Antifungal Properties of Fucus vesiculosus L. Supercritical Fluid Extract Against Fusarium culmorum and Fusarium oxysporum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fucus vesiculosus Extract
2.3. Growth Rate of Fusarium Strains on the Medium with Extract
2.4. Preparation of RB Liquid Medium
2.5. Preparation of Fusarium Strains Macroconidia
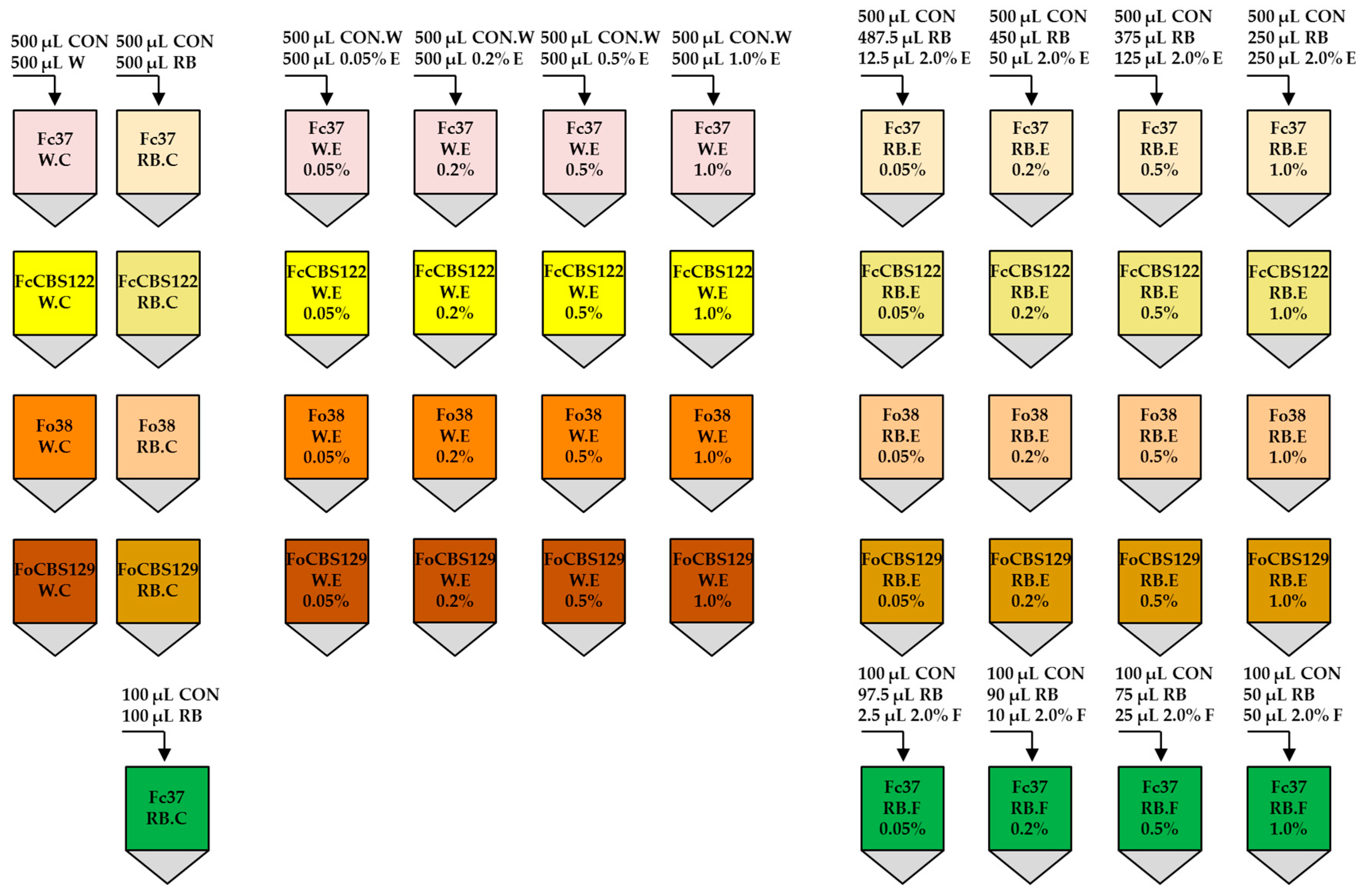
2.6. Preparation of Final Samples
2.7. Preparation of Fucosterol Standard
2.8. Evaluation of Macroconidia Germination Capacity of Fusarium spp.
2.9. Statistical Analysis
3. Results and Discussion
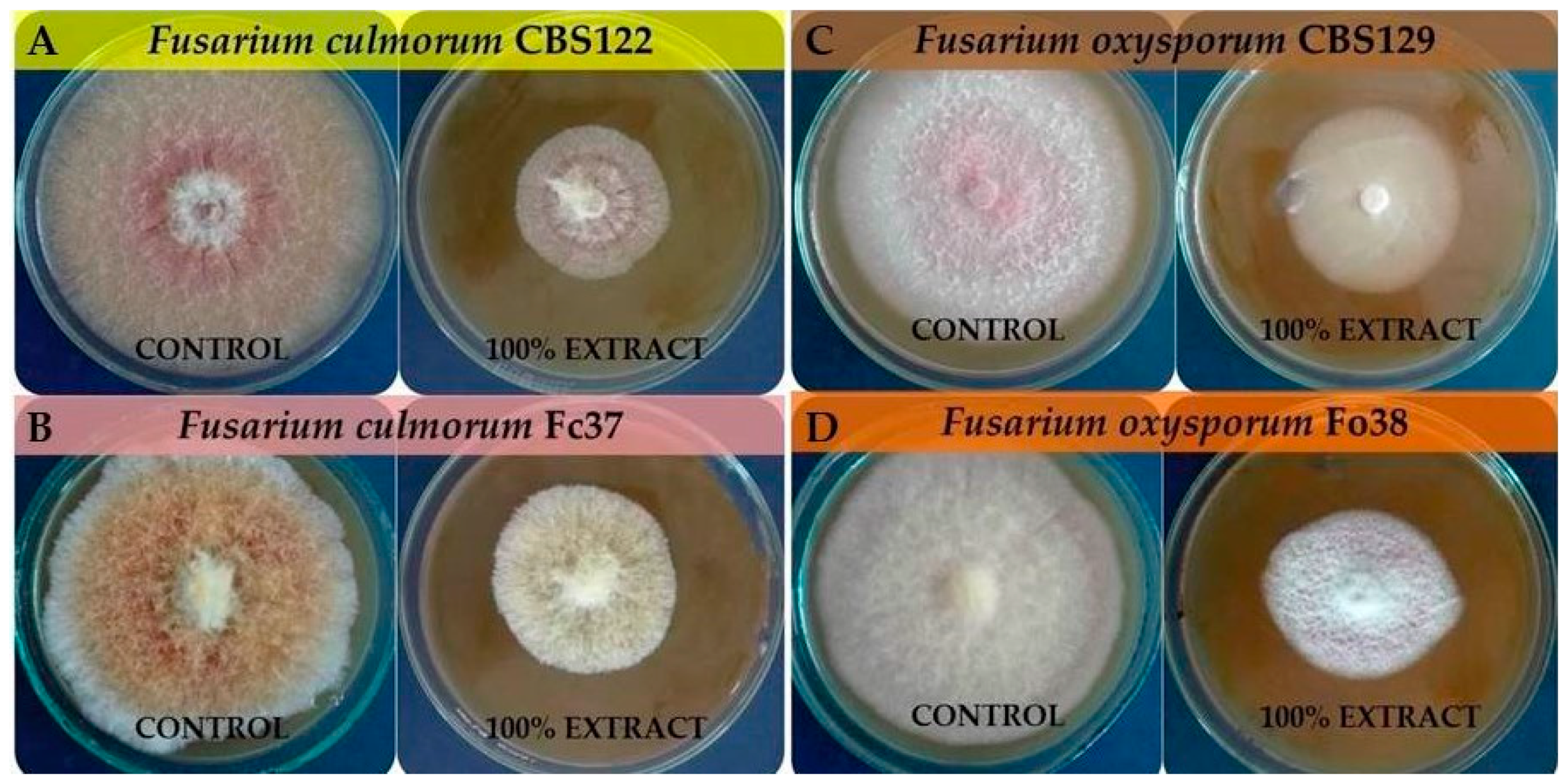
3.1. The Effect of F. vesiculosus Extract on the Mycelial Growth of Fusarium Strains
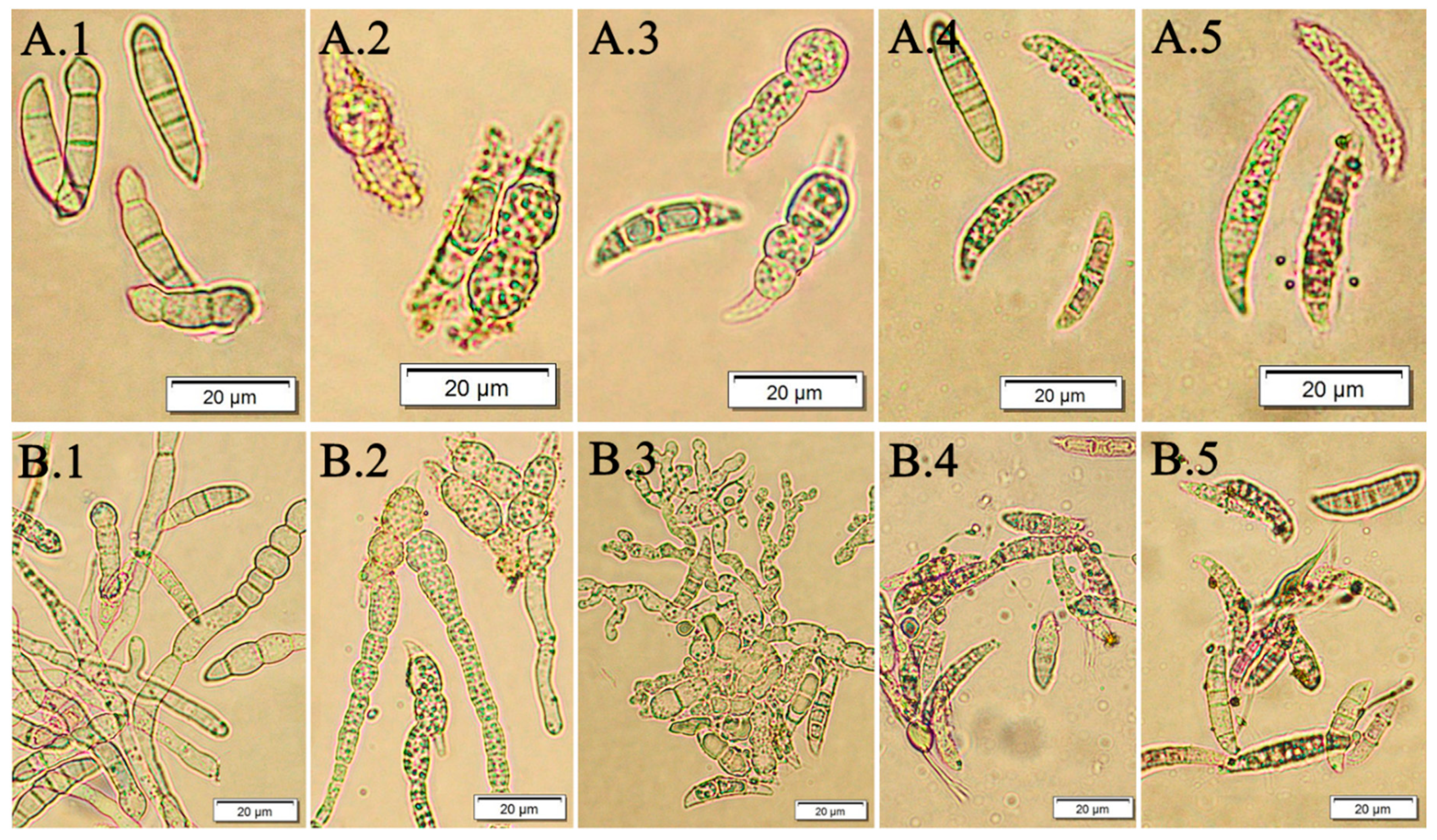
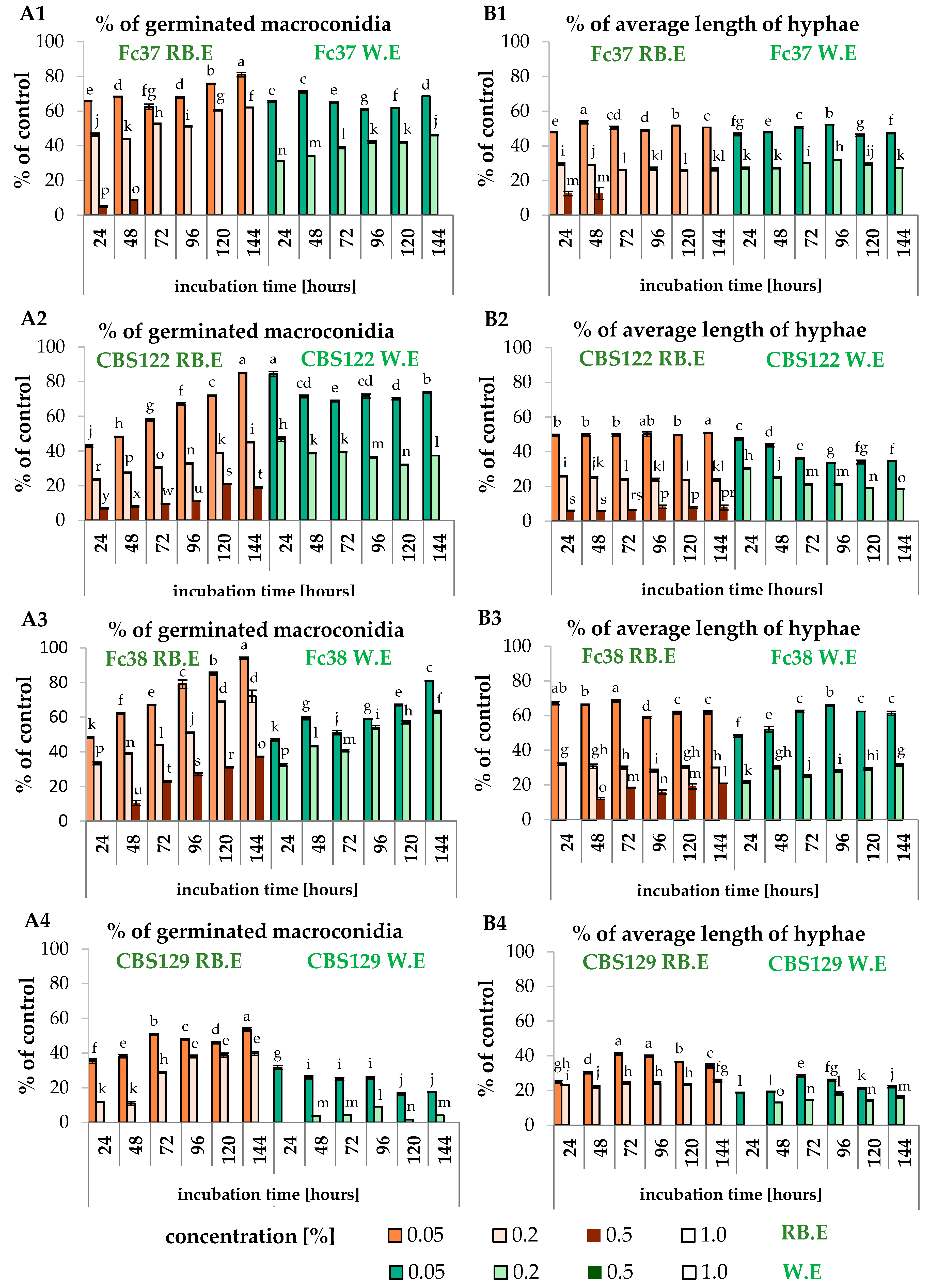
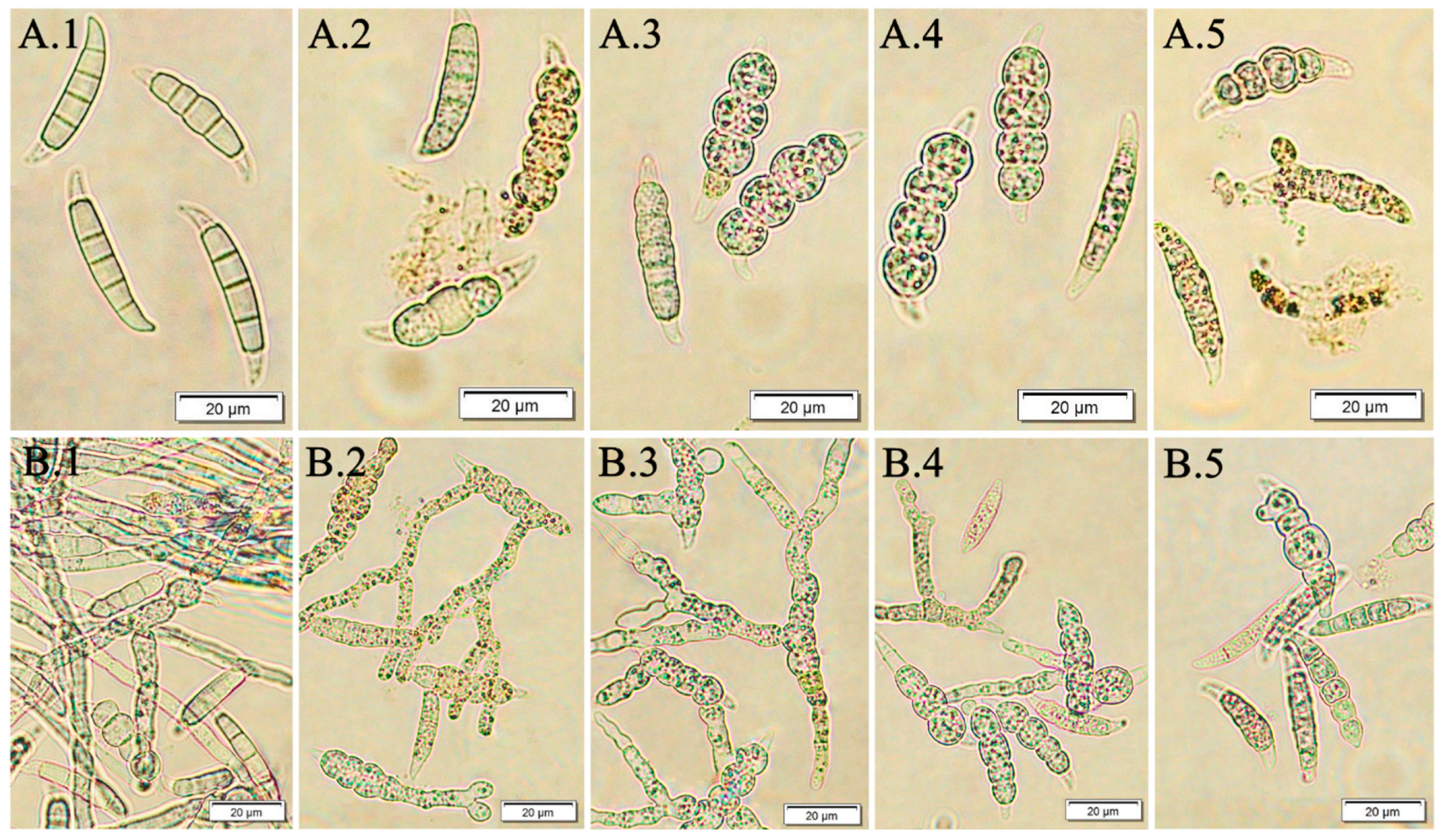
3.2. The Effect of F. vesiculosus Extract on the Fusarium Macroconidia
3.3. The Effect of Fucosterol on the Fusarium Macroconidia
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Sui, Y.; Wisniewski, M.; Droby, S.; Liu, Y. Review: utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 2013, 167, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Matny, O.N. Fusarium head blight and crown rot on wheat & barley: Losses and health risks. Adv. Plants Agric. Res. 2015, 2, 3843. [Google Scholar]
- Naeimi, S.; Okhovvat, S.M.; Javan-Nikkhah, M.; Vágvölgyi, C.; Khosravi, V.; Kredics, L. Biological control of Rhizoctonia solani AG1-1A, the causal agent of rice sheath blight with Trichoderma strains. Phytopathol. Mediterr. 2010, 49, 287–300. [Google Scholar]
- Escrivá, L.; Font, G.; Manyes, L. In vivo toxicity studies of Fusarium mycotoxins in the last decade: A review. Food Chem. Toxicol. 2015, 78, 185–206. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Mares, M.V.; Hernandez-Carlos, B. Plant-derived natural products from the American continent for the control of phytopathogenic fungi: A review. J. Glob. Innov. Agric. Soc. Sci. 2015, 3, 2312–5225. [Google Scholar]
- Saremi, H.; Saremi, H.; Okhovvat, S.M. Major Fusarium diseases on crops and their control management with soil solarisation on northwest Iran. Commun. Agric. Appl. Biol. Sci. 2008, 73, 189–199. [Google Scholar] [PubMed]
- Joshi, R. A review of Fusarium oxysporum on its plant interaction and industrial use. J. Med. Plant Res. 2018, 6, 112–115. [Google Scholar] [CrossRef]
- Awad, W.; Ghareeb, K.; Böhm, J.; Zentek, J. The toxicological impacts on the Fusarium mycotoxin, deoxynivalenol, in poultry flocks with special reference to immunotoxicity. Toxins 2013, 5, 912–925. [Google Scholar] [CrossRef]
- Huang, D.; Cui, L.; Sajid, A.; Zainab, F.; Wu, Q.; Wang, X.; Yuan, Z. The epigenetic mechanisms in Fusarium mycotoxins induced toxicities. Food Chem. Toxicol. 2019, 123, 595–601. [Google Scholar] [CrossRef]
- Prandini, A.; Sigolo, S.L.; Filippi, P.; Battilani, P.; Piva, G. Review of predictive models for Fusarium head blight and related mycotoxin contamination in wheat. Food Chem. Toxicol. 2009, 47, 927–931. [Google Scholar] [CrossRef]
- Dita, M.; Barquero, M.; Heck, D.; Mizubuti, E.S.G.; Staver, C.P. Fusarium wilt of banana: current knowledge on epidemiology and research needs toward sustainable disease management. Front Plant Sci. 2018, 9, 1468. [Google Scholar] [CrossRef] [PubMed]
- Shuping, D.S.S.; Eloff, J.N. The use of plants to protect plants and food against fungal pathogens: A review. Afr. J. Tradit. Complement Altern. Med. 2017, 14, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Nan, Z. Effects of phytopathogens on plant community dynamics: A review. Acta Ecol. Sin. 2015, 35, 177–183. [Google Scholar] [CrossRef]
- Zhang, W.J.; Jiang, F.B.; Ou, J.F. Global pesticide consumption and pollution: With China as a focus. Proc. Int. Acad. Ecol. Env. Sci. 2011, 1, 125–144. [Google Scholar]
- Hamed, S.M.; Abd El-Rhmana, A.A.; Abdel-Raoufb, N.; Ibraheem, I.B.M. Role of marine macroalgae in plant protection & improvement for sustainable agriculture technology. Beni-Suef Univ. J. Appl. Sci. 2018, 7, 104–110. [Google Scholar]
- Hong, D.D.; Hien, H.M.; Son, P.N. Seaweeds from Vietnam used for functional food, medicine and fertilizer. J. Appl. Phycol. 2007, 19, 817–826. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Davies, P.J. Plant Hormones. Biosynthesis, Signal Transduction, Action! 3rd ed.; Kluwer: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Esserti, S.; Smaili, A.; Rifai, L.A.; Koussa, T.; Makroum, K.; Belfaiza, M.; Kabil, E.; Faize, L.; Burgos, L.; Alburquerque, N.; et al. Protective effect of three brown seaweed extracts against fungal and bacterial diseases of tomato. J. Appl. Phycol. 2017, 29, 1081–1093. [Google Scholar] [CrossRef]
- Ibraheem, B.M.I.; Hamed, S.M.; Abd elrhman, A.A.; Farag, F.M.; Abdel-Raouf, N. Antimicrobial activities of some brown macroalgae against some soil borne plant pathogens and in vivo management of Solanum melongena root diseases. Aust. J. Basic Appl. Sci. 2017, 11, 157–168. [Google Scholar]
- Galal, H.R.M.; Salem, W.M.; El-Deen, N.F. Biological control of some pathogenic fungi using Marine Algae Extracts. Res. J. Microbiol. 2011, 6, 645–657. [Google Scholar] [CrossRef][Green Version]
- Ramkissoon, A.; Ramsubhag, A.; Jayaraman, J. Phytoelicitor activity of three Caribbean seaweed species on suppression of pathogenic infections in tomato plants. J. Appl. Phycol. 2017, 29, 3235–3244. [Google Scholar] [CrossRef]
- Baloch, G.N.; Tariq, S.; Ehteshamul-Haque, S.; Athar, M.; Sultana, V.; Ara, J. Management of root diseases of eggplant and watermelon with the application of asafoetida and seaweeds. J. Appl. Bot. Food Qual. 2013, 86, 138–142. [Google Scholar]
- Menshova, R.V.; Lepeshkin, F.D.; Ermakova, S.P.; Pokrovskii, O.I.; Zvyagintseva, T.N. Effect of pretreatment conditions of brown algae by supercritical fluids on yield and structural characteristics of fucoidans. Chem. Nat. Compd. 2013, 48, 923–926. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phycochemical constitutents and biological activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef] [PubMed]
- Quitain, A.T.; Kai, T.; Sasaki, M.; Goto, M. Supercritical carbon dioxide extraction of fucoxanthin from Undaria pinnatifida. J. Agric. Food Chem. 2013, 61, 5792–5797. [Google Scholar] [CrossRef]
- Taylor, S. Marine Medicinal Foods: Implications and Applications. Macro and Microalgae; Elsevier Inc.: Lincoln, NE, USA, 2011. [Google Scholar]
- Blunden, G.; Morse, P.F.; Mathe, I.; Hohmann, J.; Critchleye, A.T.; Morrell, S. Betaine yields from marine algal species utilized in the preparation of seaweed extracts used in agriculture. Nat. Prod. Commun. 2010, 5, 581–585. [Google Scholar] [CrossRef]
- Coşoveanu, A.; Axîne, O.; Iacomi, B. Antifungal Activity of Macroalgae Extracts; Sci. Pap. Series A; UASVM Bucharest: Bucharest, Romania, 2010; Volume LIII, ISSN 1222-5339. [Google Scholar]
- Michalak, I.; Górka, B.; Wieczorek, P.P.; Rój, E.; Lipok, J.; Łęska, B.; Messyasz, B.; Wilk, R.; Schroeder, G.; Dobrzyńska-Inger, A.; et al. Supercritical fluid extraction of algae enhances levels of biologically active compounds promoting plant growth. Eur. J. Phycol. 2016, 51, 243–252. [Google Scholar] [CrossRef]
- Rój, E.; Dobrzyńska-Inger, A.; Grzęda, K.; Kostrzewa, D. Supercritical extraction of plant material. Przem. Chem. 2013, 92, 1358–1363. [Google Scholar]
- Michalak, I.; Chojnacka, K.; Dmytryk, A.; Wilk, R.; Gramza, M.; Rój, E. Evaluation of supercritical fluid extract of algae as biostimulants of plant growth in field trials. Front. Plant Sci. 2016, 7, 1591. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Rój, E. The application of supercritical fluid extraction in phenolic compounds isolation from natural plant materials. Molecules 2018, 23, 2625. [Google Scholar] [CrossRef]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Karim, A.A.; Abbas, K.A.; Norulaini, N.A.N.; Omar, A.K.M. Application of supercritical CO2 in lipid extraction—A review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidović, S.; Redovniković, R.; Jokić, S. New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod. Process 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Herrero, M.; Medniola, J.A.; Cifuentes, A.; Ibáñez, E. Supercritical fluid extraction: Recent advances and applications. J. Chrom. A. 2010, 1217, 2495–2511. [Google Scholar] [CrossRef] [PubMed]
- Reyes, F.; Byrde, R.J.W. Partial purification and properties of a β-N-acetylglucosaminidase from the fungus Sclerotinia fructigena. Biochem. J. 1973, 131, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Jaroszuk-Ściseł, J.; Kurek, E.; Winiarczyk, K.; Baturo, A.; Łukanowski, A. Colonization of root tissues and protection against Fusarium wilt or rye (Secale cereale) by nonpathogenic rhizosphere strains of Fusarium culmorum. Biol. Control 2008, 45, 297–307. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Kurek, E.; Słomka, A.; Janczarek, M.; Rodzik, B. Activities of cell wall degrading enzymes in autolyzing cultures of three Fusarium culmorum isolates: growth-promoting, deleterious and pathogenic to rye (Secale cereale). Mycologia 2011, 103, 929–945. [Google Scholar] [CrossRef]
- Tyśkiewicz, K.; Konkol, M.; Wiejak, R.; Maziarczyk, I.; Rój, E.; Tyśkiewicz, R.; Jaroszuk-Ściseł, J.; Skalicka-Woźniak, K. Determination of fucosterol in the supercritical extract from Fucus vesiculosus by supercritical chromatography (Polish). Przem. Chem. 2019, 98, 70–73. [Google Scholar] [CrossRef]
- Van Lenteren, J.C. The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. BioControl. 2012, 57, 1–20. [Google Scholar] [CrossRef]
- Ropek, D.; Krysa, A.; Rola, A.; Frączek, K. Antagonistic effect of Trichoderma viride on entomopathogenic fungi Beauveria bassiana, Isaria Fumosorosea and Metarhizium anisopliae in vitro. Pol. J. Agron. 2014, 16, 57–63. [Google Scholar]
- Rahman, S.A.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef]
- Mannai, S.; Horrigue-Raouani, N.; Boughalleb-M’Hamdi, N. Effect of six fungicides against Fusarium oxysporum and Fusarium solani associated with peach seedlings decline in Tunisian nurseries. Annu. Res. Rev. Biol. 2018, 26, 1–11. [Google Scholar] [CrossRef]
- Danielewicz, B.; Gwiazdowski, R.; Bednarek-Bartsch, A. Influence of some selected fungicides on Fusarium genus cultures growth limitation (Polish). Prog. Plant Prot. 2013, 53. [Google Scholar]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Vera, J.; Castro, J.; Gonzalez, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef]
- De Corato, U.; Salimbeni, R.; de Pretis, A.; Avella, N.; Patruno, G. Antifungal activity of crude extracts from brown and red seaweeds by a supercritical carbon dioxide technique against fruit postharvest fungal diseases. Postharvest Biol. Technol. 2017, 131, 16–30. [Google Scholar] [CrossRef]
- Raj, T.S.; Graff, K.H.; Suji, H.A. Bio chemical characterization of a brown seaweed algae and its efficacy on control of rice sheath blight caused by Rhizoctonia solani Kuhn. Int. J. Trop. Agric. 2016, 34, 429–439. [Google Scholar]
- De Borba Gurpihares, D.; Rodrigues Moreira, T.; da Luz Bueno, J.; Cinelli, L.P.; Mazzola, P.G.; Pessora, A.; Sette, L.D. Algae’s sulfated polysaccharides modifications: potential use of microbial enzymes. Process Biochem. 2016, 51, 989–998. [Google Scholar] [CrossRef]
- Rodríguez-Jasso, R.M.; Mussatto, S.I.; Sepúlveda, L.; Torrado Agrasar, A.; Pastrana, L.; Aguilar, C.N.; Teixeria, J.A. Fungal fucoidanase production by solid-state fermentation in a rotating drum bioreactor using algal biomass as substrate. Food Bioprod. Process. 2013, 91, 587–594. [Google Scholar] [CrossRef]
- Alexeeva, Y.V.; Ivanova, E.P.; Bakunina, I.Y.; Zvaygintseva, T.N.; Mikhailov, V.V. Optimization of glycosidases production by Pseudoalteromonas issachenkonii KMM 3549. Lett. Appl. Microbiol. 2002, 35, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.C. Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Can. J. Plant. Pathol. Rev. Can. Phytopathol. 1982, 4, 195–209. [Google Scholar] [CrossRef]
- Popiel, D.; Kwaśna, H.; Chełkowski, J.; Stępień, Ł.; Laskowska, M. Impact of selected antagonistic fungi on Fusarium species—toxigenic cereale pathogens. Acta Mycol. 2008, 43, 29–40. [Google Scholar] [CrossRef]
- Seminghini, C.P.; Murray, N.; Harris, S.D. Inhibition of Fusarium graminearum growth and development by farnesol. FEMS Microbiol. Lett. 2008, 279, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Kontoyiannis, D.P. Fusarium infections in critically ill patients. Semin. Respir. Crit. Care Med. 2004, 25, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Aktar, M.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Neumann, A.; Bagiński, M.; Czub, J. How do sterols determine the antifungal activity of amphotericin B? Free energy of binding between the drug and its membrane targets. J. Am. Chem. Soc. 2010, 132, 18266–18272. [Google Scholar] [CrossRef] [PubMed]
- Ohvo-Rekilä, H.; Ramstedt, B.; Leppimäki, P.; Slotte, J.P. Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 2002, 41, 66–97. [Google Scholar] [CrossRef]
- Choi, N.H.; Jang, J.Y.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Nguyen, V.T.; Min, B.S.; Le Dang, Q.; Kim, J.C. Antifungal activity of sterols and dipsacus saponins isolated from Dipsacus asper roots against phytopathogenic fungi. Pestic. Biochem. Phys. 2017, 141, 103–108. [Google Scholar] [CrossRef]
- Kazan, K.; Gardiner, D.M. Targeting pathogen sterols: Defence and counterdefense? PloS Pathog. 2017. [Google Scholar] [CrossRef]
- Papadopoulou, K.; Melton, R.E.; Leggett, M.; Daniels, M.J.; Osbourn, A.E. Compromised disease resistance in saponins-deficient plants. PNAS 1999, 96, 12923–12928. [Google Scholar] [CrossRef]
- Upadhyay, S.; Jeena, G.S.; Shikha; Shukla, R.K. Recent advances in steroidal saponins biosynthesis and in vitro production. Planta 2018, 248, 519–544. [Google Scholar] [CrossRef]
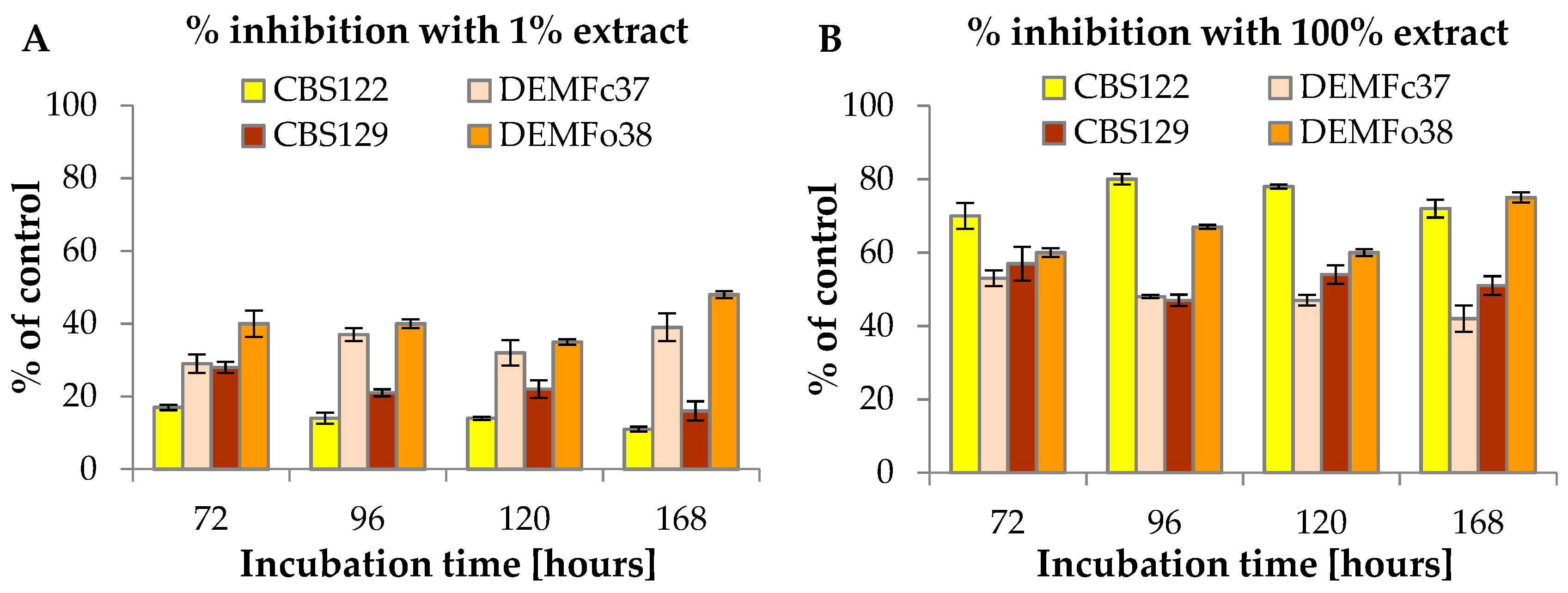



| Incubation Time [h] | Fusarium culmorum | |||||
|---|---|---|---|---|---|---|
| CBS122 | Fc37 | |||||
| R Factor (mm2 mycelium/day) | ||||||
| Control | Extract 1% | Extract 100% | Control | Extract 1% | Extract 100% | |
| 72 | 322.37 ± 8.23 | 268.21 ± 12.54 | 98.65 ± 5.76 | 285.74 ± 12.45 | 203.32 ± 5.76 | 133.97 ± 9.54 |
| 96 | 625.06 ± 23.14 | 538.71 ± 9.54 | 120.11 ± 10.54 | 358.79 ± 11.56 | 227.85 ± 14.76 | 188.40 ± 4.87 |
| 120 | 873.08 ± 15.67 | 759.35 ± 19.94 | 193.42 ± 5.76 | 430.97 ± 9.76 | 293.90 ± 10.12 | 228.75 ± 12.04 |
| 168 | 901.18 ± 8.32 | 803.06 ± 15.04 | 251.20 ± 12.67 | 590.43 ± 19.43 | 357.18 ± 11.04 | 344.50 ± 13.76 |
| Fusarium oxysporum | ||||||
| CBS129 | Fo38 | |||||
| 72 | 203.32 ± 3.98 | 146.80 ± 13.55 | 87.92 ± 6.87 | 146.80 ± 11.67 | 87.92 ± 3.67 | 58.88 ± 8.65 |
| 96 | 285.94 ± 12.56 | 227.85 ± 9.04 | 152.49 ± 12.11 | 221.03 ± 20.02 | 130.51 ± 9.54 | 73.99 ± 7.22 |
| 120 | 374.64 ± 10.54 | 293.90 ± 6.76 | 171.44 ± 13.54 | 280.25 ± 14.67 | 182.28 ± 11.05 | 113.04 ± 2.54 |
| 168 | 511.37 ± 10.32 | 437.92 ± 17.51 | 251.20 ± 10.54 | 396.54 ± 17.45 | 209.93 ± 9.94 | 100.59 ± 7.43 |
| Strains | Mycelial Growth | Macroconidia Germination | ||
|---|---|---|---|---|
| F. vesiculosus scCO2 Extract | Fucosterol | |||
| RB.E | W.E | RB.F | ||
| IC50 [%] | ||||
| CBS122 | 59.63 ± 4.32 | 0.02 ± 0.00 | 0.13 ± 0.01 | |
| DEMFc37 | 120.12 ± 4.75 | 0.19 ± 0.07 | 0.07 ± 0.00 | 0.06 ± 0.00 |
| CBS129 | 93.21 ± 5.41 | 0.25 ± 0.09 | 1.82 ± 0.12 | |
| DEMFo38 | 38.43 ± 6.22 | 0.25 ± 0.03 | 0.09 ± 0.00 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyśkiewicz, K.; Tyśkiewicz, R.; Konkol, M.; Rój, E.; Jaroszuk-Ściseł, J.; Skalicka-Woźniak, K. Antifungal Properties of Fucus vesiculosus L. Supercritical Fluid Extract Against Fusarium culmorum and Fusarium oxysporum. Molecules 2019, 24, 3518. https://doi.org/10.3390/molecules24193518
Tyśkiewicz K, Tyśkiewicz R, Konkol M, Rój E, Jaroszuk-Ściseł J, Skalicka-Woźniak K. Antifungal Properties of Fucus vesiculosus L. Supercritical Fluid Extract Against Fusarium culmorum and Fusarium oxysporum. Molecules. 2019; 24(19):3518. https://doi.org/10.3390/molecules24193518
Chicago/Turabian StyleTyśkiewicz, Katarzyna, Renata Tyśkiewicz, Marcin Konkol, Edward Rój, Jolanta Jaroszuk-Ściseł, and Krystyna Skalicka-Woźniak. 2019. "Antifungal Properties of Fucus vesiculosus L. Supercritical Fluid Extract Against Fusarium culmorum and Fusarium oxysporum" Molecules 24, no. 19: 3518. https://doi.org/10.3390/molecules24193518
APA StyleTyśkiewicz, K., Tyśkiewicz, R., Konkol, M., Rój, E., Jaroszuk-Ściseł, J., & Skalicka-Woźniak, K. (2019). Antifungal Properties of Fucus vesiculosus L. Supercritical Fluid Extract Against Fusarium culmorum and Fusarium oxysporum. Molecules, 24(19), 3518. https://doi.org/10.3390/molecules24193518






