An Update on Isolation Methods for Proteomic Studies of Extracellular Vesicles in Biofluids
Abstract
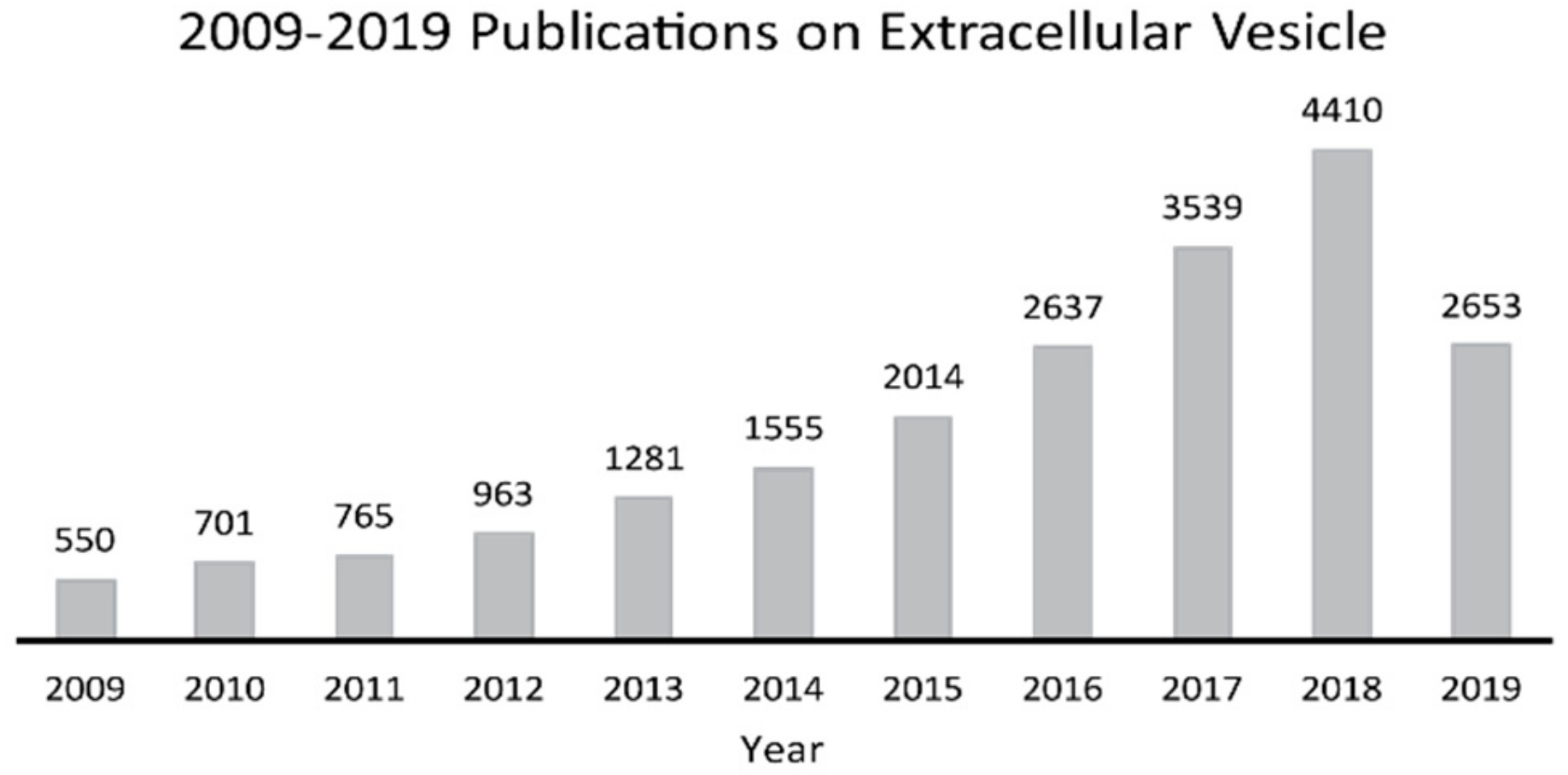
1. Introduction
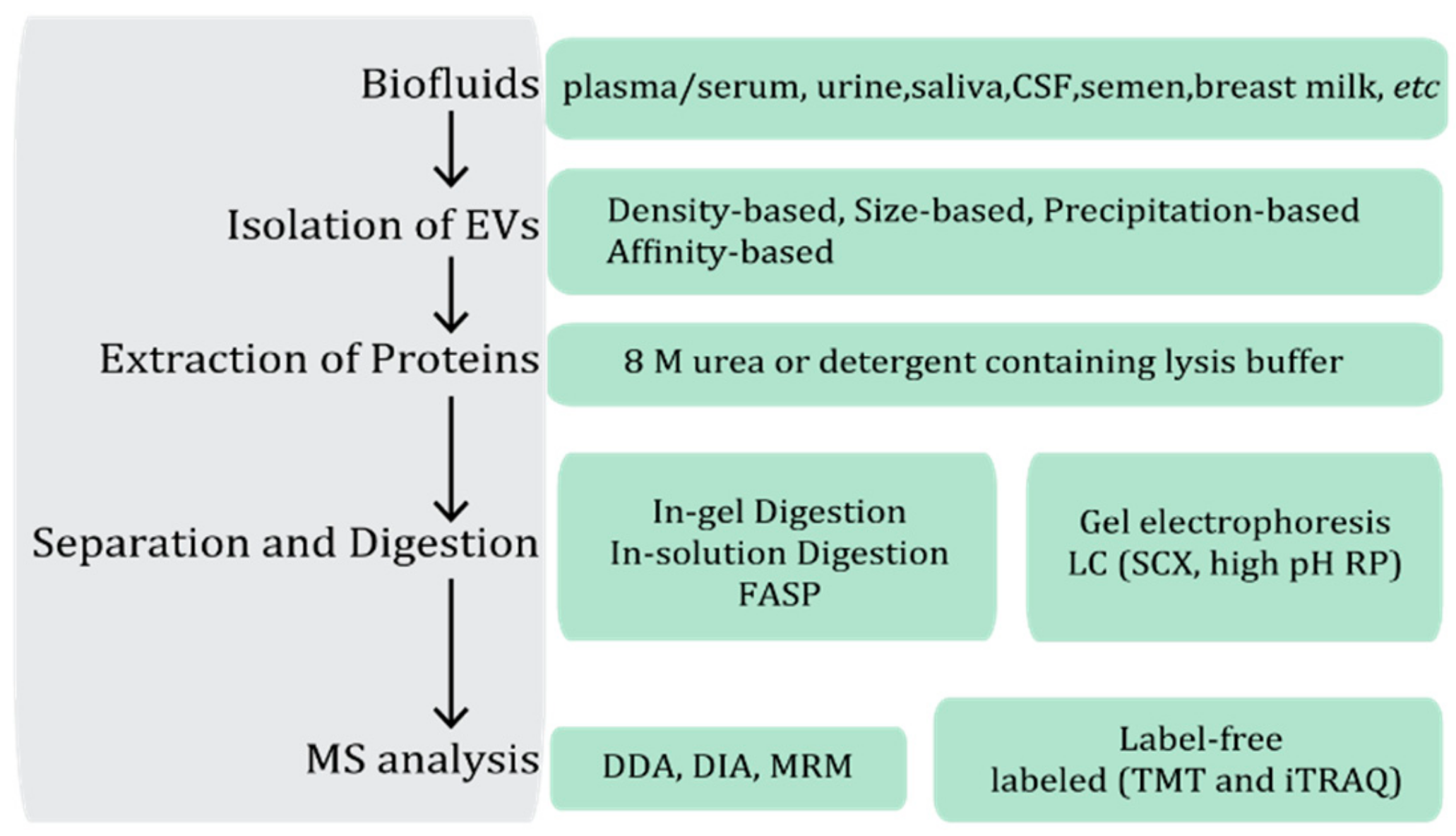
2. Isolation Strategies for Extracellular Vesicles in MS-Based Proteomic Studies
2.1. Sample Storage and Processing Conditions
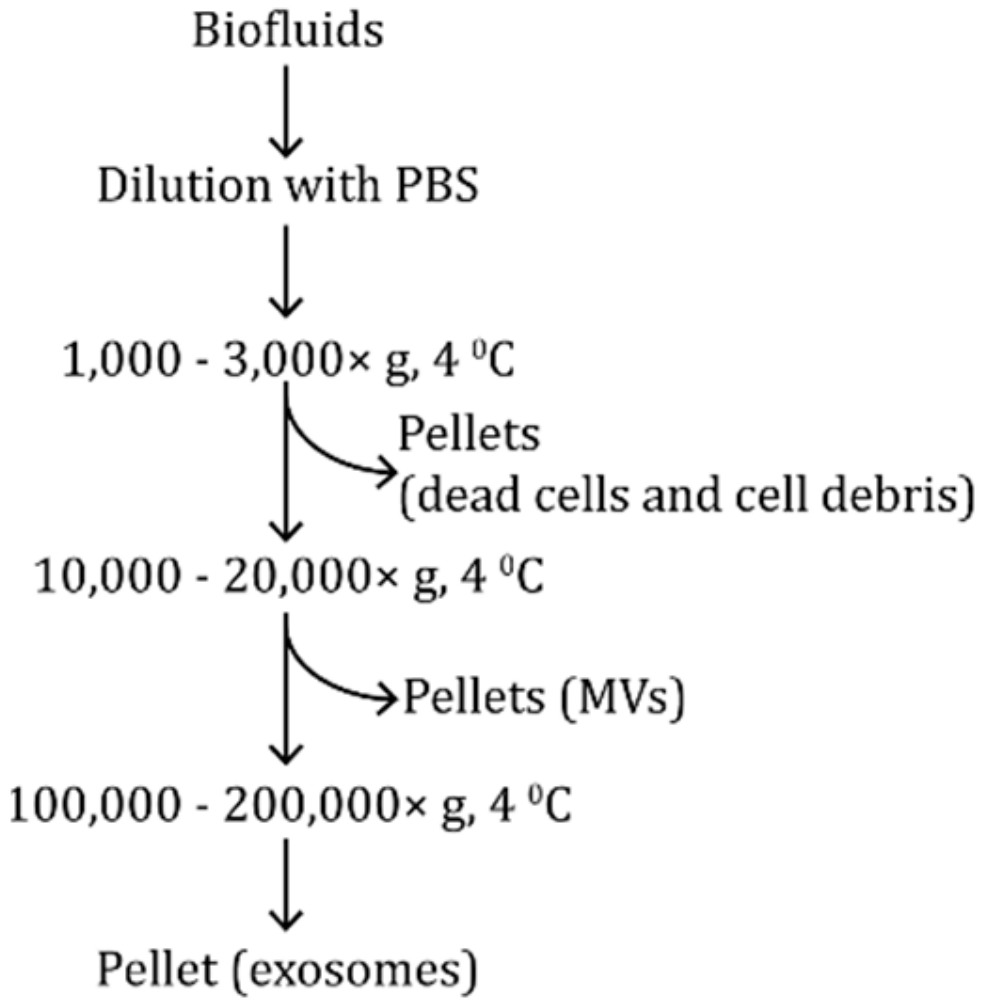
2.2. Density-Based Isolation
2.3. Size-Based Isolation
2.4. Precipitation-Based Isolation
2.5. Affinity-Based Isolation
3. Comparative Studies for Isolation Methods of EVs
4. MS Strategies Used in Proteomic Studies of Extracellular Vesicles
4.1. Sample Preparation and Separation
4.2. MS Acquisition
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Merchant, M.L.; Rood, I.M.; Deegens, J.K.J.; Klein, J.B. Isolation and Characterization of Urinary Extracellular Vesicles: Implications for Biomarker Discovery. Nat. Rev. Nephrol. 2017, 13, 731–749. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P. The Nature and Significance of Platelet Products in Human Plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular Vesicles in Physiological and Pathological Conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Patel, T.; Wong, D.; Das, S.; Freedman, J.E.; Laurent, L.C.; Carter, B.S.; Hochberg, F.; Van Keuren-Jensen, K.; Huentelman, M.; et al. Extracellular Rnas: Development as Biomarkers of Human Disease. J. Extracell Vesicles 2015, 4, 27495. [Google Scholar] [CrossRef] [PubMed]
- Loyer, X.; Vion, A.C.; Tedgui, A.; Boulanger, C.M. Microvesicles as Cell-Cell Messengers in Cardiovascular Diseases. Circ. Res. 2014, 114, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Pocsfalvi, G.; Stanly, C.; Vilasi, A.; Fiume, I.; Capasso, G.; Turiák, L.; Buzas, E.I.; Vékey, K. Mass Spectrometry of Extracellular Vesicles. Mass Spectrom. Rev. 2016, 35, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, J.B. What Are We Looking At? Extracellular Vesicles, Lipoproteins, or Both. Circ. Res. 2017, 121, 920–922. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Kalra, H.; Mathivanan, S. Exocarta as a Resource for Exosomal Research. J. Extracell Vesicles 2012, 1, 18374. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borras, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A Compendium for Extracellular Vesicles with Continuous Community Annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Lee, J.; Kim, S.R.; Choi, D.S.; Yoon, Y.J.; Kim, J.H.; Go, G.; Nhung, D.; Hong, K.; Jang, S.C.; et al. Evpedia: A Community Web Portal for Extracellular Vesicles Research. Bioinformatics 2015, 31, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Buzas, E.; Bemis, L.T.; Bora, A.; Lasser, C.; Lotvall, J.; Nolte-t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of Sample Collection, Isolation and Analysis Methods in Extracellular Vesicle Research. J. Extracell Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Raposo, G. As We Wait: Coping with an Imperfect Nomenclature for Extracellular Vesicles. J. Extracell Vesicles 2013, 2, 20389. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, M.N.; Calderon-Cruz, B.; Fernandez-Rocca, L.; Garcia, A. Application of Extracellular Vesicles Proteomics to Cardiovascular Disease: Guidelines, Data Analysis, and Future Perspectives. Proteomics 2019, 19, 1800247. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Luo, J.; Wang, S. Recent Progress in Isolation and Detection of Extracellular Vesicles for Cancer Diagnostics. Adv. Healthc. Mater. 2018, 7, e1800484. [Google Scholar] [CrossRef] [PubMed]
- Szatanek, R.; Baran, J.; Siedlar, M.; Baj-Krzyworzeka, M. Isolation of Extracellular Vesicles: Determining the Correct Approach. Int. J. Mol. Med. 2015, 36, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Abramowicz, A.; Widlak, P.; Pietrowska, M. Proteomic Analysis of Exosomal Cargo: The Challenge of High Purity Vesicle Isolation. Mol. Biosyst. 2016, 12, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Tzouanas, C.; Lim, J.S.Y.; Wen, Y.; Thiery, J.P.; Khoo, B.L. Microdevices for Non-Invasive Detection of Bladder Cancer. Chemosensors 2017, 5, 30. [Google Scholar] [CrossRef]
- Yuana, Y.; Boing, A.N.; Grootemaat, A.E.; van der Pol, E.; Hau, C.M.; Cizmar, P.; Buhr, E.; Sturk, A.; Nieuwland, R. Handling and Storage of Human Body Fluids for Analysis of Extracellular Vesicles. J. Extracell. Vesicles 2015, 4, 29260. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, R.; Judicone, C.; Mooberry, M.; Boucekine, M.; Key, N.S.; Dignat-George, F.; The ISTH SSC Workshop. Standardization of Pre-Analytical Variables in Plasma Microparticle Determination: Results of the International Society on Thrombosis and Haemostasis Ssc Collaborative Workshop. J. Thromb. Haemost. 2013, 11, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Yuana, Y.; Bertina, R.M.; Osanto, S. Pre-Analytical and Analytical Issues in the Analysis of Blood Microparticles. Thromb. Haemost. 2011, 105, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Akers, J.C.; Ramakrishnan, V.; Yang, I.; Hua, W.; Mao, Y.; Carter, B.S.; Chen, C.C. Optimizing Preservation of Extracellular Vesicular Mirnas Derived from Clinical Cerebrospinal Fluid. Cancer Biomark. 2016, 17, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Jamaly, S.; Ramberg, C.; Olsen, R.; Latysheva, N.; Webster, P.; Sovershaev, T.; Braekkan, S.K.; Hansen, J.B. Impact of Preanalytical Conditions on Plasma Concentration and Size Distribution of Extracellular Vesicles Using Nanoparticle Tracking Analysis. Sci. Rep. 2018, 8, 17216. [Google Scholar] [CrossRef] [PubMed]
- Jeyaram, A.; Jay, S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2017, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.Y.; Zhou, Y.X.; Lu, J.F.; Bai, Y.F.; Xie, X.Y.; Lu, Z.H. Mirna in Plasma Exosome Is Stable under Different Storage Conditions. Molecules 2014, 19, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Thery, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques Used for the Isolation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef] [PubMed]
- Livshts, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of Exosomes by Differential Centrifugation: Theoretical Analysis of a Commonly Used Protocol. Sci Rep. 2015, 5, 17319. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and Characterization of Exosomes from Cell Culture Supernatants and Biological Fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Trachtenberg, A.J.; Hochberg, F.H.; Skog, J.; Kuo, W.P. Impact of Biofluid Viscosity on Size and Sedimentation Efficiency of the Isolated Microvesicles. Front. Physiol. 2012, 3, 162. [Google Scholar] [CrossRef]
- Harel, M.; Oren-Giladi, P.; Kaidar-Person, O.; Shaked, Y.; Geiger, T. Proteomics of Microparticles with Silac Quantification (Promis-Quan): A Novel Proteomic Method for Plasma Biomarker Quantification. Mol. Cell Proteom. 2015, 14, 1127–1136. [Google Scholar] [CrossRef]
- Antwi-Baffour, S.; Adjei, J.K.; Agyemang-Yeboah, F.; Annani-Akollor, M.; Kyeremeh, R.; Asare, G.A.; Gyan, B. Proteomic Analysis of Microparticles Isolated from Malaria Positive Blood Samples. Proteome Sci. 2017, 15, 5. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Chutipongtanate, S.; Greis, K.D. Multiplex Biomarker Screening Assay for Urinary Extracellular Vesicles Study: A Targeted Labelfree Proteomic Approach. Sci. Rep. 2018, 8, 15039. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huo, C.; Qiao, Z.; Shang, Z.; Uzzaman, A.; Liu, S.; Jiang, X.; Fan, L.; Ji, L.; Guan, X.; et al. Comparative Proteomic Analysis of Exosomes and Microvesicles in Human Saliva for Lung Cancer. J. Proteome Res. 2018, 17, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular Vesicles Provide a Means for Tissue Crosstalk During Exercise. Cell Metab. 2018, 27, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Tan, Z.; Lubman, D.M. Exosome Enrichment of Human Serum Using Multiple Cycles of Centrifugation. Electrophoresis 2015, 36, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Langevin, S.M.; Kuhnell, D.; Orr-Asman, M.A.; Biesiada, J.; Zhang, X.; Medvedovic, M.; Thomas, H.E. Balancing Yield, Purity and Practicality: A Modified Differential Ultracentrifugation Protocol for Efficient Isolation of Small Extracellular Vesicles from Human Serum. RNA Biol. 2019, 16, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Cvjetkovic, A.; Lotvall, J.; Lasser, C. The Influence of Rotor Type and Centrifugation Time on the Yield and Purity of Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 23111. [Google Scholar] [CrossRef] [PubMed]
- Shiromizu, T.; Kume, H.; Ishida, M.; Adachi, J.; Kano, M.; Matsubara, H.; Tomonaga, T. Quantitation of Putative Colorectal Cancer Biomarker Candidates in Serum Extracellular Vesicles by Targeted Proteomics. Sci. Rep. 2017, 7, 12782. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Kume, H.; Matsuzaki, K.; Kawashima, A.; Ujike, T.; Nagahara, A.; Uemura, M.; Miyagawa, Y.; Tomonaga, T.; Nonomura, N. Proteomic Analysis of Urinary Extracellular Vesicles from High Gleason Score Prostate Cancer. Sci. Rep. 2017, 7, 42961. [Google Scholar] [CrossRef]
- Lin, Y.; Liang, A.; He, Y.; Li, Z.; Li, Z.; Wang, G.; Sun, F. Proteomic Analysis of Seminal Extracellular Vesicle Proteins Involved in Asthenozoospermia by iTRAQ. Mol. Reprod Dev. 2019. [Google Scholar] [CrossRef]
- Jiao, Y.J.; Jin, D.D.; Jiang, F.; Liu, J.X.; Qu, L.S.; Ni, W.K.; Liu, Z.X.; Lu, C.H.; Ni, R.Z.; Zhu, J.; et al. Characterization and Proteomic Profiling of Pancreatic Cancer-Derived Serum Exosomes. J. Cell Biochem. 2019, 120, 988–999. [Google Scholar] [CrossRef] [PubMed]
- Moon, P.G.; Lee, J.E.; You, S.; Kim, T.K.; Cho, J.H.; Kim, I.S.; Kwon, T.H.; Kim, C.D.; Park, S.H.; Hwang, D.; et al. Proteomic Analysis of Urinary Exosomes from Patients of Early Iga Nephropathy and Thin Basement Membrane Nephropathy. Proteomics 2011, 11, 2459–2475. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Li, J.; Rui, C.; Ji, H.; Ding, H.; Lu, Y.; De, W.; Sun, L. Comparative Proteomic Profile of the Human Umbilical Cord Blood Exosomes between Normal and Preeclampsia Pregnancies with High-Resolution Mass Spectrometry. Cell Physiol. Biochem. 2015, 36, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, T.; Gandolfo, M.T.; Das, S.; Knepper, M.A.; Bagnasco, S.M. Application of Systems Biology Principles to Protein Biomarker Discovery: Urinary Exosomal Proteome in Renal Transplantation. Proteomics Clin. Appl. 2012, 6, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Winck, F.V.; Ribeiro, A.C.P.; Domingues, R.R.; Ling, L.Y.; Riano-Pachon, D.M.; Rivera, C.; Brandao, T.B.; Gouvea, A.F.; Santos-Silva, A.R.; Coletta, R.D.; et al. Insights into Immune Responses in Oral Cancer through Proteomic Analysis of Saliva and Salivary Extracellular Vesicles. Sci. Rep. 2015, 5, 16305. [Google Scholar] [CrossRef] [PubMed]
- Ramacciotti, E.; Hawley, A.E.; Wrobleski, S.K.; Myers, D.D., Jr.; Strahler, J.R.; Andrews, P.C.; Guire, K.E.; Henke, P.K.; Wakefield, T.W. Proteomics of Microparticles after Deep Venous Thrombosis. Thromb. Res. 2010, 125, e269–e274. [Google Scholar] [CrossRef] [PubMed]
- Van Herwijnen, M.J.; Zonneveld, M.I.; Goerdayal, S.; Nolte-’t Hoen, E.N.; Garssen, J.; Stahl, B.; Maarten Altelaar, A.F.; Redegeld, F.A.; Wauben, M.H. Comprehensive Proteomic Analysis of Human Milk-Derived Extracellular Vesicles Unveils a Novel Functional Proteome Distinct from Other Milk Components. Mol. Cell. Proteom. 2016, 15, 3412–3423. [Google Scholar] [CrossRef]
- Chen, I.H.; Xue, L.; Hsu, C.C.; Paez, J.S.; Pan, L.; Andaluz, H.; Wendt, M.K.; Iliuk, A.B.; Zhu, J.K.; Tao, W.A. Phosphoproteins in Extracellular Vesicles as Candidate Markers for Breast Cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 3175–3180. [Google Scholar] [CrossRef]
- Manek, R.; Moghieb, A.; Yang, Z.; Kumar, D.; Kobessiy, F.; Sarkis, G.A.; Raghavan, V.; Wang, K.K.W. Protein Biomarkers and Neuroproteomics Characterization of Microvesicles/Exosomes from Human Cerebrospinal Fluid Following Traumatic Brain Injury. Mol. Neurobiol. 2018, 55, 6112–6128. [Google Scholar] [CrossRef]
- Greening, D.W.; Xu, R.; Ji, H.; Tauro, B.J.; Simpson, R.J. A Protocol for Exosome Isolation and Characterization: Evaluation of Ultracentrifugation, Density-Gradient Separation, and Immunoaffinity Capture Methods. Methods Mol. Biol. 2015, 1295, 179–209. [Google Scholar]
- Iwai, K.; Minamisawa, T.; Suga, K.; Yajima, Y.; Shiba, K. Isolation of Human Salivary Extracellular Vesicles by Iodixanol Density Gradient Ultracentrifugation and Their Characterizations. J. Extracell. Vesicles 2016, 5, 30829. [Google Scholar] [CrossRef] [PubMed]
- Arab, T.; Raffo-Romero, A.; Van Camp, C.; Lemaire, Q.; Le Marrec-Croq, F.; Drago, F.; Aboulouard, S.; Slomianny, C.; Lacoste, A.S.; Guigon, I.; et al. Proteomic Characterisation of Leech Microglia Extracellular Vesicles (Evs): Comparison between Differential Ultracentrifugation and Optiprep (Tm) Density Gradient Isolation. J. Extracell. Vesicles 2019, 8, 1603048. [Google Scholar] [CrossRef] [PubMed]
- Musante, L.; Saraswat, M.; Duriez, E.; Byrne, B.; Ravida, A.; Domon, B.; Holthofer, H. Biochemical and Physical Characterisation of Urinary Nanovesicles Following Chaps Treatment. PLoS ONE 2012, 7, e37279. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, M.N.; Sueiro, A.M.; Casas, V.; Izquierdo, I.; Hermida-Nogueira, L.; Guitian, E.; Casanueva, F.F.; Abian, J.; Carrascal, M.; Pardo, M.; et al. A Combination of Proteomic Approaches Identifies a Panel of Circulating Extracellular Vesicle Proteins Related to the Risk of Suffering Cardiovascular Disease in Obese Patients. Proteomics 2019, 19, e1800248. [Google Scholar] [CrossRef] [PubMed]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Moller, A. Optimized Exosome Isolation Protocol for Cell Culture Supernatant and Human Plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.L.; Powell, D.W.; Wilkey, D.W.; Cummins, T.D.; Deegens, J.K.; Rood, I.M.; McAfee, K.J.; Fleischer, C.; Klein, E.; Klein, J.B. Microfiltration Isolation of Human Urinary Exosomes for Characterization by MS. Proteom. Clin. Appl. 2010, 4, 84–96. [Google Scholar] [CrossRef]
- Musante, L.; Tataruch, D.; Gu, D.F.; Benito-Martin, A.; Calzaferri, G.; Aherne, S.; Holthofer, H. A Simplified Method to Recover Urinary Vesicles for Clinical Applications, and Sample Banking. Sci. Rep. 2014, 4, 7532. [Google Scholar] [CrossRef]
- Hu, S.; Musante, L.; Tataruch, D.; Xu, X.; Kretz, O.; Henry, M.; Meleady, P.; Luo, H.; Zou, H.; Jiang, Y.; et al. Purification and Identification of Membrane Proteins from Urinary Extracellular Vesicles Using Triton X-114 Phase Partitioning. J. Proteome Res. 2018, 17, 86–96. [Google Scholar] [CrossRef]
- Heinemann, M.L.; Ilmer, M.; Silva, L.P.; Hawke, D.H.; Recio, A.; Vorontsova, M.A.; Alt, E.; Vykoukal, J. Benchtop Isolation and Characterization of Functional Exosomes by Sequential Filtration. J. Chromatogr. A 2014, 1371, 125–135. [Google Scholar] [CrossRef]
- Osti, D.; Del Bene, M.; Rappa, G.; Santos, M.; Matafora, V.; Richichi, C.; Faletti, S.; Beznoussenko, G.V.; Mironov, A.; Bachi, A.; et al. Clinical Significance of Extracellular Vesicles in Plasma from Glioblastoma Patients. Clin. Cancer Res. 2019, 25, 266–276. [Google Scholar] [CrossRef]
- Musante, L.; Tataruch-Weinert, D.; Kerjaschki, D.; Henry, M.; Meleady, P.; Holthofer, H. Residual Urinary Extracellular Vesicles in Ultracentrifugation Supernatants after Hydrostatic Filtration Dialysis Enrichment. J. Extracell. Vesicles 2017, 6, 1267896. [Google Scholar] [CrossRef] [PubMed]
- De Menezes-Neto, A.; Saez, M.J.F.; Lozano-Ramos, I.; Segui-Barber, J.; Martin-Jaular, L.; Ullate, J.M.E.; Fernandez-Becerra, C.; Borras, F.E.; del Portillo, H.A. Size-Exclusion Chromatography as a Stand-Alone Methodology Identifies Novel Markers in Mass Spectrometry Analyses of Plasma-Derived Vesicles from Healthy Individuals. J. Extracell. Vesicles 2015, 4, 27378. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Cvjetkovic, A.; Jang, S.C.; Crescitelli, R.; Hosseinpour Feizi, M.A.; Nieuwland, R.; Lotvall, J.; Lasser, C. Detailed Analysis of the Plasma Extracellular Vesicle Proteome after Separation from Lipoproteins. Cell Mol. Life Sci. 2018, 75, 2873–2886. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, M.; Pietrowska, M.; Matysiak, N.; Mielanczyk, L.; Widlak, P. Proteome Profiling of Exosomes Purified from a Small Amount of Human Serum: The Problem of Co-Purified Serum Components. Proteomes 2019, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Aqrawi, L.A.; Galtung, H.K.; Vestad, B.; Ovstebo, R.; Thiede, B.; Rusthen, S.; Young, A.; Guerreiro, E.M.; Utheim, T.P.; Chen, X.; et al. Identification of Potential Saliva and Tear Biomarkers in Primary Sjogren’s Syndrome, Utilising the Extraction of Extracellular Vesicles and Proteomics Analysis. Arthritis Res. Ther. 2017, 19, 14. [Google Scholar] [CrossRef]
- Foers, A.D.; Chatfield, S.; Dagley, L.F.; Scicluna, B.J.; Webb, A.I.; Cheng, L.; Hill, A.F.; Wicks, I.P.; Pang, K.C. Enrichment of Extracellular Vesicles from Human Synovial Fluid Using Size Exclusion Chromatography. J. Extracell. Vesicles 2018, 7, 1490145. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Xie, Y.; Xu, L.; Zhan, S.H.; Xiao, Y.; Gao, Y.P.; Wu, B.; Ge, W. Protein Content and Functional Characteristics of Serum-Purified Exosomes from Patients with Colorectal Cancer Revealed by Quantitative Proteomics. J. Extracell. Vesicles 2017, 140, 900–913. [Google Scholar] [CrossRef]
- Zhang, W.; Ou, X.; Wu, X. Proteomics Profiling of Plasma Exosomes in Epithelial Ovarian Cancer: A Potential Role in the Coagulation Cascade, Diagnosis and Prognosis. Int. J. Oncol. 2019, 54, 1719–1733. [Google Scholar] [CrossRef]
- Tsuno, H.; Arito, M.; Suematsu, N.; Sato, T.; Hashimoto, A.; Matsui, T.; Omoteyama, K.; Sato, M.; Okamoto, K.; Tohma, S.; et al. A Proteomic Analysis of Serum-Derived Exosomes in Rheumatoid Arthritis. BMC Rheumatol. 2018, 2, 35. [Google Scholar] [CrossRef]
- Leberman, R. The Isolation of Plant Viruses by Means of “Simple” Coacervates. Virology 1966, 30, 341–347. [Google Scholar] [CrossRef]
- Ding, M.; Wang, C.; Lu, X.; Zhang, C.; Zhou, Z.; Chen, X.; Zhang, C.Y.; Zen, K.; Zhang, C. Comparison of Commercial Exosome Isolation Kits for Circulating Exosomal Microrna Profiling. Anal. Bioanal. Chem. 2018, 410, 3805–3814. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.J.; Sui, Z.G.; Shan, Y.C.; Hu, Y.C.; Chen, Y.B.; Zhang, L.H.; Zhang, Y.K. Effective Isolation of Exosomes with Polyethylene Glycol from Cell Culture Supernatant for in-Depth Proteome Profiling. Analyst 2016, 141, 4640–4646. [Google Scholar] [CrossRef] [PubMed]
- Rider, M.A.; Hurwitz, S.N.; Meckes, D.G. Extrapeg: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Sci. Rep. 2016, 6, 23978. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Han, C.; Labuz, J.M.; Kim, J.; Kim, J.; Cho, S.; Gho, Y.S.; Takayama, S.; Park, J. High-Yield Isolation of Extracellular Vesicles Using Aqueous Two-Phase System. Sci. Rep. 2015, 5, 13103. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Sun, W. Urinary Extracellular Microvesicles: Isolation Methods and Prospects for Urinary Proteome. Proteomics 2014, 14, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Hildonen, S.; Skarpen, E.; Halvorsen, T.G.; Reubsaet, L. Isolation and Mass Spectrometry Analysis of Urinary Extraexosomal Proteins. Sci. Rep. 2016, 6, 36331. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Ishikawa, N.; Tatsuguchi, A.; Saichi, N.; Fujii, R.; Nakagawa, H. Antibody-Coupled Monolithic Silica Microtips for Highthroughput Molecular Profiling of Circulating Exosomes. Sci. Rep. 2014, 4, 6232. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.D.; Gercel-Taylor, C. Microrna Signatures of Tumor-Derived Exosomes as Diagnostic Biomarkers of Ovarian Cancer. Gynecol. Oncol. 2008, 110, 13–21. [Google Scholar] [CrossRef]
- Kalra, H.; Adda, C.G.; Liem, M.; Ang, C.S.; Mechler, A.; Simpson, R.J.; Hulett, M.D.; Mathivanan, S. Comparative Proteomics Evaluation of Plasma Exosome Isolation Techniques and Assessment of the Stability of Exosomes in Normal Human Blood Plasma. Proteomics 2013, 13, 3354–3364. [Google Scholar] [CrossRef]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Mathivanan, S.; Ji, H.; Simpson, R.J. Two Distinct Populations of Exosomes Are Released from Lim1863 Colon Carcinoma Cell-Derived Organoids. Mol. Cell. Proteom. 2013, 12, 587–598. [Google Scholar] [CrossRef]
- Ghosh, A.; Davey, M.; Chute, I.C.; Griffiths, S.G.; Lewis, S.; Chacko, S.; Barnett, D.; Crapoulet, N.; Fournier, S.; Joy, A.; et al. Rapid Isolation of Extracellular Vesicles from Cell Culture and Biological Fluids Using a Synthetic Peptide with Specific Affinity for Heat Shock Proteins. PLoS ONE 2014, 9, e110443. [Google Scholar] [CrossRef] [PubMed]
- Bijnsdorp, I.V.; Maxouri, O.; Kardar, A.; Schelfhorst, T.; Piersma, S.R.; Pham, T.V.; Vis, A.; van Moorselaar, R.J.; Jimenez, C.R. Feasibility of Urinary Extracellular Vesicle Proteome Profiling Using a Robust and Simple, Clinically Applicable Isolation Method. J. Extracell. Vesicles 2017, 6, 1313091. [Google Scholar] [CrossRef] [PubMed]
- Balaj, L.; Atai, N.A.; Chen, W.L.; Mu, D.; Tannous, B.A.; Breakefield, X.O.; Skog, J.; Maguire, C.A. Heparin Affinity Purification of Extracellular Vesicles. Sci. Rep. 2015, 5, 10266. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.Y.; Jiao, F.L.; Xia, C.S.; Zhao, Y.; Ying, W.T.; Xie, Y.P.; Guan, X.Y.; Tao, M.; Zhang, Y.J.; Qin, W.J.; et al. A Novel Strategy for Facile Serum Exosome Isolation Based on Specific Interactions between Phospholipid Bilayers and TiO2. Chem. Sci. 2019, 10, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.; Tan, S.S.; Sze, S.K.; Lee, W.K.R.; Ng, M.J.; Lim, S.K. Plasma Biomarker Discovery in Preeclampsia Using a Novel Differential Isolation Technology for Circulating Extracellular Vesicles. Am. J. Obstet. Gynecol. 2014, 211, 380-e1. [Google Scholar] [PubMed]
- Nakai, W.; Yoshida, T.; Diez, D.; Miyatake, Y.; Nishibu, T.; Imawaka, N.; Naruse, K.; Sadamura, Y.; Hanayama, R. A Novel Affinity-Based Method for the Isolation of Highly Purified Extracellular Vesicles. Sci. Rep. 2016, 6, 33935. [Google Scholar] [CrossRef] [PubMed]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Comparison of Small Extracellular Vesicles Isolated from Plasma by Ultracentrifugation or Size-Exclusion Chromatography: Yield, Purity and Functional Potential. J. Extracell. Vesicles 2019, 8, 1560809. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.N.; Phillips, H.; Tomes, J.J.; Swain, M.T.; Wilkinson, T.J.; Brophy, P.M.; Morphew, R.M. The Importance of Extracellular Vesicle Purification for Downstream Analysis: A Comparison of Differential Centrifugation and Size Exclusion Chromatography for Helminth Pathogens. PLoS Negl. Trop. Dis. 2019, 13, e0007191. [Google Scholar] [CrossRef]
- Stranska, R.; Gysbrechts, L.; Wouters, J.; Vermeersch, P.; Bloch, K.; Dierickx, D.; Andrei, G.; Snoeck, R. Comparison of Membrane Affinity-Based Method with Size-Exclusion Chromatography for Isolation of Exosome-Like Vesicles from Human Plasma. J. Transl. Med. 2018, 16, 1. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative Analysis of Exosome Isolation Methods Using Culture Supernatant for Optimum Yield, Purity and Downstream Applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef]
- Antounians, L.; Tzanetakis, A.; Pellerito, O.; Catania, V.D.; Sulistyo, A.; Montalva, L.; McVey, M.J.; Zani, A. The Regenerative Potential of Amniotic Fluid Stem Cell Extracellular Vesicles: Lessons Learned by Comparing Different Isolation Techniques. Sci. Rep. 2019, 9, 1837. [Google Scholar] [CrossRef] [PubMed]
- Royo, F.; Zuniga-Garcia, P.; Sanchez-Mosquera, P.; Egia, A.; Perez, A.; Loizaga, A.; Arceo, R.; Lacasa, I.; Rabade, A.; Arrieta, E.; et al. Different Ev Enrichment Methods Suitable for Clinical Settings Yield Different Subpopulations of Urinary Extracellular Vesicles from Human Samples. J. Extracell. Vesicles 2016, 5, 29497. [Google Scholar] [CrossRef] [PubMed]
- Andreu, Z.; Rivas, E.; Sanguino-Pascual, A.; Lamana, A.; Marazuela, M.; Gonzalez-Alvaro, I.; Sanchez-Madrid, F.; de la Fuente, H.; Yanez-Mo, M. Comparative Analysis of Ev Isolation Procedures for Mirnas Detection in Serum Samples. J. Extracell. Vesicles 2016, 5, 31655. [Google Scholar] [CrossRef] [PubMed]
- Rood, I.M.; Deegens, J.K.; Merchant, M.L.; Tamboer, W.P.; Wilkey, D.W.; Wetzels, J.F.; Klein, J.B. Comparison of Three Methods for Isolation of Urinary Microvesicles to Identify Biomarkers of Nephrotic Syndrome. Kidney Int. 2010, 78, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Caradec, J.; Kharmate, G.; Hosseini-Beheshti, E.; Adomat, H.; Gleave, M.; Guns, E. Reproducibility and Efficiency of Serum-Derived Exosome Extraction Methods. Clin. Biochem. 2014, 47, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Joy, A.P.; Ayre, D.C.; Chute, I.C.; Beauregard, A.P.; Wajnberg, G.; Ghosh, A.; Lewis, S.M.; Ouellette, R.J.; Barnett, D.A. Proteome Profiling of Extracellular Vesicles Captured with the Affinity Peptide Vn96: Comparison of Laemmli and Trizol (C) Protein-Extraction Methods. J. Extracell. Vesicles 2018, 7, 1438727. [Google Scholar] [CrossRef] [PubMed]
- Fel, A.; Lewandowska, A.E.; Petrides, P.E.; Wisniewski, J.R. Comparison of Proteome Composition of Serum Enriched in Extracellular Vesicles Isolated from Polycythemia Vera Patients and Healthy Controls. Proteomes 2019, 7, 20. [Google Scholar] [CrossRef]
- Xie, X.F.; Chu, H.J.; Xu, Y.F.; Hua, L.; Wang, Z.P.; Huang, P.; Jia, H.L.; Zhang, L. Proteomics Study of Serum Exosomes in Kawasaki Disease Patients with Coronary Artery Aneurysms. Cardiol. J. 2018. [Google Scholar] [CrossRef]
- Gonzales, P.A.; Pisitkun, T.; Hoffert, J.D.; Tchapyjnikov, D.; Star, R.A.; Kleta, R.; Wang, N.S.; Knepper, M.A. Large-Scale Proteomics and Phosphoproteomics of Urinary Exosomes. J. Am. Soc. Nephrol. 2009, 20, 363–379. [Google Scholar] [CrossRef]
- Kittivorapart, J.; Crew, V.K.; Wilson, M.C.; Heesom, K.J.; Siritanaratkul, N.; Toye, A.M. Quantitative Proteomics of Plasma Vesicles Identify Novel Biomarkers for Hemoglobin E/Beta-Thalassemic Patients. Blood Adv. 2018, 2, 95–104. [Google Scholar] [CrossRef]
- Sok Hwee Cheow, E.; Hwan Sim, K.; de Kleijn, D.; Neng Lee, C.; Sorokin, V.; Sze, S.K. Simultaneous Enrichment of Plasma Soluble and Extracellular Vesicular Glycoproteins Using Prolonged Ultracentrifugation-Electrostatic Repulsion-Hydrophilic Interaction Chromatography (Puc-Erlic) Approach. Mol. Cell. Proteom. 2015, 14, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, M.; Joenvaara, S.; Musante, L.; Peltoniemi, H.; Holthofer, H.; Renkonen, R. N-Linked (N-) Glycoproteomics of Urinary Exosomes. Mol. Cell. Proteom. 2015, 14, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Braga-Lagache, S.; Buchs, N.; Iacovache, M.I.; Zuber, B.; Jackson, C.B.; Heller, M. Robust Label-Free, Quantitative Profiling of Circulating Plasma Microparticle (Mp) Associated Proteins. Mol. Cell. Proteom. 2016, 15, 3640–3652. [Google Scholar] [CrossRef] [PubMed]
- Kodidela, S.; Wang, Y.; Patters, B.J.; Gong, Y.; Sinha, N.; Ranjit, S.; Gerth, K.; Haque, S.; Cory, T.; McArthur, C.; et al. Proteomic Profiling of Exosomes Derived from Plasma of Hiv-Infected Alcohol Drinkers and Cigarette Smokers. J. Neuroimmune Pharmacol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Anderson, K.W.; Turko, I.V. Assessment of Extracellular Vesicles Purity Using Proteomic Standards. Analyt. Chem. 2017, 89, 11070–11075. [Google Scholar] [CrossRef]
- Wang, T.; Turko, I.V. Proteomic Toolbox to Standardize the Separation of Extracellular Vesicles and Lipoprotein Particles. J. Proteome Res. 2018, 17, 3104–3113. [Google Scholar] [CrossRef]

| Isolation | Proteomic Sample Preparation | Mass Spectrometry | Sample Origin | Number of Proteins | Year | Study |
|---|---|---|---|---|---|---|
| 19,000× g for 120 min | 2D-LC/MS: SCX as 1st dimensional | LTQ ion trap | plasma | 1806 proteins | 2017 | [30] |
| Sucrose cushion at 100,000× g for 90 min | 2D-LC/MS: C18-SCX stage-tip as 1st dimensional | Q-Exactive | serum | 702 proteins | 2017 | [38] |
| 100,000× g for 90 minincubation with DTT | iTRAQ 2D-LC/MS | LTQ-Orbitrap Velos Elite | urine | 4710 proteins in total and 3528 proteins for quantification | 2017 | [39] |
| Sucrose cushion at 100,000× g for 90 min | iTRAQ 2D-LC/MS: high pH as 1st dimensional | Orbitrap Fusion Lumos | semen | 3699 proteins in total | 2018 | [40] |
| 110,000× g for 70 min | FASP | Q Exactive | serum | 655 proteins | 2018 | [41] |
| 10,000× g, 20 min for MVs and at 100,000× g, 1 h for exosomes | in-solution digestion | SWATH-MS TripleTof 5600+ | urine | Targeted data analysis for 888 proteins | 2018 | [32] |
| Density ultracentrifugation at 270,000× g, 1 h and incubation with DTT | in-solution digestion | MSE | urine | 1877 proteins | 2011 | [42] |
| 100,000× g for 180 min | in-solution digestion | L Q-Exactive Orbitrap | umbilical cord blood | 211 proteins | 2015 | [43] |
| 200,000× g, 1 h and incubation with DTT | in-gel digestion | LTQ Orbitrap XL and LTQ Orbitrap Velos | urine | 1989 proteins in total | 2012 | [44] |
| 100,000× g for 90 min | in-solution digestion | LTQ Orbitrap Velos | saliva | 381 proteins | 2015 | [45] |
| 200,000× g for 90 min and incubation with KBr | iTRAQ LC off-line separation | MALDI * tandem mass spectrometry | plasma | not report | 2010 | [46] |
| Sucrose cushion at 192,000× g for 15–18 h | in-gel digestion | Q-Exactive | breast milk | 1963 proteins | 2016 | [47] |
| 20,000× g for 1 h for MVs | in-solution digestion | Q-Exactive/Plus | plasma | 3294 proteins in 4 h LC/MS | 2015 | [29] |
| 10,000 or 20,000× g, 1 h for MVs; 100,000 or 125,000× g, 2.5 h for exosomes | SDS-PAGE FASP | Q-Exactive | saliva | 785 proteins for MVs; 910 proteins for exosomes | 2018 | [33] |
| 20,000× g, 1 h for MVs; 100,000× g, 1 h for exosomes | in-solution digestion | LTQ-Orbitrap Velos Pro | plasma | 9225 phosphopeptides in MVs; 1014 phosphopeptides in exosomes | 2017 | [48] |
| 100,000× g for 70 min | in-gel digestion | LTQ-XL | CSF | 91 proteins identified from control466 proteins identified from disease | 2018 | [49] |
| Isolation Methods | Characterization Techniques | Samples | Study |
|---|---|---|---|
| dUC, SEC | NTA, Dissociation-enhanced lanthanide fluorescence immunoassay, WB, TEM | rat plasma, cell culture | [87] |
| dUC, SEC | TEM, AFM, WB, MS | cell culture | [88] |
| Affinity-based (exoEasy kit) and SEC (qEV) | WB, TEM, NTA, lipid quantification kit, RNA quality | plasma | [89] |
| dUC and Commercial kit from Invitrogen, 101Bio, Wako and iZON | Dynamic Light Scattering, immunoblot analysis, qRT-PCR, MS, Cell Proliferation Assay | cell culture | [90] |
| dUC, precipitation (ExoQuick, Total Exosome Isolation Reafent, Exo-PREP) and SEC (qEV) | TEM, NTA, WB | cell culture | [91] |
| Lectin-based, Exoquick, Total exosome Isolation and in-house modified procedure | WB, Reverse transcriptase and qPCR, EM | urine | [92] |
| dUC, precipitation (ExoQuick, Total exosome isolation, PEG, Exo-spin), filtration (ExoMir) | NTA, Flow cytometry, WB, PCR, | serum | [93] |
| dUC, filtration (Stirred cell and Centricon), OptiPrep DG, ExoQuick, Exo-spin, SEC | Tunable resistive pulse sensing, EM, WB | cell culture and plasma | [55] |
| SEC and Exo-Spin | NTA, Flow cytometry, MS | plasma | [62] |
| dUC, anti-EpCAM, OptiPrep DG | MS, WB, TEM | plasma | [79] |
| Nanomembrane ultrafiltration, dUC and dUC-SEC | MS, TEM, WB | urine | [94] |
| dUC, anti-EpCAM, OptiPrep DG | TEM, CryoEM, MS | cell culture | [50] |
| Sucrose DG and ExoQuick | TEM, NTA, WB | serum | [95] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; He, X.; Deng, Y.; Yang, C. An Update on Isolation Methods for Proteomic Studies of Extracellular Vesicles in Biofluids. Molecules 2019, 24, 3516. https://doi.org/10.3390/molecules24193516
Li J, He X, Deng Y, Yang C. An Update on Isolation Methods for Proteomic Studies of Extracellular Vesicles in Biofluids. Molecules. 2019; 24(19):3516. https://doi.org/10.3390/molecules24193516
Chicago/Turabian StyleLi, Jing, Xianqing He, Yuanyuan Deng, and Chenxi Yang. 2019. "An Update on Isolation Methods for Proteomic Studies of Extracellular Vesicles in Biofluids" Molecules 24, no. 19: 3516. https://doi.org/10.3390/molecules24193516
APA StyleLi, J., He, X., Deng, Y., & Yang, C. (2019). An Update on Isolation Methods for Proteomic Studies of Extracellular Vesicles in Biofluids. Molecules, 24(19), 3516. https://doi.org/10.3390/molecules24193516






