Zirconocene-Catalyzed Dimerization of α-Olefins: DFT Modeling of the Zr-Al Binuclear Reaction Mechanism
Abstract
1. Introduction
2. Results
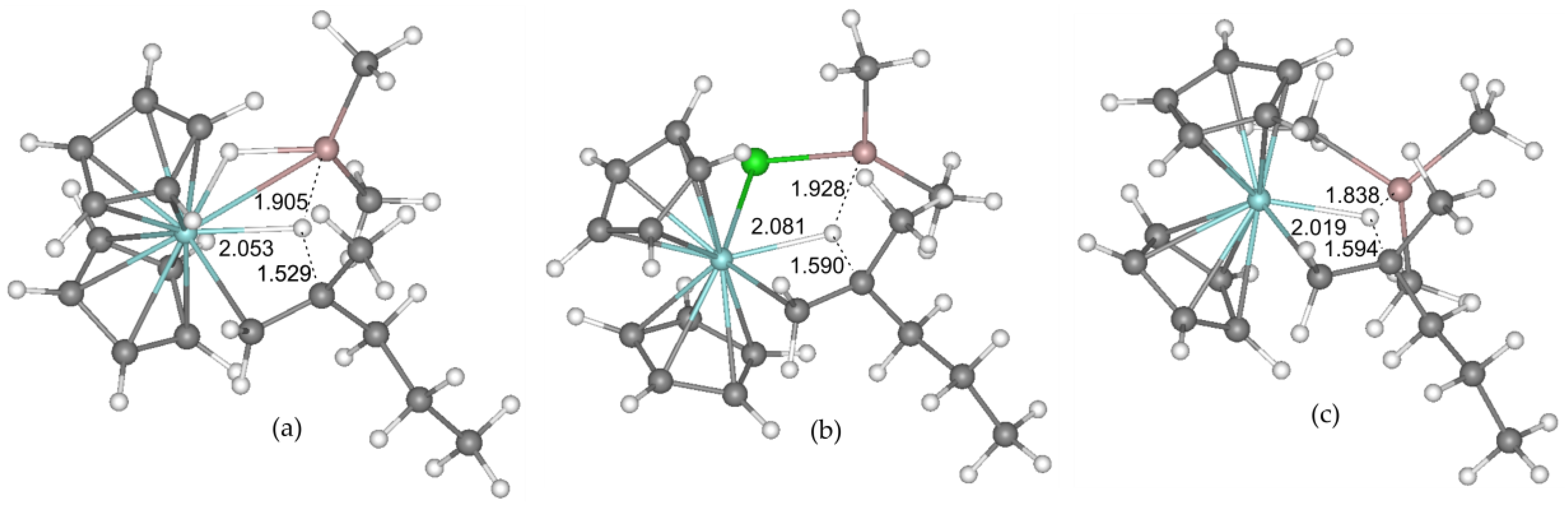
2.1. DFT Modeling of the Initiation Stage
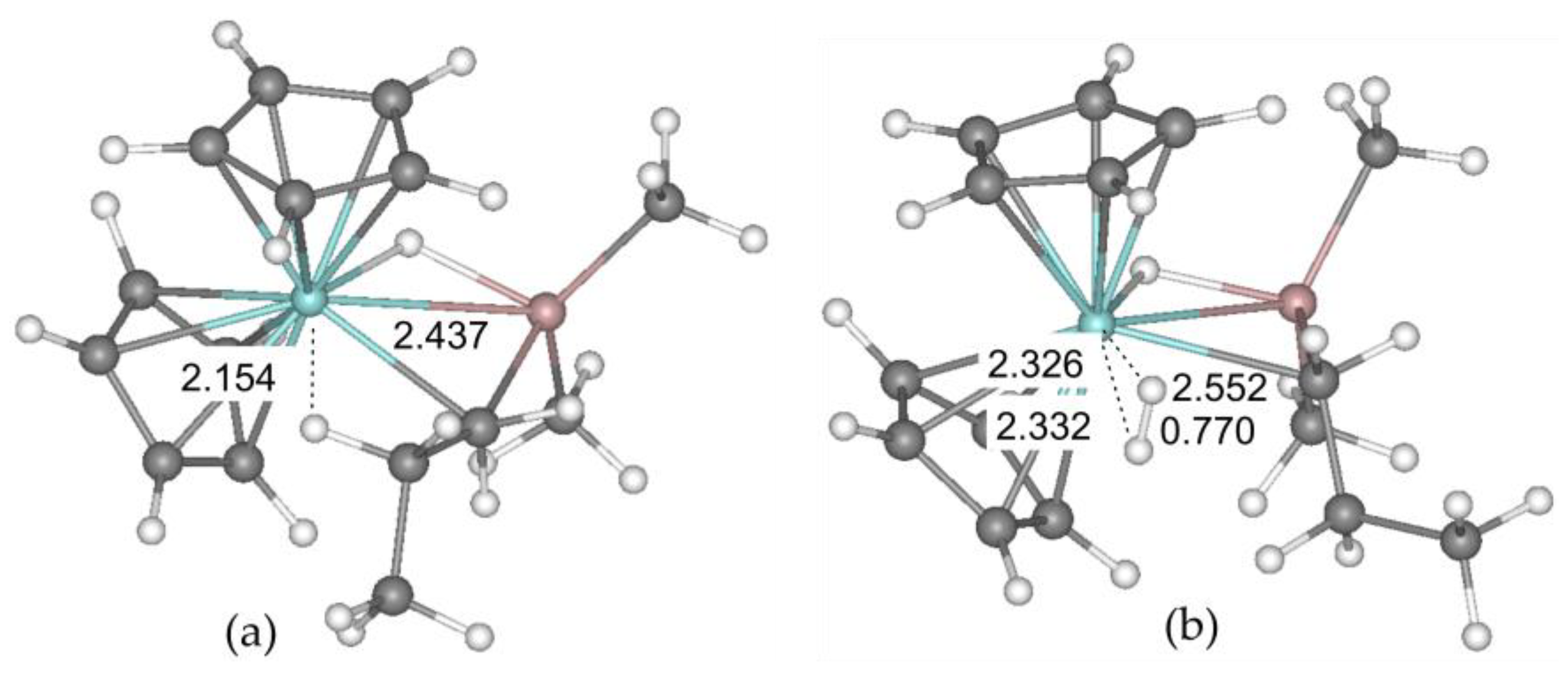
2.2. DFT Modeling of the Propagation Stage and Dimer Formation
2.3. Dimerization and Oligomerization of 1-Hexene: Experimental Study
3. Discussion
4. Materials and Methods
4.1. DFT Calculations
4.2. General Experimentsl Remarks
4.3. Dimerization and Oligomerization Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borrelli, M.; Busico, V.; Cipullo, R.; Ronca, S.; Budzelaar, P.H.M. Selectivity of metallocene-catalyzed olefin polymerization: a combined experimental and quantum mechanical study. 1. Nonchiral bis(cyclopentadienyl) systems. Macromolecules 2002, 35, 2835–2844. [Google Scholar] [CrossRef]
- Moscardi, G.; Resconi, L.; Cavallo, L. Propene polymerization with the isospecific, highly regioselective rac-Me2C(3-t-bu-1-Ind)2ZrCl2/MAO catalyst. 2. Combined DFT/MM analysis of chain propagation and chain release reactions. Organometallics 2001, 20, 1918–1931. [Google Scholar] [CrossRef]
- Silanes, I.; Ugalde, J.M. Comparative study of various mechanisms for metallocene-catalyzed α-olefin polymerization. Organometallics 2005, 24, 3233–3246. [Google Scholar] [CrossRef]
- Chan, M.S.W.; Vanka, K.; Pye, C.C.; Ziegler, T. Density functional study on activation and ion-pair formation in group iv metallocene and related olefin polymerization catalysts. Organometallics 1999, 18, 4624–4636. [Google Scholar] [CrossRef]
- Woo, T.K.; Fan, L.; Ziegler, T. A density functional study of chain growing and chain terminating steps in olefin polymerization by metallocene and constrained geometry catalysts. Organometallics 1994, 13, 2252–2261. [Google Scholar] [CrossRef]
- Silanes, I.; Mercero, J.M.; Ugalde, J.M. Comparison of Ti, Zr, and Hf as cations for metallocene-catalyzed olefin polymerization. Organometallics 2006, 25, 4483–4490. [Google Scholar] [CrossRef]
- Laine, A.; Linnolahti, M.; Pakkanen, T.A.; Severn, J.R.; Kokko, E.; Pakkanen, A. Comparative theoretical study on homopolymerization of α-olefins by bis(cyclopentadienyl) zirconocene and hafnocene: elemental propagation and termination reactions between monomers and metals. Organometallics 2010, 29, 1541–1550. [Google Scholar] [CrossRef]
- Slaugh, L.H.; Schoenthal, G.W. Vinylidene Olefin Process. US4658078 (A), 1987. [Google Scholar]
- Christoffers, J.; Bergman, R.G. Catalytic dimerization reactions of α-olefins and α,ω-dienes with Cp2ZrCl2/poly(methylalumoxane): Formation of dimers, carbocycles, and oligomers. J. Am. Chem. Soc. 1996, 118. [Google Scholar] [CrossRef]
- Christoffers, J.; Bergman, R.G. Zirconocene-alumoxane (1:1) – a catalyst for the selective dimerization of α-olefins. Inorg. Chim. Acta 1998, 270, 20–27. [Google Scholar] [CrossRef]
- Kissin, Y.V.; Schwab, F.C. Post-oligomerization of alpha-olefin oligomers: a route to single-component and multicomponent synthetic lubricating oils. J. Appl. Polym. Sci. 2009, 111, 273–280. [Google Scholar] [CrossRef]
- Janiak, C. Metallocene and related catalysts for olefin, alkyne and silane dimerization and oligomerization. Coord. Chem. Rev. 2006, 250, 66–94. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Ivchenko, P.V. Zirconocene-catalyzed dimerization of 1-hexene: Two-stage activation and structure–catalytic performance relationship. Catal. Commun. 2016, 79, 6–10. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Panchenko, V.N.; Timofeeva, M.N.; Zakharov, V.A.; Brintzinger, H.H. Novel zirconocene hydride complexes in homogeneous and in SiO2-supported olefin-polymerization catalysts modified with diisobutylaluminium hydride or triisobutylaluminum. Macromol. Chem. Phys. 2008, 209, 1210–1219. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Khalilov, L.M.; Dzhemilev, U.M. Mechanisms of reactions of organoaluminium compounds with alkenes and alkynes catalyzed by Zr complexes. Russ. Chem. Rev. 2012, 81, 524–548. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Balaev, A.V.; Gubaidullin, I.M.; Abzalilova, L.R.; Pechatkina, S.V.; Khalilov, L.M.; Spivak, S.I.; Dzhemilev, U.M. Kinetic model of olefin hydroalumination by HAlBui2 and AlBui3 in the presence of Cp2ZrCl2 catalyst. Int. J. Chem. Kinet. 2007, 39, 333–339. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Nifant’ev, I.E.; Khalilov, L.M.; Dzhemilev, U.M. Role of Zr,Al hydride intermediate structure and dynamics in alkene hydroalumination with XAlBui2 (X = H, Cl, Bui), catalyzed by Zr η5 complexes. Organometallics 2015, 34, 3559–3570. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Tyumkina, T.V.; Islamov, D.N.; Lyapina, A.R.; Karchevsky, S.G.; Ivchenko, P.V. Reactions of bimetallic Zr, Al-hydride complexes with methylaluminoxane: NMR and DFT study. J. Organomet. Chem. 2017, 851, 30–39. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Vil’danova, R.F.; Pechatkina, S.V.; Khalilov, L.M.; Dzhemilev, U.M. New effective reagent [Cp2ZrH2 · ClAlEt2]2 for alkene hydrometallation. J. Organomet. Chem. 2007, 692, 3424–3429. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Talsi, E.P.; Semikolenova, N.V.; Zakharov, V.A.; Brand, J.; Alonso-Moreno, C.; Bochmann, M. Formation and structures of cationic zirconium complexes in ternary systems rac-(SBI)ZrX2/AlBu3i/[CPh3][B(C6F5)4] (X = Cl, Me). J. Organomet. Chem. 2007, 692, 859–868. [Google Scholar] [CrossRef][Green Version]
- Baldwin, S.M.; Bercaw, J.E.; Brintzinger, H.H. Cationic alkylaluminum-complexed zirconocene hydrides as participants in olefin polymerization catalysis. J. Am. Chem. Soc. 2010, 132, 13969–13971. [Google Scholar] [CrossRef] [PubMed]
- Parfenova, L.V.; Gabdrakhmanov, V.Z.; Khalilov, L.M.; Dzhemilev, U.M. On study of chemoselectivity of reaction of trialkylalanes with alkenes, catalyzed with Zr η-complexes. J. Organomet. Chem. 2009, 694, 3725–3731. [Google Scholar] [CrossRef]
- Pankratyev, E.Yu.; Tyumkina, T.V.; Parfenova, L.V.; Khalilov, L.M.; Khursan, S.L.; Dzhemilev, U.M. DFT study on mechanism of olefin hydroalumination by XAlBui2 in the presence of Cp2ZrCl2 catalyst. I. simulation of intermediate formation in reaction of HAlBui2 with Cp2ZrCl2. Organometallics 2009, 28, 968–977. [Google Scholar] [CrossRef]
- Pankratyev, E.Yu.; Tyumkina, T.V.; Parfenova, L.V.; Khalilov, L.M.; Khursan, S.L.; Dzhemilev, U.M. DFT and Ab initio study on mechanism of olefin hydroalumination by XAlBui2 in the presence of Cp2ZrCl2 catalyst. II. olefin interaction with catalytically active centers. Organometallics 2011, 30, 6078–6089. [Google Scholar] [CrossRef]
- Tyumkina, T.V.; Islamov, D.N.; Parfenova, L.V.; Whitby, R.J.; Khalilov, L.M.; Dzhemilev, U.M. Mechanistic aspects of chemo- and regioselectivity in Cp2ZrCl2-catalyzed alkene cycloalumination by AlEt3. J. Organomet. Chem. 2016, 822, 135–143. [Google Scholar] [CrossRef]
- Tyumkina, T.V.; Islamov, D.N.; Parfenova, L.V.; Karchevsky, S.G.; Khalilov, L.M.; Dzhemilev, U.M. Mechanism of Cp2ZrCl2-Catalyzed olefin cycloalumination with AlEt3: Quantum chemical approach. Organometallics 2018, 37, 2406–2418. [Google Scholar] [CrossRef]
- Baldwin, S.M.; Bercaw, J.E.; Brintzinger, H.H. Alkylaluminum-complexed zirconocene hydrides: Identification of hydride-bridged species by nmr spectroscopy. J. Am. Chem. Soc. 2008, 130, 17423–17433. [Google Scholar] [CrossRef] [PubMed]
- Nifant’ev, I.; Ivchenko, P.; Tavtorkin, A.; Vinogradov, A.; Vinogradov, A. Non-traditional Ziegler-Natta catalysis in α-olefin transformations: Reaction mechanisms and product design. Pure Appl. Chem. 2017, 89, 1017–1032. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Semikolenova, N.V.; Zakharov, V.A.; Talsi, E.P. Mechanism of dimethylzirconocene activation with methylaluminoxane: NMR monitoring of intermediates at high Al/Zr ratios. Macromol. Chem. Phys. 2000, 201, 558–567. [Google Scholar] [CrossRef]
- Babushkin, D.E.; Brintzinger, H.-H. Activation of dimethyl zirconocene by methylaluminoxane (MAO)-size estimate for Me-MAO– anions by pulsed field-gradient NMR. J. Am. Chem. Soc. 2002, 124, 12869–12873. [Google Scholar] [CrossRef]
- Theurkauff, G.; Bader, M.; Marquet, N.; Bondon, A.; Roisnel, T.; Guegan, J.-P.; Amar, A.; Boucekkine, A.; Carpentier, J.-F.; Kirillov, E. Discrete ionic complexes of highly isoselective zirconocenes. solution dynamics, trimethylaluminum adducts, and implications in propylene polymerization. Organometallics 2016, 35, 258–276. [Google Scholar] [CrossRef]
- Theurkauff, G.; Bondon, A.; Dorcet, V.; Carpentier, J.-F.; Kirillov, E. Heterobi- and -trimetallic ion pairs of zirconocene-based isoselective olefin polymerization catalysts with AlMe3. Angew. Chem. Int. Ed. 2015, 54, 6343–6346. [Google Scholar] [CrossRef] [PubMed]
- Ehm, C.; Cipullo, R.; Budzelaar, P.H.M.; Busico, V. Role of TMA in polymerization. Dalton Trans. 2016, 45, 6847–6855. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; He, F.; Zhang, Z.; Khan, A.; Fu, Z.; Xu, J.; Fan, Z. Influence of trimethylaluminum on kinetics of rac-Et(Ind)ZrCl2/aluminoxane catalyzed ethylene polymerization. J. Organomet. Chem. 2016, 808, 109–116. [Google Scholar] [CrossRef]
- Collins, S.; Linnolahti, M.; Zamora, M.G.; Zijlstra, H.S.; Hernández, M.T.R.; Perez-Camacho, O. Activation of Cp2ZrX2 (X = Me, Cl) by methylaluminoxane as studied by electrospray ionization mass spectrometry: relationship to polymerization catalysis. Macromolecules 2017, 50, 8871–8884. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Revision, A.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other fun. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Godbout, N.; Salahub, D.R.; Andzelm, J.; Wimmer, E. Optimization of gaussian-type basis sets for local spin density functional calculations. Part, I. Boron through neon, optimization technique and validation. Can. J. Chem. 1992, 70, 560–571. [Google Scholar] [CrossRef]
- Kissin, Y.V. Detailed kinetics of 1-hexene oligomerization reaction with (n-Bu-Cp)2ZrCl2–MAO catalyst. Macromol. Chem. Phys. 2009, 210, 1241–1246. [Google Scholar] [CrossRef]
- Kissin, Y.V. Oligomerization reactions of 1-hexene with metallocene catalysts: Detailed data on reaction chemistry and kinetics. Mol. Catal. 2019, 463, 87–93. [Google Scholar] [CrossRef]
- Hirvi, J.T.; Bochmann, M.; Severn, J.R.; Linnolahti, M. Formation of octameric methylaluminoxanes by hydrolysis of trimethylaluminum and the mechanisms of catalyst activation in single-site α-olefin polymerization catalysis. Chem. Phys. Chem. 2014, 15, 2732–2742. [Google Scholar] [CrossRef]
- Kuklin, M.S.; Hirvi, J.T.; Bochmann, M.; Linnolahti, M. Toward controlling the metallocene/methylaluminoxane-catalyzed olefin polymerization process by a computational approach. Organometallics 2015, 34, 3586–3597. [Google Scholar] [CrossRef]
- Ghiotto, F.; Pateraki, C.; Tanskanen, J.; Severn, J.R.; Luehmann, N.; Kusmin, A.; Stellbrink, J.; Linnolahti, M.; Bochmann, M. Probing the structure of methylalumoxane (MAO) by a combined chemical, spectroscopic, neutron scattering, and computational approach. Organometallics 2013, 32, 3354–3362. [Google Scholar] [CrossRef]
- Trefz, T.K.; Henderson, M.A.; Linnolahti, M.; Collins, S.; McIndoe, J.S. Mass spectrometric characterization of methylaluminoxane-activated metallocene complexes. Chem. Eur. J. 2015, 21, 2980–2991. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Fujikawa, S. Base Oil for Oil Drilling Fluid and Oil Drilling Fluid Composition. Patent Appl. US2011251445 (A1), 2011. [Google Scholar]
- Fujikawa, S.; Okamoto, T.; Yokota, K. Process for Producing Unsaturated Hydrocarbon Compound. Patent US8119850 (B2), 2012. [Google Scholar]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Ivchenko, P.V. Production Method of the Vinylidene Olefins. Patent RU2652118 (C2), 2018. [Google Scholar]
- Laikov, D.N.; Ustynyuk, Y.A. PRIRODA-04: A quantum-chemical program suite. New possibilities in the study of molecular systems with the application of parallel computing. Russ. Chem. Bull. 2005, 54, 820–826. [Google Scholar] [CrossRef]
- Sosa, C.; Andzelm, J.; Elkin, B.C.; Wimmer, E.; Dobbs, K.D.; Dixon, D.A. A local density functional study of the structure and vibrational frequencies of molecular transition-metal compounds. J. Phys. Chem. 1992, 96, 6630–6636. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, H. Performance of density functionals for activation energies of Zr-mediated reactions. J. Chem. Theory Comput. 2013, 9, 4735–4743. [Google Scholar] [CrossRef]
Sample Availability: Samples of vinylidene oligomers of 1-hexene are available from the authors. |







| Structure | Cationic Mechanism | R = Me | R = iBu | |||||
|---|---|---|---|---|---|---|---|---|
| X=H | X=Cl | X=Me | X=H | X=Cl | X=Me | |||
| I-0 I-0X | G | 27.5 | −8.4 | −4.6 | −2.7 | −11.8 | −6.3 | −3.5 |
| H | 40.8 | −9.9 | −6.7 | −2.6 | −11.2 | −8.6 | −6.6 | |
| I-1 I-1X | G | 10.0 | −13.5 | 0.0 | 5.1 | −14.9 | −1.8 | 5.3 |
| H | 9.8 | −26.7 | −14.0 | −1.6 | −29.7 | −17.3 | −12.2 | |
| TS-1 TS-1X | G | 12.1 | −7.0 | 4.3 | 10.6 | −9.2 | 3.7 | 11.8 |
| H | 11.4 | −21.9 | −10.9 | −4.1 | −25.2 | −12.8 | −6.9 | |
| I-2_b I-2X_bi | G | 0.0 | −13.1 | −7.0 | −1.6 | --16.3 | −9.8 | −1.4 |
| H | 0.0 | −26.9 | −19.5 | −11.6 | −28.9 | --23.0 | −15.2 | |
| I-2+_a I-2X_a | G | 10.6 | −22.1 | −13.0 | −7.8 | −21.0 | −12.7 | −6.1 |
| H | 10.6 | −35.9 | −28.4 | −22.6 | −36.7 | −29.3 | −24.7 | |
| I-2_b I-2X_bo | G | 0.0 | −28.0 | −10.4 | −9.1 | −28.1 | −11.0 | −6.8 |
| H | 0.0 | −41.9 | −26.9 | −24.0 | −43.7 | −28.8 | −26.7 | |
| I-3 | G | −4.4 | ||||||
| H | −16.3 | |||||||
| I-3_b | G | −3.2 | ||||||
| H | −15.8 | |||||||
| TS-2 | G | 6.0 | ||||||
| H | −9.3 | |||||||
| TS-3 | G | 12.7 | 12.7 | 12.7 | 12.7 | 12.7 | 12.7 | 12.7 |
| H | −1.9 | −1.9 | −1.9 | −1.9 | −1.9 | −1.9 | −1.9 | |
| I-4_b I-4X_bi | G | −12.5 | −27.1 | −19.1 | −12.7 | −28.0 | −22.4 | −13.8 |
| H | −26.4 | −52.0 | −45.9 | −38.0 | −55.1 | −49.8 | −43.3 | |
| I-4_a I-4X_a | G | −3.0 | −34.8 | −23.6 | −20.7 | −33.9 | −22.8 | −19.0 |
| H | −15.7 | −61.9 | −51.7 | −48.2 | −62.8 | −53.8 | −50.6 | |
| I-4_b I-4X_bo | G | −12.5 | −39.7 | −22.4 | −20.8 | −42.5 | −23.7 | −18.7 |
| H | −26.4 | −68.3 | −51.8 | −49.1 | −71.0 | −53.9 | −51.6 | |
| TS-4 TS-4X | G | −3.8 | −22.0 | −9.9 | −4.6 | −21.4 | −6.5 | 0.3 |
| H | −17.9 | −50.5 | −38.9 | −32.1 | −51.6 | −38.3 | −33.2 | |
| I-5 I-5X | G | −8.2 | −30.5 | −18.6 | −12.3 | −31.2 | −17.4 | −10.4 |
| H | −19.7 | −56.6 | −45.0 | −37.7 | −59.3 | −45.6 | −40.2 | |
| I-6 | G | −15.7 | ||||||
| H | −41.2 | |||||||
| I-6_b | G | −16.0 | ||||||
| H | −41.3 | |||||||
| TS-5 | G | −4.1 | ||||||
| H | −32.5 | |||||||
| Run | MMAO-12 eq. | R2AlX eq. | 1-Hexene Conv., % | Product Distribution (wt.%) 2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| 2-C6 | C12 | C18 | C24 | C30 | Higher Oligomers | ||||
| 1 | 200 | 0 | 99 | 1.7 | 53.8 | 13.2 | 5.3 | 3.2 | 21.7 |
| 2 | 100 | 0 | 98 | 1.9 | 69.5 | 7.5 | 3.8 | 2.4 | 14.1 |
| 3 | 50 | 0 | 96 | 2.0 | 75.1 | 6.9 | 3.1 | 1.6 | 7.7 |
| 4 | 20 | 0 | 93 | 2.0 | 77.8 | 5.2 | 2.3 | 1.1 | 4.8 |
| 5 | 10 | 0 | 97 | 3.4 | 82.3 | 5.9 | 1.5 | 0.3 | 3.6 |
| 6 | 5 | 0 | 95 | 3.8 | 81.6 | 5.4 | 1.3 | 0.3 | 2.6 |
| 7 | 10 | Me2AlCl, 1 | 98 | 3.6 | 87.4 | 3.2 | 1.1 | 0.3 | 2.5 |
| 8 | 10 | Et2AlCl, 1 | 88 | 3.6 | 78.1 | 2.9 | 1.0 | 0.3 | 2.2 |
| 9 | 10 | Et2AlCl, 2 | 97 | 3.8 | 85.7 | 3.3 | 1.2 | 0.4 | 2.7 |
| 10 | 10 | Et2AlCl, 5 | 59 | 2.0 | 53.2 | 1.1 | 0.3 | <0.1 | <0.2 |
| 11 | 10 | Et2AlCl, 10 | 31 | 0.6 | 29.1 | 0.3 | <0.1 | <0.1 | <0.2 |
| 12 | 10 | Me3Al, 2 | 61 | 0.9 | 36.6 | 9.6 | 3.3 | 1.4 | 9.2 |
| 13 3 | 10 | 10 | 99 | 2.8 | 88.8 | 3.2 | 1.4 | 0.6 | 2.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Karchevsky, S.; Ivchenko, P. Zirconocene-Catalyzed Dimerization of α-Olefins: DFT Modeling of the Zr-Al Binuclear Reaction Mechanism. Molecules 2019, 24, 3565. https://doi.org/10.3390/molecules24193565
Nifant’ev I, Vinogradov A, Vinogradov A, Karchevsky S, Ivchenko P. Zirconocene-Catalyzed Dimerization of α-Olefins: DFT Modeling of the Zr-Al Binuclear Reaction Mechanism. Molecules. 2019; 24(19):3565. https://doi.org/10.3390/molecules24193565
Chicago/Turabian StyleNifant’ev, Ilya, Alexander Vinogradov, Alexey Vinogradov, Stanislav Karchevsky, and Pavel Ivchenko. 2019. "Zirconocene-Catalyzed Dimerization of α-Olefins: DFT Modeling of the Zr-Al Binuclear Reaction Mechanism" Molecules 24, no. 19: 3565. https://doi.org/10.3390/molecules24193565
APA StyleNifant’ev, I., Vinogradov, A., Vinogradov, A., Karchevsky, S., & Ivchenko, P. (2019). Zirconocene-Catalyzed Dimerization of α-Olefins: DFT Modeling of the Zr-Al Binuclear Reaction Mechanism. Molecules, 24(19), 3565. https://doi.org/10.3390/molecules24193565