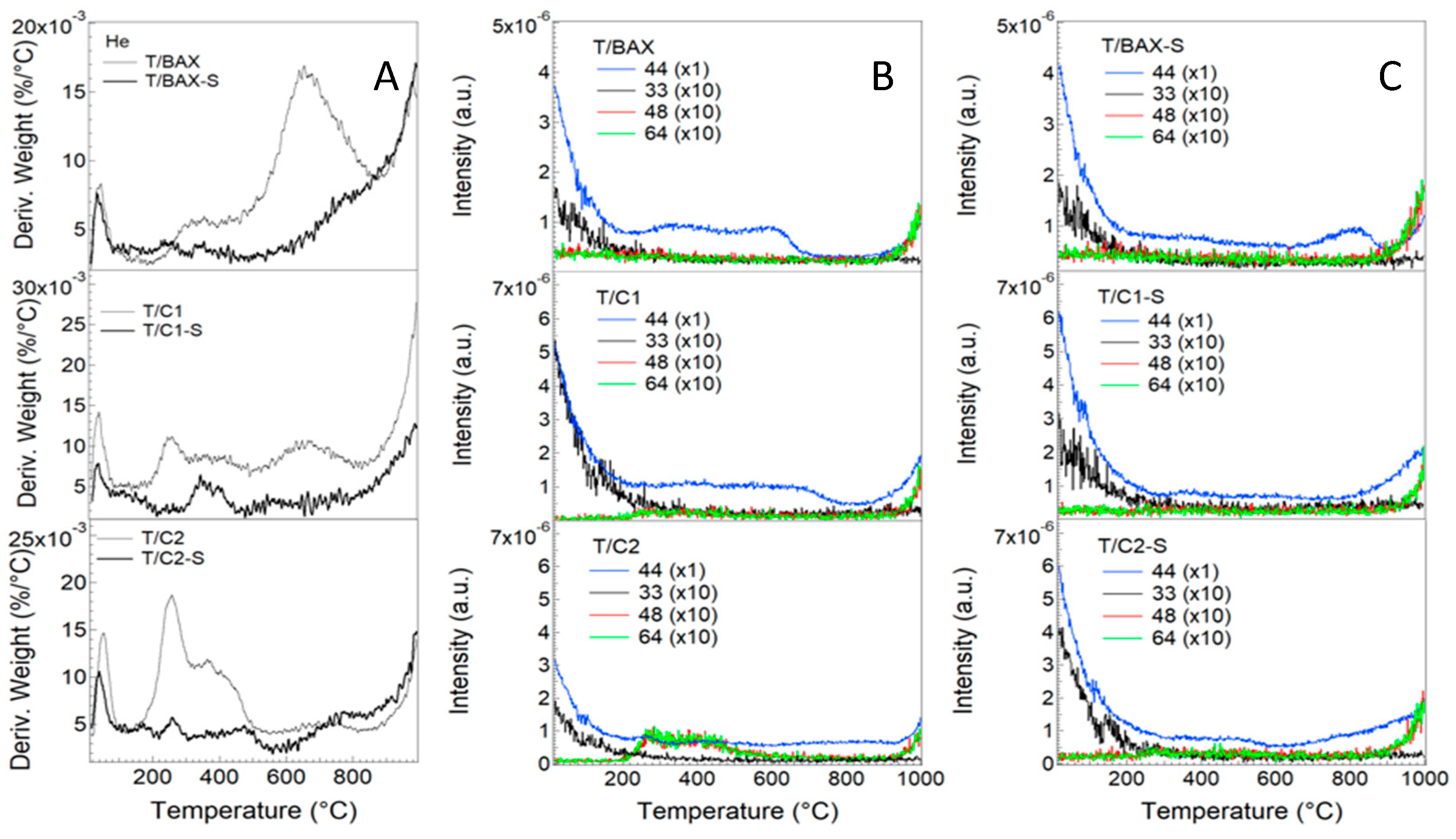
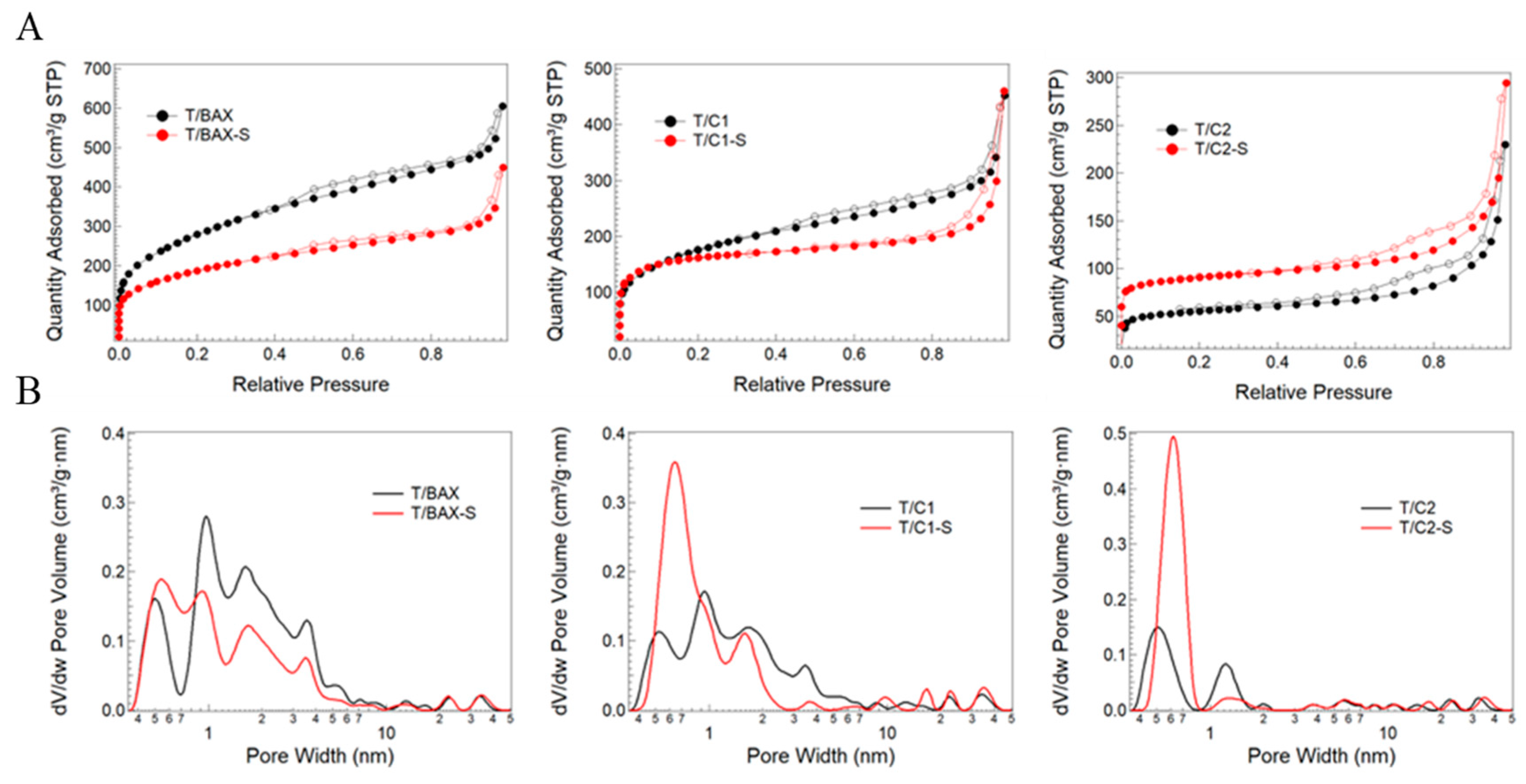
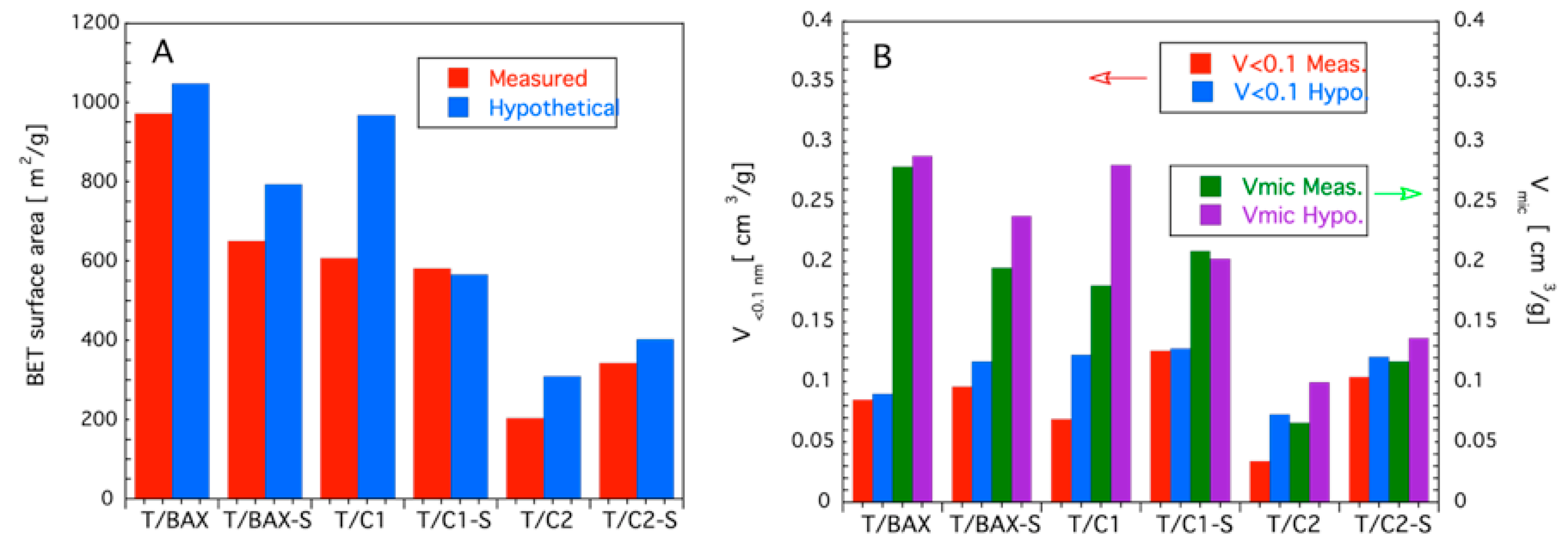
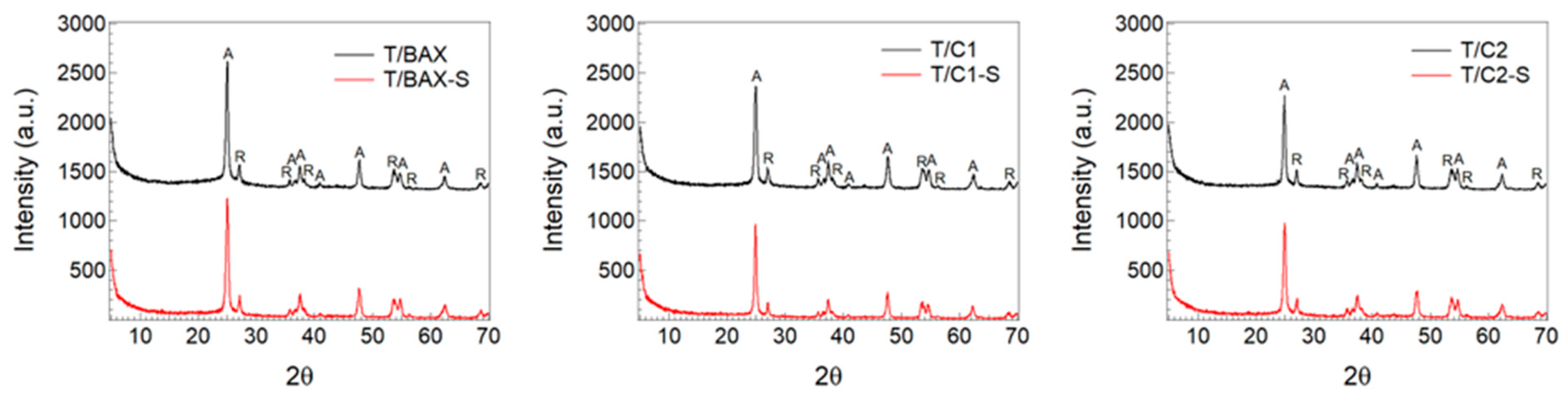
3.2.1. Porosity and Texture
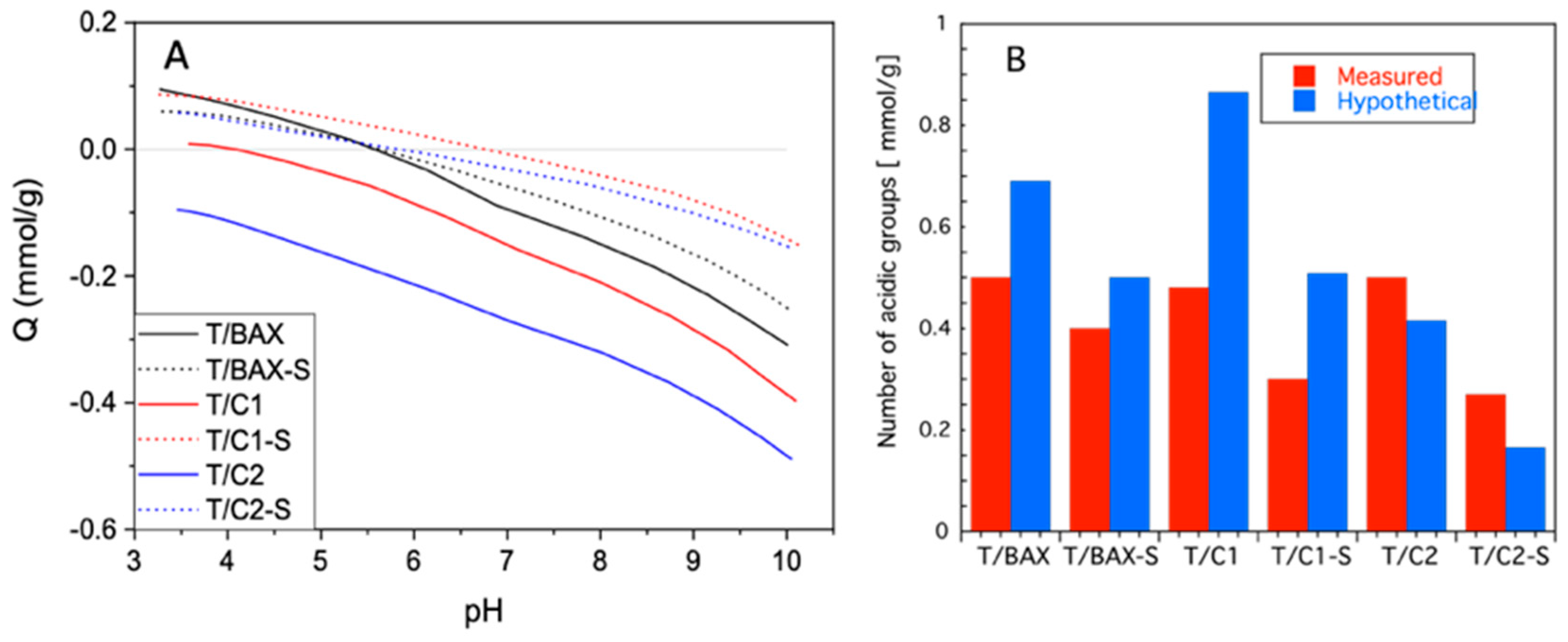
3.2.2. Surface Chemistry and Bulk Chemical Features
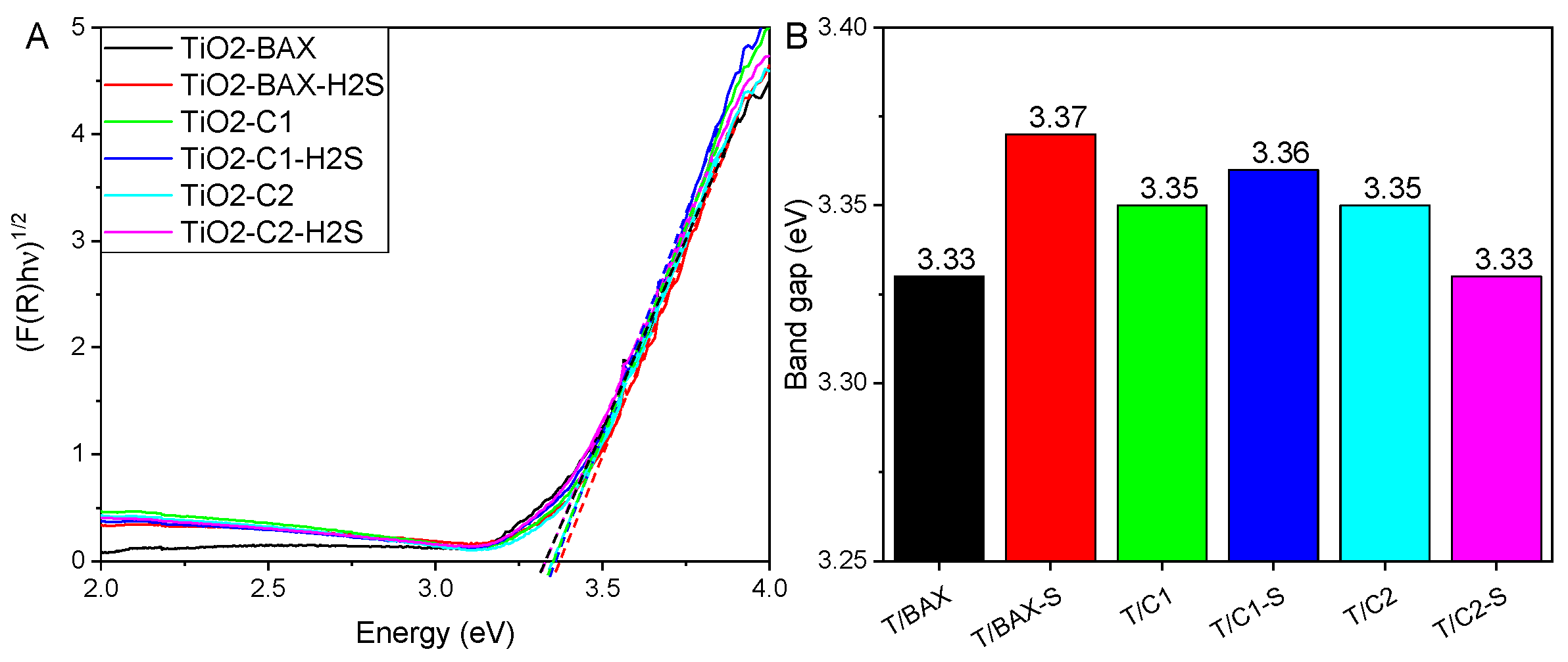
3.2.3. Optical Features/Band Gap Estimation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging pollutants in the environment: A challenge for water resource management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Basheer, A.A. New generation nano-adsorbents for the removal of emerging contaminants in water. J. Mol. Liq. 2018, 261, 583–593. [Google Scholar] [CrossRef]
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; Rahman, U.U.; Soares, B.C.V.; Souza, S.L.Q.; Pimentel, T.C.; Scudino, H.; Guimarães, J.T.; Esmerino, E.A.; et al. Treatment and utilization of dairy industrial waste: A review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Jeon, T.H.; Koo, M.S.; Kim, H.; Choi, W. Dual-functional photocatalytic and photoelectrocatalytic systems for energy- and resource-recovering water treatment. ACS Catal. 2018, 8, 11542–11563. [Google Scholar] [CrossRef]
- Puga, A.V. Photocatalytic production of hydrogen from biomass-derived feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Matos, J.; Ocares-Riquelme, J.; Poon, P.S.; Montaña, R.; García, X.; Campos, K.; Hernández-Garrido, J.C.; Titirici, M.M. C-doped anatase TiO2: Adsorption kinetics and photocatalytic degradation of methylene blue and phenol, and correlations with DFT estimations. J. Colloid Int. Sci. 2019, 547, 14–29. [Google Scholar] [CrossRef]
- La France va Interdire le Dioxyde de Titane à Compter de 2020. Available online: https://www.europeanscientist.com/fr/sante/la-france-va-interdire-le-dioxyde-de-titane-a-compter-de-2020/ (accessed on 22 April 2019).
- Matos, J.; Laine, J.; Herrmann, J.-M. Effect of the type of Activated Carbons on the Photocatalytic Degradation of Aqueous Organic Pollutants by UV-Irradiated Titania. J. Catal. 2001, 200, 10–20. [Google Scholar] [CrossRef]
- Matos, J.; Fierro, V.; Montaña, R.; Rivero, E.; Martínez de Yuso, A.; Zhao, W.; Celzard, A. High surface area microporous carbons as photoreactors for the catalytic photodegradation of methylene blue under UV-vis irradiation. Appl. Catal. A Gen. 2016, 517, 1–11. [Google Scholar] [CrossRef]
- Matos, J.; Miralles-Cuevas, S.; Ruíz-Delgado, A.; Oller, I.; Malato, S. Development of TiO2-C photocatalysts for solar treatment of polluted water. Carbon 2017, 122, 361–373. [Google Scholar] [CrossRef]
- Velasco, L.F.; Fonseca, I.M.; Parra, J.B.; Lima, J.C.; Ania, C.O. Photochemical behavior of activated carbons under UV irradiation. Carbon 2012, 50, 249–258. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Matos, J.; Seredych, M.; Islam, M.S.Z.; Alfano, R. Photoactivity of S-doped nanoporous activated carbons: A new perspective for harvesting solar energy on carbon-based semiconductors. Appl. Catal. A Gen. 2012, 445–446, 159–165. [Google Scholar] [CrossRef]
- Velasco, L.F.; Maurino, V.; Laurenti, E.; Ania, C.O. Light-induced generation of radicals on semiconductor-free carbon photocatalysts. Appl. Catal. A Gen. 2013, 453, 310–315. [Google Scholar] [CrossRef]
- Velasco, L.F.; Carmona, R.J.; Matos, J.; Ania, C.O. Performance of activated carbons in consecutive phenol photooxidation cycles. Carbon 2014, 73, 206–215. [Google Scholar] [CrossRef]
- Andrade, M.A.; Carmona, R.J.; Mestre, A.S.; Matos, J.; Carvalho, A.P.; Ania, C.O. Visible light driven photooxidation of phenol on TiO2/Cu-loaded carbon catalysts. Carbon 2014, 76, 183–192. [Google Scholar] [CrossRef][Green Version]
- Wei, W.; Yu, C.; Zhao, Q.; Qian, X.; Li, G.; Wan, Y. Synergy effect in photodegradation of contaminants from water using ordered mesoporous carbon-based titania catalyst. Appl. Catal. B Environ. 2014, 146, 151–161. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite titanium dioxide nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Li, X.; Shen, R.; Ma, S.; Chen, X.; Xie, J. Graphene-based heterojunction photocatalysts. Appl. Surf. Sci. 2018, 430, 53–107. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Ania, C.O. Origin and perspectives of the photochemical activity of nanoporous carbons. Adv. Sci. 2018, 1800293. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.; Pastrana-Martínez, L.M.; Pereira, M.F.R.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M.T. N/S-doped graphene derivatives and TiO2 for catalytic ozonation and photocatalysis of water pollutants. Chem. Eng. J. 2018, 348, 888–897. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Ruksana; Alhabarah, A.N.; Alshehri, S.M. N/S doped highly porous magnetic carbo aerogel derived from sugarcane bagasse cellulose for the removal of bisphenol-A. Int. J. Biol. Macromol. 2019, 132, 1031–1038. [Google Scholar] [CrossRef]
- Gomis-Berenguer, A.; Seredych, M.; Iniesta, J.; Lima, J.C.; Bandosz, T.J.; Ania, C.O. Sulfur-mediated photochemical energy harvesting in nanoporous carbons. Carbon 2016, 104, 253–259. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Policicchio, A.; Florent, M.; Li, W.; Poon, P.S.; Matos, J. Solar light-driven photocatalytic degradation of phenol on S-doped nanoporous carbons: The Role of functional groups in governing activity and selectivity. Carbon 2019, 156, 10–23. [Google Scholar] [CrossRef]
- Matos, J.; García, A.; Poon, P.S. Environmental green chemistry applications of nanoporous carbons. J. Mater. Sci. 2010, 45, 4934–4944. [Google Scholar] [CrossRef]
- Matos, J.; Hofman, M.; Pietrzak, R. Synergy effect in the photocatalytic degradation of methylene blue on a suspended mixture of TiO2 and N-containing carbons. Carbon 2013, 54, 460–471. [Google Scholar] [CrossRef]
- Huang, H.; Song, Y.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. One-step in-situ preparation of N-doped TiO2@C derived from Ti3C2 MXene for enhanced visible-light driven photodegradation. Appl. Catal. B Environ. 2019, 251, 154–161. [Google Scholar] [CrossRef]
- Torres-Pinto, A.; Sampaio, M.J.; Silva, C.G.; Faria, J.L.; Silva, A.M.T. Metal-free carbon nitride photocatalysis with in situ hydrogen peroxide generation for the degradation of aromatic compounds. Appl. Catal. B Environ. 2019, 252, 128–137. [Google Scholar] [CrossRef]
- Matos, J.; Poon, P.S.; Montaña, R.; Romero, R.; Gonçalves, G.R.; Schettino, M.A., Jr.; Passamani, E.C.; Freitas, J.C.C. Photocatalytic activity of P-Fe/activated carbon nanocomposites under artificial solar irradiation. Catal. Today 2019. [Google Scholar] [CrossRef]
- Al-Kahtani, A.A.; Alshehri, S.M.; Naushad, M.; Ruksana; Ahamad, T. Fabrication of highly porous N/S doped carbon embedded with ZnS as highly efficient photocatalyst for degradation of bisphenol. Int. J. Biol. Macromol. 2019, 121, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Xu, Q.; Li, Y.; Fu, T.; Jiang, G.; Li, Y.; Zhao, Z.; Wei, Y. Novel visible-light-driven S-doped carbon dots/BiOI nanocomposites: Improved photocatalytic activity and mechanism insight. J. Mater. Sci. 2017, 52, 7282–7293. [Google Scholar] [CrossRef]
- Lv, K.; Guo, X.; Wu, X.; Li, Q.; Ho, W.; Li, M.; Ye, H.; Du, D. Photocatalytic selective oxidation of phenol to produce dihydroxybenzenes in a TiO2/UV system: Hydroxyl radical versus hole. Appl. Catal. B Environ. 2016, 199, 405–411. [Google Scholar] [CrossRef]
- Kim, B.-J.; Park, E.-H.; Kang, K.-S. Optical properties of soluble polythiophene for flexible solar cell. Curr. Photovolt. Res. 2018, 6, 91–93. [Google Scholar]
- Seredych, M.; Singh, K.; Bandosz, T.J. Insight into the capacitive performance of sulfur-doped nanoporous carbons modified by addition of graphene phase. Electroanalysis 2014, 26, 109–120. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. Carbon slit pore model incorporating surface energetical heterogeneity and geometrical corrugation. Adsorption 2013, 19, 777–783. [Google Scholar] [CrossRef]
- Ijadpanah-Saravy, H.; Safari, M.; Khodadadi-Darban, A.; Rezaei, A. Synthesis of titanium dioxide nanoparticles for photocatalytic degradation of cyanide in wastewater. Anal. Lett. 2014, 47, 1772–1782. [Google Scholar] [CrossRef]
- Cordero, T.; Chovelon, J.-M.; Duchamp, C.; Ferronato, C.; Matos, J. Surface nano-aggregation and photocatalytic activity of TiO2 on H-type activated carbons. Appl. Catal. B Environ. 2007, 73, 227–235. [Google Scholar] [CrossRef]
- Jagiello, J. Stable numerical solution of the adsorption integral equation using splines. Langmuir 1994, 10, 2778–2885. [Google Scholar] [CrossRef]
- Jagiello, J.; Bandosz, T.J. Carbon surface characterization in terms of its acidity constant distribution. Carbon 1994, 32, 1026–1028. [Google Scholar] [CrossRef]
- Seredych, M.; Rodriguez-Castellon, E.; Bandosz, T.J. Alterations of S-doped porous carbon-rGO composites surface features upon CO2 adsorption at ambient conditions. Carbon 2016, 107, 501–509. [Google Scholar] [CrossRef]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Band gap narrowing of titanium dioxide by sulfur doping. Appl. Phys. Lett. 2002, 81, 454–456. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
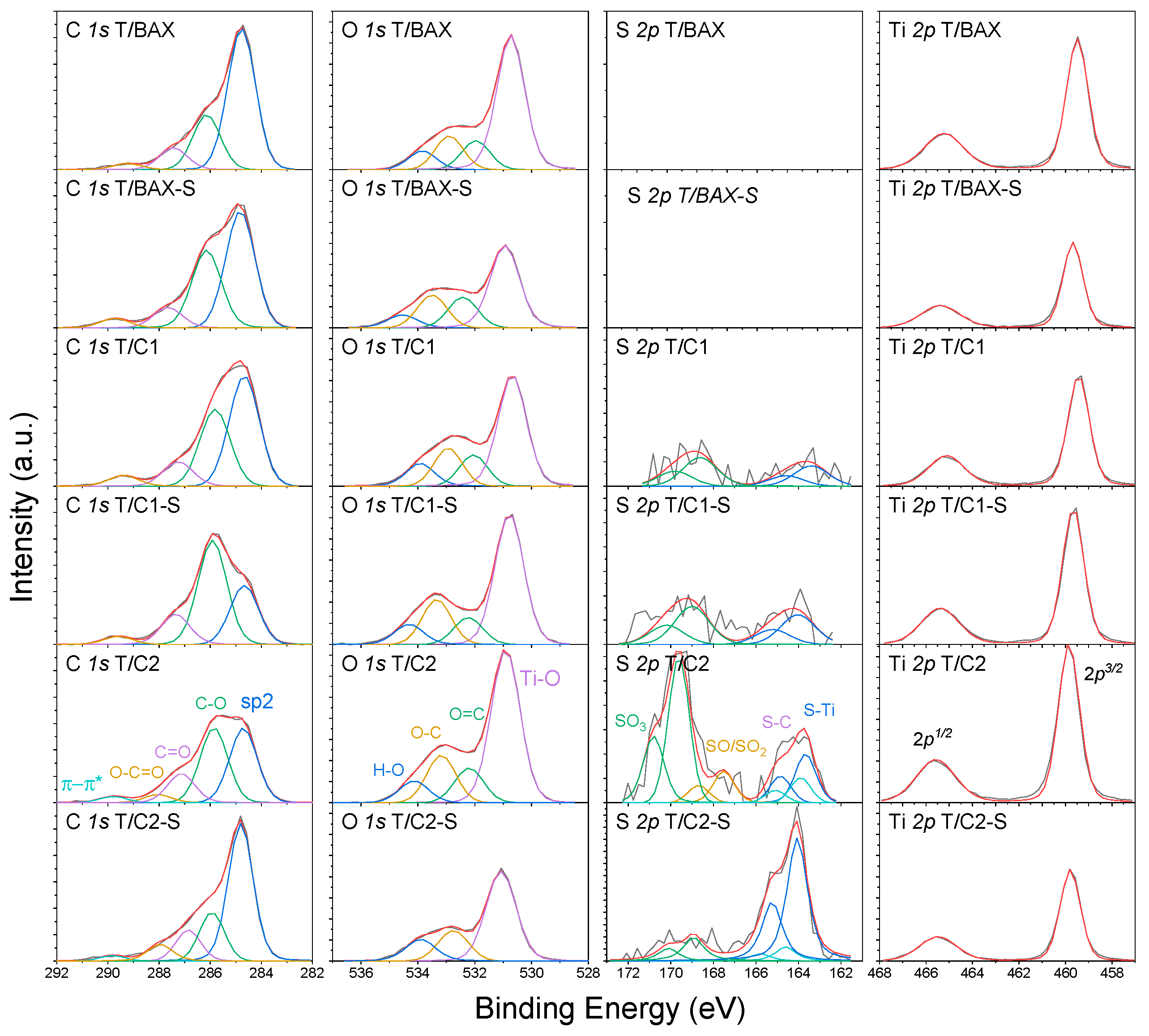
| Energy, eV | Bond Assignment | T/BAX | T/BAX-S | T/C1 | T/C1-S | T/C2 | T/C2-S |
|---|---|---|---|---|---|---|---|
| C 1s | 66.4 | 72.6 | 66.5 | 60.7 | 55.7 | 72.0 | |
| 284.8 | C-(C, S) (graphitic carbon) | 63.1 | 50.9 | 49.3 | 28.4 | 38.1 | 57.8 |
| 286.1 | C-O, C-H (phenolic, alcoholic, etheric) | 24.1 | 35.5 | 34.4 | 51.7 | 38.3 | 19.3 |
| 287.0 | C=O (carbonyl or quinone) | 10.0 | 9.3 | 11.4 | 15.8 | 15.6 | 12.7 |
| 288.0 | O-C=O (carboxyl or ester) | 2.8 | 4.3 | 4.9 | 4.1 | 4.6 | 7.8 |
| 289.0 | π-π * | --- | --- | --- | --- | 3.4 | 2.4 |
| O 1s | 26.1 | 21.9 | 25.7 | 30.0 | 32.4 | 20.2 | |
| 530.9 | TiO2 | 63.9 | 53.2 | 55.9 | 59.2 | 60.0 | 63.1 |
| 532.4 | O=C/O=S (in carboxyl/carbonyl or sulfoxides/sulfones) | 12.8 | 18.4 | 14.5 | 11.7 | 13.2 | 20.5 |
| 533.5 | O-C/O-S (in phenol/epoxy or thioesters/sulfonic) | 14.8 | 19.6 | 17.6 | 19.6 | 18.1 | 16.4 |
| 534.5 | -O- (in water or chemisorbed oxygen species) | 8.5 | 8.8 | 12.0 | 9.5 | 8.7 | --- |
| S 2p3/2 | 0.3 | 0.5 | 1.1 | 1.6 | |||
| 163.4 | Ti-S | --- | --- | 41.5 | 44.0 | 20.1 | 75.0 |
| 164.6 | R-S-S-, C-S-C (in bisulfides/thiophenes configurations) | --- | --- | --- | --- | 10.0 | 8.9 |
| 166.8 | C-S-O, R2-S=O/R-SO2-R (in sulfoxides, sulfones) | --- | --- | 13.4 | |||
| 168.8 | Sulfonic acid | --- | --- | 58.5 | 56.0 | 56.5 | 16.1 |
| Ti 2p3/2 | 7.5 | 5.5 | 7.5 | 8.8 | 10.8 | 6.2 | |
| 459.5 | TiO2 | 100 | 100 | 100 | 100 | 100 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandosz, T.J.; Policicchio, A.; Florent, M.; Poon, P.S.; Matos, J. TiO2/S-Doped Carbons Hybrids: Analysis of Their Interfacial and Surface Features. Molecules 2019, 24, 3585. https://doi.org/10.3390/molecules24193585
Bandosz TJ, Policicchio A, Florent M, Poon PS, Matos J. TiO2/S-Doped Carbons Hybrids: Analysis of Their Interfacial and Surface Features. Molecules. 2019; 24(19):3585. https://doi.org/10.3390/molecules24193585
Chicago/Turabian StyleBandosz, Teresa J., Alfonso Policicchio, Marc Florent, Po S. Poon, and Juan Matos. 2019. "TiO2/S-Doped Carbons Hybrids: Analysis of Their Interfacial and Surface Features" Molecules 24, no. 19: 3585. https://doi.org/10.3390/molecules24193585
APA StyleBandosz, T. J., Policicchio, A., Florent, M., Poon, P. S., & Matos, J. (2019). TiO2/S-Doped Carbons Hybrids: Analysis of Their Interfacial and Surface Features. Molecules, 24(19), 3585. https://doi.org/10.3390/molecules24193585